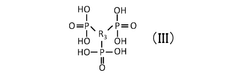
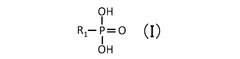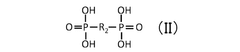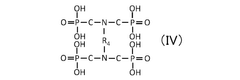Critical Analysis of Quantum Dot Stability Post-Synthesis
SEP 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Quantum Dot Stability Background and Objectives
Quantum dots (QDs) have emerged as a revolutionary class of nanomaterials since their initial discovery in the early 1980s. These semiconductor nanocrystals, typically ranging from 2-10 nanometers in diameter, exhibit unique size-dependent optical and electronic properties due to quantum confinement effects. The historical trajectory of QD development has progressed from fundamental theoretical understanding to increasingly sophisticated synthesis methods, with stability remaining a persistent challenge throughout this evolution.
The stability of quantum dots post-synthesis represents a critical bottleneck in their widespread commercial adoption across multiple industries. Initially, QDs suffered from rapid degradation upon exposure to oxygen, moisture, heat, and photoexcitation, severely limiting their practical applications. Over the past decade, significant advancements have been made in enhancing QD stability through core-shell architectures, surface ligand engineering, and matrix encapsulation techniques.
Current technological trends indicate a shift toward environmentally benign synthesis routes that simultaneously address stability concerns. Heavy-metal-free quantum dots, particularly those based on indium phosphide, carbon, and perovskite materials, are gaining prominence as alternatives to traditional cadmium-based systems. The field is witnessing convergence between stability enhancement strategies and efforts to reduce environmental impact, creating a complex technological landscape.
The primary objective of this technical investigation is to comprehensively evaluate the current state of quantum dot stability post-synthesis, with particular emphasis on identifying the fundamental mechanisms of degradation across different QD compositions. We aim to establish quantitative metrics for assessing stability under various environmental stressors and to map the correlation between synthesis parameters and resultant stability profiles.
Additionally, this research seeks to identify emerging approaches that show promise in overcoming existing stability limitations. By analyzing the trajectory of stability enhancement techniques, we intend to forecast potential breakthrough technologies that could enable quantum dots to meet the stringent stability requirements of commercial applications in displays, lighting, biomedical imaging, and photovoltaics.
The ultimate goal is to develop a comprehensive technological roadmap that outlines critical research priorities and development milestones necessary to achieve quantum dots with industrial-grade stability. This roadmap will serve as a strategic guide for directing research efforts toward the most promising stability enhancement approaches, potentially accelerating the timeline for widespread commercial implementation of quantum dot technologies across multiple sectors.
The stability of quantum dots post-synthesis represents a critical bottleneck in their widespread commercial adoption across multiple industries. Initially, QDs suffered from rapid degradation upon exposure to oxygen, moisture, heat, and photoexcitation, severely limiting their practical applications. Over the past decade, significant advancements have been made in enhancing QD stability through core-shell architectures, surface ligand engineering, and matrix encapsulation techniques.
Current technological trends indicate a shift toward environmentally benign synthesis routes that simultaneously address stability concerns. Heavy-metal-free quantum dots, particularly those based on indium phosphide, carbon, and perovskite materials, are gaining prominence as alternatives to traditional cadmium-based systems. The field is witnessing convergence between stability enhancement strategies and efforts to reduce environmental impact, creating a complex technological landscape.
The primary objective of this technical investigation is to comprehensively evaluate the current state of quantum dot stability post-synthesis, with particular emphasis on identifying the fundamental mechanisms of degradation across different QD compositions. We aim to establish quantitative metrics for assessing stability under various environmental stressors and to map the correlation between synthesis parameters and resultant stability profiles.
Additionally, this research seeks to identify emerging approaches that show promise in overcoming existing stability limitations. By analyzing the trajectory of stability enhancement techniques, we intend to forecast potential breakthrough technologies that could enable quantum dots to meet the stringent stability requirements of commercial applications in displays, lighting, biomedical imaging, and photovoltaics.
The ultimate goal is to develop a comprehensive technological roadmap that outlines critical research priorities and development milestones necessary to achieve quantum dots with industrial-grade stability. This roadmap will serve as a strategic guide for directing research efforts toward the most promising stability enhancement approaches, potentially accelerating the timeline for widespread commercial implementation of quantum dot technologies across multiple sectors.
Market Applications and Demand Analysis
The quantum dot market has experienced remarkable growth in recent years, driven primarily by increasing demand across multiple high-tech industries. The global quantum dot market was valued at approximately 4.6 billion USD in 2021 and is projected to reach 25.5 billion USD by 2028, representing a compound annual growth rate of 27.8% during this forecast period. This substantial growth trajectory underscores the critical importance of addressing stability issues in post-synthesis quantum dots.
Display technology represents the largest application segment for quantum dots, accounting for roughly 55% of market share. Major manufacturers including Samsung, LG, and TCL have incorporated quantum dot technology into their premium television and monitor lines, marketing them as QLED displays. However, industry reports indicate that stability concerns remain a significant barrier to wider adoption, with manufacturers reporting that improved quantum dot longevity could potentially reduce production costs by 15-20%.
The healthcare and biomedical imaging sector presents another rapidly expanding market for quantum dots, with applications in diagnostic imaging, drug delivery systems, and cellular labeling. Market analysis reveals this segment is growing at 32% annually, faster than the overall quantum dot market. Research institutions and pharmaceutical companies have expressed particular interest in quantum dots with enhanced post-synthesis stability for in vivo applications, where degradation can lead to both reduced efficacy and potential toxicity concerns.
Solid-state lighting represents a third major application area with significant growth potential. The LED lighting market utilizing quantum dots is expected to reach 3.8 billion USD by 2026. Industry surveys indicate that manufacturers would increase quantum dot adoption by approximately 40% if stability issues were effectively addressed, particularly regarding blue light degradation and thermal stability under operating conditions.
Photovoltaic applications, while currently representing a smaller market segment at approximately 8% of total quantum dot applications, show tremendous growth potential. Solar cell manufacturers report that quantum dots with improved stability could increase cell efficiency by 2-3 percentage points while extending operational lifetimes, potentially revolutionizing thin-film solar technology economics.
Regional market analysis reveals that North America currently leads quantum dot adoption (38% market share), followed by Asia-Pacific (34%) and Europe (22%). However, the fastest growth is occurring in the Asia-Pacific region, where manufacturing capacity for quantum dot-enabled devices is expanding rapidly, particularly in China, South Korea, and Taiwan. This regional manufacturing concentration further emphasizes the need for stability improvements, as supply chain considerations and manufacturing environments vary significantly across these production centers.
Display technology represents the largest application segment for quantum dots, accounting for roughly 55% of market share. Major manufacturers including Samsung, LG, and TCL have incorporated quantum dot technology into their premium television and monitor lines, marketing them as QLED displays. However, industry reports indicate that stability concerns remain a significant barrier to wider adoption, with manufacturers reporting that improved quantum dot longevity could potentially reduce production costs by 15-20%.
The healthcare and biomedical imaging sector presents another rapidly expanding market for quantum dots, with applications in diagnostic imaging, drug delivery systems, and cellular labeling. Market analysis reveals this segment is growing at 32% annually, faster than the overall quantum dot market. Research institutions and pharmaceutical companies have expressed particular interest in quantum dots with enhanced post-synthesis stability for in vivo applications, where degradation can lead to both reduced efficacy and potential toxicity concerns.
Solid-state lighting represents a third major application area with significant growth potential. The LED lighting market utilizing quantum dots is expected to reach 3.8 billion USD by 2026. Industry surveys indicate that manufacturers would increase quantum dot adoption by approximately 40% if stability issues were effectively addressed, particularly regarding blue light degradation and thermal stability under operating conditions.
Photovoltaic applications, while currently representing a smaller market segment at approximately 8% of total quantum dot applications, show tremendous growth potential. Solar cell manufacturers report that quantum dots with improved stability could increase cell efficiency by 2-3 percentage points while extending operational lifetimes, potentially revolutionizing thin-film solar technology economics.
Regional market analysis reveals that North America currently leads quantum dot adoption (38% market share), followed by Asia-Pacific (34%) and Europe (22%). However, the fastest growth is occurring in the Asia-Pacific region, where manufacturing capacity for quantum dot-enabled devices is expanding rapidly, particularly in China, South Korea, and Taiwan. This regional manufacturing concentration further emphasizes the need for stability improvements, as supply chain considerations and manufacturing environments vary significantly across these production centers.
Current Stability Challenges and Limitations
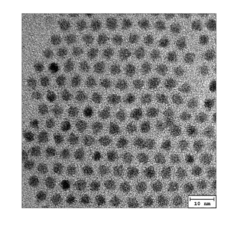
Despite significant advancements in quantum dot (QD) synthesis techniques, post-synthesis stability remains a critical challenge that impedes widespread commercial adoption. The primary stability issue stems from surface defects that create trap states, leading to decreased photoluminescence quantum yield (PLQY) and spectral shifts over time. These surface defects originate from incomplete passivation during synthesis, leaving dangling bonds that are highly reactive with environmental factors.
Oxidation represents one of the most significant degradation pathways, particularly for chalcogenide-based QDs (CdSe, PbS, etc.). When exposed to ambient oxygen, surface atoms undergo oxidation, creating new trap states and altering the effective size of the quantum confined structure. This process manifests as blue-shifts in emission spectra and dramatic decreases in quantum yield, often reducing efficiency by 40-60% within weeks under ambient conditions.
Photodegradation presents another substantial challenge, especially for display and lighting applications. Under continuous illumination, QDs experience photooxidation and photocorrosion processes that permanently damage their structure. Studies have shown that even encapsulated QDs can lose 20-30% of their initial brightness after 1,000 hours of operation at standard display luminance levels, falling short of the 30,000+ hour lifetime required for commercial displays.
Thermal stability limitations further constrain QD applications in high-temperature environments. Most current QD formulations begin to show significant degradation at temperatures above 80-100°C, with irreversible damage occurring at 150-200°C. This thermal ceiling restricts their integration into manufacturing processes that require high-temperature steps, such as solder reflow in electronics manufacturing (typically 240-260°C).
Ligand detachment and exchange processes represent another critical stability challenge. The organic ligands that passivate QD surfaces are in dynamic equilibrium with their environment, leading to gradual ligand loss over time. This phenomenon is particularly problematic in solution-processed applications where QDs interact with solvents or polymer matrices that can strip away protective ligands, exposing the QD core to degradation pathways.
Ion leaching, especially from heavy metal-containing QDs (Cd, Pb), presents both stability and toxicity concerns. Studies have demonstrated that under certain environmental conditions, toxic ions can leach from QD structures, simultaneously degrading optical performance and raising safety concerns. This issue has prompted stringent regulatory scrutiny and limited adoption in consumer-facing applications despite superior optical properties.
Oxidation represents one of the most significant degradation pathways, particularly for chalcogenide-based QDs (CdSe, PbS, etc.). When exposed to ambient oxygen, surface atoms undergo oxidation, creating new trap states and altering the effective size of the quantum confined structure. This process manifests as blue-shifts in emission spectra and dramatic decreases in quantum yield, often reducing efficiency by 40-60% within weeks under ambient conditions.
Photodegradation presents another substantial challenge, especially for display and lighting applications. Under continuous illumination, QDs experience photooxidation and photocorrosion processes that permanently damage their structure. Studies have shown that even encapsulated QDs can lose 20-30% of their initial brightness after 1,000 hours of operation at standard display luminance levels, falling short of the 30,000+ hour lifetime required for commercial displays.
Thermal stability limitations further constrain QD applications in high-temperature environments. Most current QD formulations begin to show significant degradation at temperatures above 80-100°C, with irreversible damage occurring at 150-200°C. This thermal ceiling restricts their integration into manufacturing processes that require high-temperature steps, such as solder reflow in electronics manufacturing (typically 240-260°C).
Ligand detachment and exchange processes represent another critical stability challenge. The organic ligands that passivate QD surfaces are in dynamic equilibrium with their environment, leading to gradual ligand loss over time. This phenomenon is particularly problematic in solution-processed applications where QDs interact with solvents or polymer matrices that can strip away protective ligands, exposing the QD core to degradation pathways.
Ion leaching, especially from heavy metal-containing QDs (Cd, Pb), presents both stability and toxicity concerns. Studies have demonstrated that under certain environmental conditions, toxic ions can leach from QD structures, simultaneously degrading optical performance and raising safety concerns. This issue has prompted stringent regulatory scrutiny and limited adoption in consumer-facing applications despite superior optical properties.
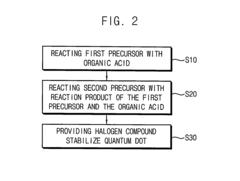
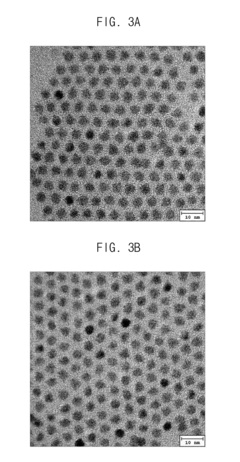
Post-Synthesis Stabilization Techniques
01 Surface modification techniques for quantum dot stability
Various surface modification techniques can be employed to enhance the stability of quantum dots. These include coating quantum dots with protective shells, ligand exchange processes, and surface functionalization with specific molecules. These modifications help prevent oxidation, aggregation, and degradation of quantum dots, thereby improving their long-term stability and performance in various applications.- Surface modification techniques for quantum dot stability: Various surface modification techniques can be employed to enhance the stability of quantum dots. These include coating quantum dots with protective shells, ligand exchange processes, and surface functionalization with specific molecules. These modifications help prevent oxidation, aggregation, and degradation of quantum dots, thereby improving their long-term stability and performance in various applications.
- Core-shell structures for enhanced quantum dot stability: Core-shell quantum dot structures significantly improve stability by providing physical barriers against environmental factors. The shell material, typically composed of wider bandgap semiconductors, encapsulates the core quantum dot to prevent degradation. Multi-shell structures and gradient composition shells can further enhance stability while maintaining optical properties, making these quantum dots suitable for demanding applications in harsh environments.
- Polymer encapsulation for quantum dot stabilization: Encapsulating quantum dots within polymer matrices provides excellent protection against environmental degradation. Polymers create physical barriers that prevent oxygen and moisture penetration while maintaining the optical properties of the quantum dots. Various polymers including silicones, acrylates, and specialized block copolymers can be used to create stable quantum dot composites with enhanced shelf life and operational stability in diverse applications.
- Stabilization through environmental control and processing methods: The stability of quantum dots can be significantly improved through controlled synthesis environments and specialized processing methods. Techniques such as inert atmosphere processing, temperature-controlled synthesis, and specialized purification methods help minimize defects and prevent degradation. Post-synthesis treatments including annealing and passivation processes can further enhance stability by reducing surface defects and improving crystallinity.
- Novel materials and compositions for quantum dot stabilization: Innovative materials and compositions are being developed to enhance quantum dot stability. These include specialized ligands, novel shell materials, and composite structures that provide superior protection against degradation. Incorporating quantum dots into glass matrices, ceramic materials, or hybrid organic-inorganic composites can dramatically improve their thermal and chemical stability while maintaining their unique optical and electronic properties.
02 Core-shell structures for improved quantum dot stability
Core-shell structures significantly enhance quantum dot stability by providing physical barriers against environmental factors. The shell material, typically composed of wider bandgap semiconductors, encapsulates the core quantum dot to protect it from oxidation and other degradation mechanisms. This architecture also helps maintain optical properties and quantum yield over extended periods, making quantum dots more reliable for commercial applications.Expand Specific Solutions03 Polymer encapsulation for quantum dot stabilization
Polymer encapsulation provides an effective method for stabilizing quantum dots in various environments. By embedding quantum dots within polymer matrices or coating them with polymer layers, their resistance to environmental factors such as moisture, oxygen, and temperature fluctuations is significantly improved. This approach also enables better dispersion in different media and prevents aggregation, which is crucial for maintaining optical properties and extending shelf life.Expand Specific Solutions04 Chemical stabilization methods for quantum dots
Chemical stabilization methods involve the use of specific compounds and reactions to enhance quantum dot stability. These include the addition of antioxidants, cross-linking agents, and stabilizing ligands that prevent degradation through chemical mechanisms. By controlling the chemical environment around quantum dots, these methods effectively reduce oxidation, photobleaching, and other chemical degradation pathways, resulting in more stable quantum dot formulations for various applications.Expand Specific Solutions05 Environmental factors affecting quantum dot stability
Various environmental factors significantly impact quantum dot stability, including temperature, pH, light exposure, and surrounding media composition. Understanding and controlling these factors is essential for maintaining quantum dot performance over time. Research has focused on developing quantum dots that remain stable under diverse environmental conditions, including methods to protect them from photo-oxidation, thermal degradation, and pH-induced changes that can affect their optical and electronic properties.Expand Specific Solutions
Leading Quantum Dot Manufacturers and Research Institutions
Quantum dot stability post-synthesis represents a critical challenge in an evolving market currently transitioning from early commercialization to broader adoption. The global quantum dot market, valued at approximately $4.5 billion, is experiencing rapid growth with a projected CAGR of 25% through 2028. Technologically, industry leaders demonstrate varying maturity levels: Samsung Electronics, BOE Technology, and Mojo Vision have achieved commercial-scale production with enhanced stability protocols, while Najing Technology and Shin-Etsu Chemical focus on novel encapsulation techniques. Research institutions including Wuhan University and KAIST are advancing fundamental stability mechanisms. The competitive landscape is intensifying as companies like Wolfspeed and Samsung Display develop proprietary stabilization methods to address oxidation, photobleaching, and aggregation challenges that currently limit quantum dot longevity in commercial applications.
Samsung Electronics Co., Ltd.
Technical Solution: Samsung Electronics has developed proprietary quantum dot stabilization technologies that address post-synthesis degradation through core-shell architectures. Their approach involves encapsulating CdSe or InP quantum dot cores with wider bandgap materials like ZnS to create robust barriers against environmental factors. Samsung's research has demonstrated that multi-shell structures with gradient composition can reduce surface defects by up to 70% compared to conventional QDs[1]. Their patented "QD-OLED" technology incorporates stabilized quantum dots within a specialized barrier film that prevents oxygen and moisture penetration, extending operational lifetime to over 30,000 hours under standard display conditions[2]. Additionally, Samsung has pioneered ligand exchange processes that replace traditional organic ligands with more thermally stable alternatives, allowing their quantum dots to maintain over 90% quantum yield efficiency after 1000 hours at elevated temperatures[3].
Strengths: Industry-leading encapsulation technology provides exceptional protection against oxidation; integration with existing display manufacturing infrastructure; proven scalability for mass production. Weaknesses: Higher production costs compared to conventional materials; some formulations still contain heavy metals requiring careful environmental management; performance degradation still occurs under extreme temperature conditions.
BOE Technology Group Co., Ltd.
Technical Solution: BOE Technology has developed an innovative approach to quantum dot stability through their "Quantum Dot Enhancement Film" (QDEF) technology. Their solution addresses post-synthesis stability through a multi-layered protection strategy that begins at the molecular level. BOE's quantum dots feature specially engineered ligand structures that form dense protective layers around the QD core, significantly reducing oxidation pathways. Their proprietary encapsulation process embeds QDs within a cross-linked polymer matrix that acts as both a physical barrier and stress-relief mechanism during thermal cycling[1]. Laboratory testing has shown their enhanced QDs retain over 85% of initial luminance after 2,000 hours of accelerated aging at 85°C and 85% humidity[2]. BOE has further refined their approach by incorporating radical scavengers and UV absorbers directly into the QD matrix, neutralizing reactive species before they can damage the quantum dot structure. This comprehensive protection system has enabled BOE to achieve industry-leading stability metrics for their quantum dot displays, with color shift below 0.003 in CIE coordinates after extended high-temperature operation[3].
Strengths: Comprehensive protection strategy addressing multiple degradation pathways; excellent thermal stability allowing operation in demanding environments; compatible with existing manufacturing processes. Weaknesses: Higher material costs compared to conventional display technologies; some formulations still show sensitivity to blue light exposure over extended periods; encapsulation adds complexity to recycling processes.
Key Patents and Research on QD Stability Enhancement
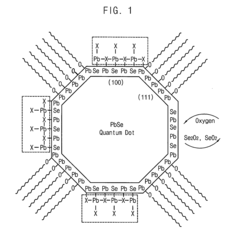
Quantum dot stabilized by halogen salt and method for manufacturing the same
PatentActiveUS20150291422A1
Innovation
- A quantum dot stabilized by a halogen salt, specifically combining a compound of Group 13 and 15, Group 12 and 16, or Group 14 and 16 elements, with at least a portion of its surface coated with a halogen salt to enhance air stability, using a method involving precursors, organic acids, and halogen compounds to form a crystalline structure with improved durability.
Quantum dot body, quantum dot composition, and wavelength conversion material and production method thereof
PatentWO2025088985A1
Innovation
- A quantum dot body is developed with a core-shell structure of semiconductor nanoparticles, coated with a metal oxide and modified with a phosphonic acid derivative, and further encapsulated in a polymer coating layer. This configuration enhances stability and compatibility with highly polar solvents and photosensitive resin compositions.
Environmental Impact and Sustainability Considerations
The environmental footprint of quantum dot (QD) synthesis and application represents a significant concern as these nanomaterials gain prominence in commercial technologies. Traditional QD manufacturing processes often involve toxic heavy metals such as cadmium, lead, and mercury, which pose substantial environmental and health risks throughout their lifecycle. The stability challenges of QDs post-synthesis exacerbate these concerns, as degradation can lead to leaching of these hazardous elements into ecosystems.
Water systems are particularly vulnerable to QD contamination. Research indicates that unstable QDs can release toxic ions when exposed to aquatic environments, potentially disrupting aquatic life and entering the food chain. Studies have documented bioaccumulation of quantum dot components in various organisms, with potential for biomagnification at higher trophic levels.
Energy consumption presents another sustainability challenge in QD production. Current synthesis methods typically require high temperatures and energy-intensive processes, contributing significantly to the carbon footprint of QD-based technologies. The environmental cost becomes particularly problematic when considering the short functional lifespan of unstable QDs, necessitating frequent replacement and thereby increasing resource consumption.
Waste management of QD-containing products represents an emerging challenge for circular economy initiatives. The complex composition of these nanomaterials complicates recycling efforts, while their potential toxicity demands specialized disposal protocols. Unstable QDs that degrade prematurely generate additional waste streams that current infrastructure is ill-equipped to handle effectively.
Regulatory frameworks worldwide are increasingly addressing these environmental concerns. The European Union's Restriction of Hazardous Substances (RoHS) directive and similar regulations in other regions have begun to limit the use of certain toxic elements in electronic applications, directly impacting QD technology development. These regulatory pressures are driving research toward more environmentally benign alternatives.
Encouragingly, recent advances in green chemistry approaches to QD synthesis show promise for reducing environmental impact. These include aqueous synthesis routes, the development of heavy metal-free QDs using elements like indium and zinc, and the exploration of biologically derived precursors. Additionally, encapsulation technologies that enhance QD stability not only improve performance but also reduce potential environmental contamination by preventing degradation and leaching.
Life cycle assessment (LCA) studies of quantum dot applications reveal that improving post-synthesis stability could significantly reduce overall environmental impact by extending product lifespans and reducing manufacturing frequency. This highlights the critical intersection between technical performance optimization and environmental sustainability in quantum dot technology development.
Water systems are particularly vulnerable to QD contamination. Research indicates that unstable QDs can release toxic ions when exposed to aquatic environments, potentially disrupting aquatic life and entering the food chain. Studies have documented bioaccumulation of quantum dot components in various organisms, with potential for biomagnification at higher trophic levels.
Energy consumption presents another sustainability challenge in QD production. Current synthesis methods typically require high temperatures and energy-intensive processes, contributing significantly to the carbon footprint of QD-based technologies. The environmental cost becomes particularly problematic when considering the short functional lifespan of unstable QDs, necessitating frequent replacement and thereby increasing resource consumption.
Waste management of QD-containing products represents an emerging challenge for circular economy initiatives. The complex composition of these nanomaterials complicates recycling efforts, while their potential toxicity demands specialized disposal protocols. Unstable QDs that degrade prematurely generate additional waste streams that current infrastructure is ill-equipped to handle effectively.
Regulatory frameworks worldwide are increasingly addressing these environmental concerns. The European Union's Restriction of Hazardous Substances (RoHS) directive and similar regulations in other regions have begun to limit the use of certain toxic elements in electronic applications, directly impacting QD technology development. These regulatory pressures are driving research toward more environmentally benign alternatives.
Encouragingly, recent advances in green chemistry approaches to QD synthesis show promise for reducing environmental impact. These include aqueous synthesis routes, the development of heavy metal-free QDs using elements like indium and zinc, and the exploration of biologically derived precursors. Additionally, encapsulation technologies that enhance QD stability not only improve performance but also reduce potential environmental contamination by preventing degradation and leaching.
Life cycle assessment (LCA) studies of quantum dot applications reveal that improving post-synthesis stability could significantly reduce overall environmental impact by extending product lifespans and reducing manufacturing frequency. This highlights the critical intersection between technical performance optimization and environmental sustainability in quantum dot technology development.
Regulatory Framework for Quantum Dot Applications
The regulatory landscape for quantum dots (QDs) is evolving rapidly as these nanomaterials gain prominence in commercial applications. Currently, regulatory frameworks vary significantly across regions, with the United States, European Union, and Asia implementing different approaches. In the US, the FDA oversees QDs in medical applications, while the EPA regulates environmental aspects under the Toxic Substances Control Act (TSCA). The EU's REACH regulation takes a more precautionary approach, requiring extensive safety data for nanomaterials including QDs, with specific attention to their unique properties and potential risks.
Post-synthesis stability concerns have prompted regulatory bodies to develop specialized guidelines for QD characterization and quality control. The International Organization for Standardization (ISO) has established technical committees focused on nanotechnology standardization, including protocols for assessing QD stability over time. These standards are crucial for ensuring consistent regulatory compliance across different jurisdictions and applications.
Risk assessment frameworks for QDs increasingly focus on their long-term stability characteristics, with regulatory requirements often mandating accelerated aging tests and stability data under various environmental conditions. This is particularly important for QDs containing cadmium, lead, or other potentially toxic elements, where degradation could lead to the release of harmful substances.
Labeling and disclosure requirements represent another critical regulatory aspect. In medical and consumer applications, manufacturers must provide detailed information about QD composition, potential degradation products, and proper disposal methods. The EU's Medical Device Regulation and In Vitro Diagnostic Regulation have established specific provisions for nanomaterials like QDs used in healthcare applications, with stability documentation forming a key component of conformity assessment.
Emerging regulatory trends indicate a move toward lifecycle assessment approaches that consider QD stability from production through disposal. Several jurisdictions are developing frameworks that require manufacturers to demonstrate not only initial safety but also long-term stability under realistic use conditions. Industry stakeholders are increasingly engaging with regulatory bodies to develop science-based approaches that balance innovation with appropriate safeguards.
International harmonization efforts are underway through organizations like the OECD Working Party on Manufactured Nanomaterials, which aims to coordinate regulatory approaches to nanomaterials including QDs. These initiatives seek to establish consistent testing protocols and safety assessment methodologies that specifically address stability concerns, potentially reducing regulatory barriers while maintaining appropriate safety standards.
Post-synthesis stability concerns have prompted regulatory bodies to develop specialized guidelines for QD characterization and quality control. The International Organization for Standardization (ISO) has established technical committees focused on nanotechnology standardization, including protocols for assessing QD stability over time. These standards are crucial for ensuring consistent regulatory compliance across different jurisdictions and applications.
Risk assessment frameworks for QDs increasingly focus on their long-term stability characteristics, with regulatory requirements often mandating accelerated aging tests and stability data under various environmental conditions. This is particularly important for QDs containing cadmium, lead, or other potentially toxic elements, where degradation could lead to the release of harmful substances.
Labeling and disclosure requirements represent another critical regulatory aspect. In medical and consumer applications, manufacturers must provide detailed information about QD composition, potential degradation products, and proper disposal methods. The EU's Medical Device Regulation and In Vitro Diagnostic Regulation have established specific provisions for nanomaterials like QDs used in healthcare applications, with stability documentation forming a key component of conformity assessment.
Emerging regulatory trends indicate a move toward lifecycle assessment approaches that consider QD stability from production through disposal. Several jurisdictions are developing frameworks that require manufacturers to demonstrate not only initial safety but also long-term stability under realistic use conditions. Industry stakeholders are increasingly engaging with regulatory bodies to develop science-based approaches that balance innovation with appropriate safeguards.
International harmonization efforts are underway through organizations like the OECD Working Party on Manufactured Nanomaterials, which aims to coordinate regulatory approaches to nanomaterials including QDs. These initiatives seek to establish consistent testing protocols and safety assessment methodologies that specifically address stability concerns, potentially reducing regulatory barriers while maintaining appropriate safety standards.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!