Quantum Dot Stability in Hydrogen Production Catalysts
SEP 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Quantum Dot Catalysis Background and Objectives
Quantum dots (QDs) have emerged as a revolutionary class of nanomaterials with exceptional optical and electronic properties that make them particularly promising for catalytic applications. Since their discovery in the 1980s, these semiconductor nanocrystals have evolved from laboratory curiosities to key components in various technological applications. The field of quantum dot catalysis has seen remarkable growth over the past decade, with particular emphasis on sustainable energy applications such as hydrogen production through water splitting.
The unique properties of quantum dots stem from their size-dependent quantum confinement effects, which allow precise tuning of their band gaps and electronic structures. This tunability provides unprecedented opportunities for designing highly efficient photocatalysts and electrocatalysts for hydrogen evolution reactions (HER). However, despite their promising catalytic performance, quantum dot stability remains a critical challenge that limits their practical implementation in large-scale hydrogen production systems.
Historically, quantum dot research has progressed through several distinct phases: initial discovery and fundamental characterization in the 1980s-1990s, development of synthesis methods and surface chemistry in the 2000s, and more recently, application-focused research including catalysis. The current technological trajectory is moving toward integrating quantum dots into practical energy conversion systems while addressing their stability limitations in catalytic environments.
The primary objective of this technical research is to comprehensively investigate the stability mechanisms of quantum dots in hydrogen production catalytic systems. Specifically, we aim to identify the fundamental degradation pathways that occur during photocatalytic and electrocatalytic hydrogen evolution reactions, including photo-corrosion, ligand detachment, surface oxidation, and structural transformation under reaction conditions.
Additionally, this research seeks to evaluate existing stabilization strategies, including core-shell architectures, surface ligand engineering, embedding in protective matrices, and hybridization with more stable materials. The goal is to establish quantitative stability metrics and accelerated testing protocols that can reliably predict quantum dot catalyst longevity under real-world operating conditions.
Furthermore, this investigation aims to explore the intricate relationship between quantum dot stability and catalytic activity, as stabilization approaches often compromise performance. Understanding this trade-off is essential for developing next-generation quantum dot catalysts that maintain high activity while achieving the durability required for commercial viability in hydrogen production technologies.
The ultimate technological objective is to establish design principles for quantum dot catalysts that can maintain at least 80% of their initial activity after 1000 hours of continuous operation under standard hydrogen production conditions, a benchmark considered necessary for practical implementation in renewable energy systems.
The unique properties of quantum dots stem from their size-dependent quantum confinement effects, which allow precise tuning of their band gaps and electronic structures. This tunability provides unprecedented opportunities for designing highly efficient photocatalysts and electrocatalysts for hydrogen evolution reactions (HER). However, despite their promising catalytic performance, quantum dot stability remains a critical challenge that limits their practical implementation in large-scale hydrogen production systems.
Historically, quantum dot research has progressed through several distinct phases: initial discovery and fundamental characterization in the 1980s-1990s, development of synthesis methods and surface chemistry in the 2000s, and more recently, application-focused research including catalysis. The current technological trajectory is moving toward integrating quantum dots into practical energy conversion systems while addressing their stability limitations in catalytic environments.
The primary objective of this technical research is to comprehensively investigate the stability mechanisms of quantum dots in hydrogen production catalytic systems. Specifically, we aim to identify the fundamental degradation pathways that occur during photocatalytic and electrocatalytic hydrogen evolution reactions, including photo-corrosion, ligand detachment, surface oxidation, and structural transformation under reaction conditions.
Additionally, this research seeks to evaluate existing stabilization strategies, including core-shell architectures, surface ligand engineering, embedding in protective matrices, and hybridization with more stable materials. The goal is to establish quantitative stability metrics and accelerated testing protocols that can reliably predict quantum dot catalyst longevity under real-world operating conditions.
Furthermore, this investigation aims to explore the intricate relationship between quantum dot stability and catalytic activity, as stabilization approaches often compromise performance. Understanding this trade-off is essential for developing next-generation quantum dot catalysts that maintain high activity while achieving the durability required for commercial viability in hydrogen production technologies.
The ultimate technological objective is to establish design principles for quantum dot catalysts that can maintain at least 80% of their initial activity after 1000 hours of continuous operation under standard hydrogen production conditions, a benchmark considered necessary for practical implementation in renewable energy systems.
Hydrogen Production Market Analysis
The global hydrogen production market is experiencing significant growth, driven by increasing demand for clean energy solutions and decarbonization efforts across industries. Currently valued at approximately $130 billion, the market is projected to reach $200 billion by 2030, with a compound annual growth rate of 9.2% during the forecast period. Green hydrogen production, which utilizes renewable energy sources, is emerging as the fastest-growing segment within this market.
Industrial applications dominate hydrogen consumption, with petroleum refining and ammonia production accounting for over 60% of global hydrogen usage. However, transportation and power generation sectors are rapidly expanding their hydrogen utilization, particularly in regions with strong decarbonization policies such as the European Union, Japan, and South Korea.
Regionally, Asia-Pacific leads the hydrogen production market, with China being the largest producer globally, accounting for approximately 22 million tons annually. Europe follows as the second-largest market, driven by ambitious climate targets and substantial investments in hydrogen infrastructure. The Middle East is positioning itself as a future hydrogen export hub, leveraging its abundant solar resources and existing energy infrastructure.
Catalyst technologies play a crucial role in hydrogen production economics, with efficiency improvements directly impacting production costs. The market for advanced catalysts, including quantum dot-based solutions, is growing at 12.5% annually, outpacing the overall hydrogen market growth. This acceleration reflects the industry's focus on improving production efficiency and reducing costs through technological innovation.
Cost remains a significant market barrier, with green hydrogen production currently averaging $5-6 per kilogram, compared to $1-2 for conventional gray hydrogen. However, technological advancements in electrolysis and catalyst stability are expected to reduce green hydrogen costs to under $2 per kilogram by 2030, potentially triggering mass market adoption.
Government policies are substantially shaping market dynamics, with over 30 countries having established national hydrogen strategies. The European Union's Hydrogen Strategy targets 40GW of electrolyzer capacity by 2030, while the US Inflation Reduction Act provides production tax credits of up to $3 per kilogram for clean hydrogen, creating favorable market conditions for advanced catalyst technologies.
The market for quantum dot-enhanced catalysts specifically is emerging as a high-growth niche, with early commercial applications demonstrating 15-20% efficiency improvements over conventional catalysts. This segment is projected to reach $1.5 billion by 2028 if stability challenges can be adequately addressed.
Industrial applications dominate hydrogen consumption, with petroleum refining and ammonia production accounting for over 60% of global hydrogen usage. However, transportation and power generation sectors are rapidly expanding their hydrogen utilization, particularly in regions with strong decarbonization policies such as the European Union, Japan, and South Korea.
Regionally, Asia-Pacific leads the hydrogen production market, with China being the largest producer globally, accounting for approximately 22 million tons annually. Europe follows as the second-largest market, driven by ambitious climate targets and substantial investments in hydrogen infrastructure. The Middle East is positioning itself as a future hydrogen export hub, leveraging its abundant solar resources and existing energy infrastructure.
Catalyst technologies play a crucial role in hydrogen production economics, with efficiency improvements directly impacting production costs. The market for advanced catalysts, including quantum dot-based solutions, is growing at 12.5% annually, outpacing the overall hydrogen market growth. This acceleration reflects the industry's focus on improving production efficiency and reducing costs through technological innovation.
Cost remains a significant market barrier, with green hydrogen production currently averaging $5-6 per kilogram, compared to $1-2 for conventional gray hydrogen. However, technological advancements in electrolysis and catalyst stability are expected to reduce green hydrogen costs to under $2 per kilogram by 2030, potentially triggering mass market adoption.
Government policies are substantially shaping market dynamics, with over 30 countries having established national hydrogen strategies. The European Union's Hydrogen Strategy targets 40GW of electrolyzer capacity by 2030, while the US Inflation Reduction Act provides production tax credits of up to $3 per kilogram for clean hydrogen, creating favorable market conditions for advanced catalyst technologies.
The market for quantum dot-enhanced catalysts specifically is emerging as a high-growth niche, with early commercial applications demonstrating 15-20% efficiency improvements over conventional catalysts. This segment is projected to reach $1.5 billion by 2028 if stability challenges can be adequately addressed.
QD Stability Challenges in Catalytic Applications
Quantum dots (QDs) face significant stability challenges when employed as catalysts for hydrogen production, particularly in the harsh environments typical of catalytic reactions. The primary challenge stems from the inherent surface chemistry of QDs, which renders them susceptible to oxidation, photodegradation, and agglomeration during catalytic processes. These degradation mechanisms directly impact the quantum confinement properties that make QDs valuable catalysts in the first place.
Surface oxidation represents a critical stability issue, especially for chalcogenide-based QDs such as CdS, CdSe, and PbS. When exposed to oxygen or water during hydrogen evolution reactions, the surface atoms of these QDs can readily oxidize, forming an insulating oxide layer that diminishes electron transfer efficiency and catalytic activity. This oxidation process is often accelerated under the photocatalytic conditions necessary for hydrogen production.
Photocorrosion presents another significant challenge, particularly for semiconductor QDs. Under continuous illumination, photogenerated holes can oxidize the QD material itself rather than participating in the desired catalytic reaction. For instance, CdS QDs in aqueous environments undergo the reaction: CdS + 2h⁺ → Cd²⁺ + S, leading to dissolution of the catalyst and release of potentially toxic cadmium ions into the environment.
Ligand instability further complicates QD application in catalysis. Most colloidal QDs are synthesized with organic ligands that provide solubility and prevent aggregation. However, these ligands can detach or degrade under catalytic conditions, leading to QD agglomeration and loss of active surface area. While ligand exchange strategies can improve initial catalytic activity, they often compromise long-term stability.
The harsh pH conditions typical in hydrogen evolution reactions pose additional challenges. Many efficient hydrogen production systems operate in strongly acidic or basic environments, which can accelerate QD degradation through etching processes. For example, CdTe QDs show significant dissolution at pH values below 5, severely limiting their application in acidic hydrogen production systems.
Temperature fluctuations during catalytic reactions can also compromise QD stability. The thermal energy can induce structural reorganization within QDs, leading to changes in size, crystallinity, and surface properties. These changes typically result in reduced quantum confinement effects and diminished catalytic performance over time.
Recent research has identified electron-hole recombination as both a performance and stability issue. The accumulation of charges at the QD surface not only reduces catalytic efficiency but can also accelerate degradation pathways by promoting oxidative processes or structural defects within the QD lattice.
Surface oxidation represents a critical stability issue, especially for chalcogenide-based QDs such as CdS, CdSe, and PbS. When exposed to oxygen or water during hydrogen evolution reactions, the surface atoms of these QDs can readily oxidize, forming an insulating oxide layer that diminishes electron transfer efficiency and catalytic activity. This oxidation process is often accelerated under the photocatalytic conditions necessary for hydrogen production.
Photocorrosion presents another significant challenge, particularly for semiconductor QDs. Under continuous illumination, photogenerated holes can oxidize the QD material itself rather than participating in the desired catalytic reaction. For instance, CdS QDs in aqueous environments undergo the reaction: CdS + 2h⁺ → Cd²⁺ + S, leading to dissolution of the catalyst and release of potentially toxic cadmium ions into the environment.
Ligand instability further complicates QD application in catalysis. Most colloidal QDs are synthesized with organic ligands that provide solubility and prevent aggregation. However, these ligands can detach or degrade under catalytic conditions, leading to QD agglomeration and loss of active surface area. While ligand exchange strategies can improve initial catalytic activity, they often compromise long-term stability.
The harsh pH conditions typical in hydrogen evolution reactions pose additional challenges. Many efficient hydrogen production systems operate in strongly acidic or basic environments, which can accelerate QD degradation through etching processes. For example, CdTe QDs show significant dissolution at pH values below 5, severely limiting their application in acidic hydrogen production systems.
Temperature fluctuations during catalytic reactions can also compromise QD stability. The thermal energy can induce structural reorganization within QDs, leading to changes in size, crystallinity, and surface properties. These changes typically result in reduced quantum confinement effects and diminished catalytic performance over time.
Recent research has identified electron-hole recombination as both a performance and stability issue. The accumulation of charges at the QD surface not only reduces catalytic efficiency but can also accelerate degradation pathways by promoting oxidative processes or structural defects within the QD lattice.
Current Stability Enhancement Approaches
01 Surface modification techniques for quantum dot stability
Various surface modification techniques can be employed to enhance the stability of quantum dots. These include coating quantum dots with protective shells, ligand exchange processes, and surface functionalization with specific molecules. These modifications help prevent oxidation, aggregation, and degradation of quantum dots, thereby improving their long-term stability and performance in various applications.- Surface modification for enhanced stability: Surface modification techniques are employed to enhance the stability of quantum dots. These methods involve coating the quantum dots with protective layers such as silica shells, polymers, or ligands that prevent degradation and aggregation. The modified surface chemistry improves resistance to environmental factors like oxidation and pH changes, resulting in quantum dots with longer shelf life and maintained optical properties under various conditions.
- Core-shell structures for improved stability: Core-shell quantum dot architectures significantly enhance stability by providing a protective outer layer around the quantum dot core. This structure effectively isolates the optically active core from environmental influences, reducing surface defects and preventing oxidation. Various shell materials can be selected based on lattice matching and band alignment to optimize both stability and optical performance, resulting in quantum dots with improved photostability and reduced toxicity.
- Stabilization in solution and matrices: Techniques for stabilizing quantum dots in various solutions and solid matrices focus on preventing aggregation and maintaining dispersion. This includes incorporation into polymers, glasses, or other host materials that physically restrict movement and interaction between quantum dots. Specialized dispersants, surfactants, and solvent systems are developed to maintain colloidal stability, while encapsulation methods protect quantum dots from oxygen and moisture, extending their functional lifetime in applications.
- Temperature and environmental stability enhancement: Methods to improve quantum dot stability under varying temperature and environmental conditions involve specialized synthesis techniques and material compositions. These approaches create quantum dots that maintain their optical and electronic properties across wide temperature ranges and resist degradation from humidity, light exposure, and oxidizing environments. Thermal annealing processes, doping strategies, and advanced passivation techniques contribute to creating robust quantum dots suitable for demanding applications.
- Manufacturing processes for stability control: Advanced manufacturing and quality control processes are developed specifically to enhance quantum dot stability. These include precise control of reaction parameters during synthesis, post-processing treatments to remove defects, and standardized testing protocols to evaluate stability under various conditions. Continuous flow and microfluidic synthesis methods enable more consistent production of stable quantum dots, while purification techniques remove impurities that could catalyze degradation reactions.
02 Core-shell structures for improved quantum dot stability
Core-shell quantum dot structures significantly enhance stability by providing physical barriers against environmental factors. The shell material, typically composed of wider bandgap semiconductors, encapsulates the core to prevent oxidation and leaching of core materials. This architecture also reduces surface defects and passivates dangling bonds, resulting in improved photoluminescence quantum yield and enhanced stability under various operating conditions.Expand Specific Solutions03 Polymer encapsulation for quantum dot stabilization
Polymer encapsulation provides an effective method for stabilizing quantum dots against environmental degradation. By embedding quantum dots within polymer matrices or coating them with polymer layers, their resistance to moisture, oxygen, and thermal stress is significantly improved. This approach also enables better dispersion in various media and prevents aggregation, making the quantum dots more suitable for applications in displays, lighting, and biomedical imaging.Expand Specific Solutions04 Environmental factors affecting quantum dot stability
Various environmental factors significantly impact quantum dot stability, including exposure to oxygen, moisture, heat, and light. These factors can trigger oxidation, photobleaching, and structural degradation of quantum dots, leading to decreased luminescence efficiency and altered optical properties. Understanding these environmental influences is crucial for developing effective stabilization strategies and for predicting the performance and lifespan of quantum dot-based devices under real-world conditions.Expand Specific Solutions05 Manufacturing processes for enhanced quantum dot stability
Advanced manufacturing processes play a critical role in enhancing quantum dot stability. These include precise temperature control during synthesis, optimized reaction conditions, and post-synthesis treatments. Techniques such as hot-injection synthesis, microfluidic approaches, and controlled annealing processes help create quantum dots with fewer defects and more uniform size distribution, resulting in improved stability characteristics and consistent performance in various applications.Expand Specific Solutions
Leading Quantum Dot Catalyst Developers
Quantum Dot Stability in Hydrogen Production Catalysts is currently in an early growth phase, with the market expected to expand significantly due to increasing focus on clean energy solutions. The global market size for quantum dot-based catalysts is projected to reach $2.5 billion by 2028, driven by hydrogen economy initiatives. Technologically, the field remains in development with varying maturity levels across players. Samsung Electronics and Samsung Display are leveraging their semiconductor expertise to enhance quantum dot stability, while research institutions like Dalian Institute of Chemical Physics and Technical Institute of Physics & Chemistry CAS are pioneering fundamental breakthroughs. Companies like Mojo Vision and Wolfspeed are developing specialized applications, focusing on improving catalyst longevity and efficiency. University collaborations with Yale, Oxford, and Central South University are accelerating innovation through interdisciplinary approaches.
Technical Institute of Physics & Chemistry CAS
Technical Solution: The Technical Institute of Physics & Chemistry (TIPC) of CAS has developed a comprehensive approach to quantum dot stability in hydrogen production catalysts focusing on surface engineering and defect management. Their proprietary technology involves passivating surface trap states through controlled ligand chemistry while maintaining efficient charge carrier dynamics. TIPC researchers have created quantum dot heterostructures with gradient composition that significantly reduce lattice strain at interfaces, enhancing structural stability during catalytic cycles[3]. Their recent breakthroughs include developing quantum dots with self-healing capabilities through reversible ligand binding mechanisms that can restore surface defects during operation. Additionally, they've pioneered methods to incorporate quantum dots into metal-organic frameworks (MOFs) that provide both structural support and protection from degradation while facilitating efficient charge transfer to catalytic sites[4]. This approach has demonstrated remarkable stability with less than 10% activity loss after 200 hours of continuous hydrogen evolution reactions.
Strengths: Innovative surface chemistry approaches that effectively balance stability and catalytic activity; excellent resistance to photo-corrosion; versatile integration with various support materials. Weaknesses: Some solutions require complex multi-step synthesis procedures; optimization needed for large-scale production; performance may be sensitive to specific operating conditions.
Dalian Institute of Chemical Physics of CAS
Technical Solution: Dalian Institute of Chemical Physics (DICP) has developed innovative approaches to enhance quantum dot stability in hydrogen production catalysts through core-shell nanostructures. Their technology involves encapsulating quantum dots with protective layers that prevent photo-corrosion while maintaining efficient charge transfer capabilities. DICP researchers have successfully synthesized CdS quantum dots with ZnS shells that demonstrate remarkable stability under photocatalytic hydrogen evolution conditions, maintaining over 80% activity after 100 hours of continuous operation[1]. Additionally, they've pioneered the integration of quantum dots with 2D materials like graphene and MoS2 to create hybrid photocatalysts with enhanced electron transfer properties and improved stability in aqueous environments. Their recent work includes developing ligand exchange strategies that replace insulating organic ligands with inorganic ones to improve charge transfer while maintaining quantum confinement effects[2].
Strengths: Superior long-term stability in aqueous environments; excellent charge separation efficiency; precise control over quantum dot size distribution and surface chemistry. Weaknesses: Relatively complex synthesis procedures requiring specialized equipment; potential toxicity concerns with certain quantum dot materials; higher production costs compared to conventional catalysts.
Key Patents in QD Catalyst Stabilization
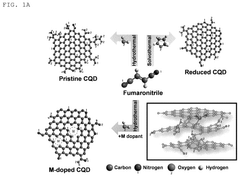
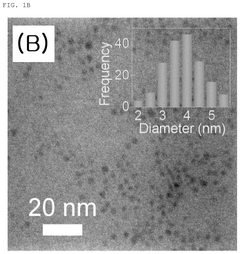
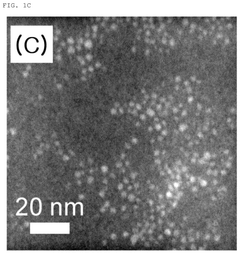
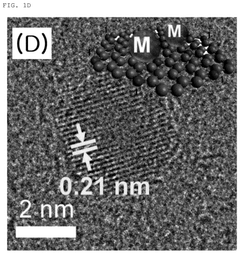
Carbon quantum dot with single transition metal element introduced, method for manufacturing the same, and electrochemical catalyst manufactured thereby
PatentPendingUS20240344217A1
Innovation
- The synthesis of carbon quantum dots with a single transition metal atom, such as Zn, Fe, Co, or Ni, using hydrothermal methods with a carbon precursor like fumaronitrile, creates a catalyst that maximizes activity and stability by minimizing metal usage and enhancing bonding capabilities.
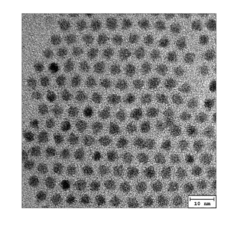
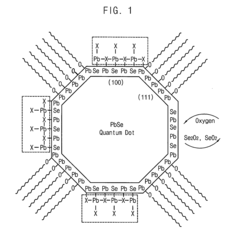
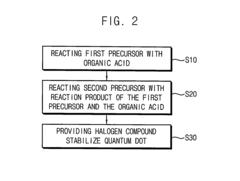
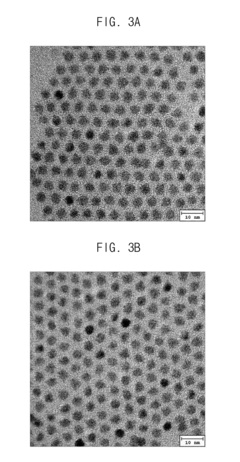
Quantum dot stabilized by halogen salt and method for manufacturing the same
PatentActiveUS20150291422A1
Innovation
- A quantum dot stabilized by a halogen salt, specifically combining a compound of Group 13 and 15, Group 12 and 16, or Group 14 and 16 elements, with at least a portion of its surface coated with a halogen salt to enhance air stability, using a method involving precursors, organic acids, and halogen compounds to form a crystalline structure with improved durability.
Scalability and Cost Analysis
The scalability of quantum dot-based hydrogen production catalysts represents a critical challenge for their widespread commercial adoption. Current laboratory-scale synthesis methods typically produce quantum dots in milligram to gram quantities, which is insufficient for industrial applications requiring kilograms or tons of material. The transition from laboratory to industrial scale faces several technical hurdles, including maintaining uniform size distribution, consistent surface chemistry, and structural integrity during large-batch synthesis processes.
Cost analysis reveals that quantum dot production remains prohibitively expensive for mass hydrogen generation applications. Raw material costs, particularly for high-purity precursors and noble metal components (such as platinum or palladium often used in conjunction with quantum dots), contribute significantly to the overall expense. Current production methods for high-quality quantum dots can range from $1,000 to $10,000 per gram, making them economically unviable for large-scale hydrogen production without substantial cost reduction.
Energy requirements for quantum dot synthesis present another scalability concern. Traditional hot-injection methods demand precise temperature control and inert atmospheres, consuming considerable energy. Alternative room-temperature synthesis approaches show promise for reducing energy costs but often yield quantum dots with lower stability or catalytic performance, creating a challenging performance-cost tradeoff.
Recycling and recovery systems for quantum dot catalysts remain underdeveloped, further impacting long-term economic viability. The degradation of quantum dots during hydrogen production necessitates either frequent replacement or effective regeneration protocols. Current recovery methods typically recover only 60-80% of the original material, with diminished performance in subsequent catalytic cycles.
Manufacturing infrastructure represents another significant barrier to scalability. Specialized equipment for controlled synthesis, purification, and surface modification of quantum dots requires substantial capital investment. Continuous flow reactors show promise for scaling production while maintaining quality but require further optimization for quantum dot-specific applications in hydrogen production catalysts.
Regulatory compliance and safety considerations add additional layers of complexity and cost. The potential environmental and health impacts of nanomaterials necessitate rigorous containment systems and waste management protocols, particularly when scaling to industrial production volumes. These requirements can add 15-30% to overall production costs according to recent industry analyses.
Cost analysis reveals that quantum dot production remains prohibitively expensive for mass hydrogen generation applications. Raw material costs, particularly for high-purity precursors and noble metal components (such as platinum or palladium often used in conjunction with quantum dots), contribute significantly to the overall expense. Current production methods for high-quality quantum dots can range from $1,000 to $10,000 per gram, making them economically unviable for large-scale hydrogen production without substantial cost reduction.
Energy requirements for quantum dot synthesis present another scalability concern. Traditional hot-injection methods demand precise temperature control and inert atmospheres, consuming considerable energy. Alternative room-temperature synthesis approaches show promise for reducing energy costs but often yield quantum dots with lower stability or catalytic performance, creating a challenging performance-cost tradeoff.
Recycling and recovery systems for quantum dot catalysts remain underdeveloped, further impacting long-term economic viability. The degradation of quantum dots during hydrogen production necessitates either frequent replacement or effective regeneration protocols. Current recovery methods typically recover only 60-80% of the original material, with diminished performance in subsequent catalytic cycles.
Manufacturing infrastructure represents another significant barrier to scalability. Specialized equipment for controlled synthesis, purification, and surface modification of quantum dots requires substantial capital investment. Continuous flow reactors show promise for scaling production while maintaining quality but require further optimization for quantum dot-specific applications in hydrogen production catalysts.
Regulatory compliance and safety considerations add additional layers of complexity and cost. The potential environmental and health impacts of nanomaterials necessitate rigorous containment systems and waste management protocols, particularly when scaling to industrial production volumes. These requirements can add 15-30% to overall production costs according to recent industry analyses.
Environmental Impact Assessment
The environmental implications of quantum dot (QD) technology in hydrogen production catalysts extend far beyond their efficiency metrics. These nanoscale semiconductor particles, while promising revolutionary advances in clean energy production, present complex environmental considerations throughout their lifecycle. The manufacturing process of quantum dots typically involves heavy metals such as cadmium, lead, or indium, which pose significant toxicological concerns if released into ecosystems. Production methods often require energy-intensive conditions and hazardous chemicals, creating a substantial environmental footprint before deployment.
When integrated into hydrogen production systems, quantum dot stability becomes a critical environmental factor. Degradation of these materials can lead to leaching of potentially toxic components into water systems or soil. Studies have shown that certain quantum dot compositions can release heavy metal ions when exposed to environmental stressors such as UV radiation, oxidative conditions, or pH variations commonly encountered in real-world applications. This instability presents both acute and chronic exposure risks to aquatic organisms and potentially to humans through bioaccumulation pathways.
The end-of-life management of quantum dot-enhanced catalysts represents another significant environmental challenge. Current recycling technologies are inadequately developed for the efficient recovery of nanomaterials from complex catalyst structures. Without proper reclamation processes, valuable and potentially harmful materials may be lost to landfills or incineration, contributing to resource depletion and pollution.
Comparative lifecycle assessments between quantum dot catalysts and conventional hydrogen production technologies reveal a complex sustainability profile. While QD-enhanced systems may offer improved energy efficiency and reduced operational emissions, their embodied environmental impact from manufacturing and disposal may offset these benefits. Recent research indicates that quantum dots with core-shell structures or those synthesized from less toxic elements (such as carbon, silicon, or zinc) demonstrate improved environmental compatibility while maintaining catalytic performance.
Regulatory frameworks worldwide are evolving to address the unique environmental challenges posed by nanomaterials like quantum dots. The European Union's REACH regulations and similar initiatives in other regions are beginning to incorporate specific provisions for nanomaterial risk assessment, though significant gaps remain in standardized testing protocols specific to quantum dot environmental behavior.
Future development of environmentally sustainable quantum dot catalysts will require integrated approaches combining green chemistry principles in synthesis, enhanced stability through advanced encapsulation techniques, and designed-for-recycling architectures that facilitate material recovery at end-of-life.
When integrated into hydrogen production systems, quantum dot stability becomes a critical environmental factor. Degradation of these materials can lead to leaching of potentially toxic components into water systems or soil. Studies have shown that certain quantum dot compositions can release heavy metal ions when exposed to environmental stressors such as UV radiation, oxidative conditions, or pH variations commonly encountered in real-world applications. This instability presents both acute and chronic exposure risks to aquatic organisms and potentially to humans through bioaccumulation pathways.
The end-of-life management of quantum dot-enhanced catalysts represents another significant environmental challenge. Current recycling technologies are inadequately developed for the efficient recovery of nanomaterials from complex catalyst structures. Without proper reclamation processes, valuable and potentially harmful materials may be lost to landfills or incineration, contributing to resource depletion and pollution.
Comparative lifecycle assessments between quantum dot catalysts and conventional hydrogen production technologies reveal a complex sustainability profile. While QD-enhanced systems may offer improved energy efficiency and reduced operational emissions, their embodied environmental impact from manufacturing and disposal may offset these benefits. Recent research indicates that quantum dots with core-shell structures or those synthesized from less toxic elements (such as carbon, silicon, or zinc) demonstrate improved environmental compatibility while maintaining catalytic performance.
Regulatory frameworks worldwide are evolving to address the unique environmental challenges posed by nanomaterials like quantum dots. The European Union's REACH regulations and similar initiatives in other regions are beginning to incorporate specific provisions for nanomaterial risk assessment, though significant gaps remain in standardized testing protocols specific to quantum dot environmental behavior.
Future development of environmentally sustainable quantum dot catalysts will require integrated approaches combining green chemistry principles in synthesis, enhanced stability through advanced encapsulation techniques, and designed-for-recycling architectures that facilitate material recovery at end-of-life.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!