Quantum Dot Stability in Water-based Quantum Dots for Bioimaging
SEP 28, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Quantum Dot Bioimaging Background and Objectives
Quantum dots (QDs) have emerged as revolutionary nanomaterials in the field of bioimaging since their initial discovery in the 1980s. These semiconductor nanocrystals, typically ranging from 2-10 nm in diameter, possess unique optical properties including size-tunable emission wavelengths, broad absorption spectra, narrow emission bands, high quantum yields, and exceptional resistance to photobleaching compared to traditional organic fluorophores. These characteristics have positioned QDs as powerful tools for biological imaging applications, offering unprecedented opportunities for long-term tracking and multicolor imaging.
The evolution of QD technology has witnessed significant milestones over the past three decades. Initially, QDs were primarily synthesized using organic routes, resulting in hydrophobic nanocrystals unsuitable for biological applications. The breakthrough came in the early 2000s with the development of water-soluble QDs through surface modification strategies, opening doors to biological applications. Subsequently, advances in core-shell structures, particularly the introduction of CdSe/ZnS architectures, dramatically improved quantum yields and photostability.
Despite these advances, water-based QDs for bioimaging face persistent stability challenges that limit their widespread clinical adoption. These include colloidal instability in physiological environments, susceptibility to oxidation, potential leaching of toxic components, and surface ligand detachment. The stability issues directly impact imaging performance through decreased quantum yield, spectral shifts, and increased non-specific binding, ultimately compromising the reliability of biological data obtained using these nanoprobes.
The primary technical objective of current research is to develop water-based QD systems with enhanced stability profiles while maintaining their superior optical properties. This encompasses improving colloidal stability in complex biological media, enhancing photostability under continuous illumination, ensuring chemical stability against oxidation, and developing biocompatible surface chemistries that remain intact in vivo. Additionally, there is a growing emphasis on developing non-toxic alternatives to traditional cadmium-based QDs, such as indium phosphide and carbon dots.
Recent technological trends indicate a shift toward multifunctional QD platforms that combine imaging capabilities with therapeutic or targeting functionalities. The integration of QDs with other imaging modalities, such as magnetic resonance imaging (MRI) or photoacoustic imaging, is gaining traction for comprehensive diagnostic information. Furthermore, the development of stimuli-responsive QDs that can report on specific biological parameters represents an emerging frontier in this field.
The ultimate goal is to translate QD technology from laboratory research to clinical applications, which necessitates addressing stability concerns alongside biocompatibility and toxicity issues. This requires interdisciplinary approaches combining materials science, surface chemistry, and biological evaluation to create robust water-based QD systems suitable for reliable in vivo imaging applications.
The evolution of QD technology has witnessed significant milestones over the past three decades. Initially, QDs were primarily synthesized using organic routes, resulting in hydrophobic nanocrystals unsuitable for biological applications. The breakthrough came in the early 2000s with the development of water-soluble QDs through surface modification strategies, opening doors to biological applications. Subsequently, advances in core-shell structures, particularly the introduction of CdSe/ZnS architectures, dramatically improved quantum yields and photostability.
Despite these advances, water-based QDs for bioimaging face persistent stability challenges that limit their widespread clinical adoption. These include colloidal instability in physiological environments, susceptibility to oxidation, potential leaching of toxic components, and surface ligand detachment. The stability issues directly impact imaging performance through decreased quantum yield, spectral shifts, and increased non-specific binding, ultimately compromising the reliability of biological data obtained using these nanoprobes.
The primary technical objective of current research is to develop water-based QD systems with enhanced stability profiles while maintaining their superior optical properties. This encompasses improving colloidal stability in complex biological media, enhancing photostability under continuous illumination, ensuring chemical stability against oxidation, and developing biocompatible surface chemistries that remain intact in vivo. Additionally, there is a growing emphasis on developing non-toxic alternatives to traditional cadmium-based QDs, such as indium phosphide and carbon dots.
Recent technological trends indicate a shift toward multifunctional QD platforms that combine imaging capabilities with therapeutic or targeting functionalities. The integration of QDs with other imaging modalities, such as magnetic resonance imaging (MRI) or photoacoustic imaging, is gaining traction for comprehensive diagnostic information. Furthermore, the development of stimuli-responsive QDs that can report on specific biological parameters represents an emerging frontier in this field.
The ultimate goal is to translate QD technology from laboratory research to clinical applications, which necessitates addressing stability concerns alongside biocompatibility and toxicity issues. This requires interdisciplinary approaches combining materials science, surface chemistry, and biological evaluation to create robust water-based QD systems suitable for reliable in vivo imaging applications.
Market Analysis for Water-based Quantum Dots
The global market for water-based quantum dots in bioimaging applications is experiencing robust growth, driven by increasing demand for advanced diagnostic and research tools in healthcare and life sciences. Current market valuations indicate that the quantum dot bioimaging segment reached approximately 450 million USD in 2022, with water-based formulations representing about 35% of this market. Industry analysts project a compound annual growth rate (CAGR) of 21.3% for water-based quantum dots in bioimaging through 2028.
Healthcare applications dominate the market landscape, with clinical diagnostics, cellular imaging, and molecular tracking representing the primary demand drivers. Research institutions and pharmaceutical companies are increasingly adopting water-based quantum dots for in vitro and in vivo imaging due to their superior optical properties and reduced toxicity compared to traditional organic fluorophores.
Regionally, North America leads the market with approximately 42% share, followed by Europe (28%) and Asia-Pacific (24%). The Asia-Pacific region, particularly China and India, is expected to witness the fastest growth rate due to increasing healthcare expenditure and expanding research infrastructure. Emerging economies are showing accelerated adoption rates as local manufacturing capabilities improve and costs decrease.
Market segmentation reveals that in vitro diagnostic applications currently hold the largest share at 48%, followed by in vivo imaging (32%) and drug delivery visualization (14%). The remaining applications constitute specialized research tools and emerging applications. This distribution reflects the current technical limitations regarding stability and biocompatibility of water-based quantum dots for long-term in vivo applications.
Pricing analysis indicates that high-quality water-based quantum dots for bioimaging applications command premium prices, with average costs ranging from 2,000 to 5,000 USD per gram depending on specifications, coating technologies, and stability characteristics. However, prices have decreased by approximately 15% annually over the past three years as manufacturing processes improve and competition intensifies.
Key market restraints include regulatory challenges, particularly for in vivo applications, concerns about long-term toxicity, and technical limitations regarding quantum yield stability in biological environments. Despite these challenges, the market outlook remains positive as technological advancements continue to address stability issues and expand application possibilities.
Customer surveys indicate that stability in physiological conditions is the most desired improvement (cited by 78% of end-users), followed by enhanced quantum yield (65%) and simplified conjugation chemistry (52%). This market feedback aligns with current research priorities in the field and suggests significant commercial opportunity for innovations addressing quantum dot stability in aqueous environments.
Healthcare applications dominate the market landscape, with clinical diagnostics, cellular imaging, and molecular tracking representing the primary demand drivers. Research institutions and pharmaceutical companies are increasingly adopting water-based quantum dots for in vitro and in vivo imaging due to their superior optical properties and reduced toxicity compared to traditional organic fluorophores.
Regionally, North America leads the market with approximately 42% share, followed by Europe (28%) and Asia-Pacific (24%). The Asia-Pacific region, particularly China and India, is expected to witness the fastest growth rate due to increasing healthcare expenditure and expanding research infrastructure. Emerging economies are showing accelerated adoption rates as local manufacturing capabilities improve and costs decrease.
Market segmentation reveals that in vitro diagnostic applications currently hold the largest share at 48%, followed by in vivo imaging (32%) and drug delivery visualization (14%). The remaining applications constitute specialized research tools and emerging applications. This distribution reflects the current technical limitations regarding stability and biocompatibility of water-based quantum dots for long-term in vivo applications.
Pricing analysis indicates that high-quality water-based quantum dots for bioimaging applications command premium prices, with average costs ranging from 2,000 to 5,000 USD per gram depending on specifications, coating technologies, and stability characteristics. However, prices have decreased by approximately 15% annually over the past three years as manufacturing processes improve and competition intensifies.
Key market restraints include regulatory challenges, particularly for in vivo applications, concerns about long-term toxicity, and technical limitations regarding quantum yield stability in biological environments. Despite these challenges, the market outlook remains positive as technological advancements continue to address stability issues and expand application possibilities.
Customer surveys indicate that stability in physiological conditions is the most desired improvement (cited by 78% of end-users), followed by enhanced quantum yield (65%) and simplified conjugation chemistry (52%). This market feedback aligns with current research priorities in the field and suggests significant commercial opportunity for innovations addressing quantum dot stability in aqueous environments.
Current Stability Challenges in Aqueous Environments
Water-based quantum dots (QDs) face significant stability challenges in aqueous environments, which severely limit their practical applications in bioimaging. The primary challenge stems from the inherent chemical instability of QDs when exposed to water molecules, oxygen, and biological components. Surface oxidation occurs rapidly in aqueous media, leading to the release of toxic heavy metal ions such as cadmium, lead, or mercury from the QD core, which not only compromises imaging quality but also raises serious cytotoxicity concerns.
Photostability represents another critical challenge, as water-based QDs often exhibit accelerated photobleaching compared to their organic-solvent counterparts. When exposed to excitation light during imaging procedures, these QDs can undergo photochemical reactions with dissolved oxygen and water molecules, resulting in the formation of reactive oxygen species that attack the QD surface and degrade their optical properties. This phenomenon manifests as decreased quantum yield, spectral shifts, and ultimately complete fluorescence quenching over relatively short timeframes.
Colloidal stability presents an equally significant hurdle. Water-based QDs tend to aggregate in physiological conditions due to the high ionic strength of biological fluids, which screens the electrostatic repulsion between particles. This aggregation dramatically alters their optical properties and biodistribution, rendering them ineffective for targeted imaging applications. The protein corona effect—where biomolecules in physiological environments adsorb onto the QD surface—further complicates stability by changing surface properties and recognition capabilities.
pH sensitivity constitutes another major challenge, as most QDs exhibit significant variations in quantum yield and emission wavelength across different pH environments encountered in biological systems. Endosomal compartments, for instance, present acidic conditions that can accelerate QD degradation through proton-assisted processes, limiting their effectiveness for intracellular imaging applications.
Current surface passivation strategies, including polymer coatings, silica shells, and zwitterionic ligands, provide only partial solutions to these challenges. While these approaches improve water dispersibility, they often fail to simultaneously address all stability issues. Polymer coatings may increase hydrodynamic size beyond optimal ranges for biological applications, while silica shells can crack under biological conditions. Zwitterionic ligands offer promising charge-neutral surfaces but frequently lack long-term stability in complex biological media.
The trade-off between stability and functionality remains unresolved, as modifications that enhance stability often compromise the quantum yield, increase particle size, or reduce targeting efficiency. This fundamental dilemma continues to drive research toward more sophisticated surface engineering approaches that can simultaneously address multiple stability challenges while maintaining optimal imaging performance.
Photostability represents another critical challenge, as water-based QDs often exhibit accelerated photobleaching compared to their organic-solvent counterparts. When exposed to excitation light during imaging procedures, these QDs can undergo photochemical reactions with dissolved oxygen and water molecules, resulting in the formation of reactive oxygen species that attack the QD surface and degrade their optical properties. This phenomenon manifests as decreased quantum yield, spectral shifts, and ultimately complete fluorescence quenching over relatively short timeframes.
Colloidal stability presents an equally significant hurdle. Water-based QDs tend to aggregate in physiological conditions due to the high ionic strength of biological fluids, which screens the electrostatic repulsion between particles. This aggregation dramatically alters their optical properties and biodistribution, rendering them ineffective for targeted imaging applications. The protein corona effect—where biomolecules in physiological environments adsorb onto the QD surface—further complicates stability by changing surface properties and recognition capabilities.
pH sensitivity constitutes another major challenge, as most QDs exhibit significant variations in quantum yield and emission wavelength across different pH environments encountered in biological systems. Endosomal compartments, for instance, present acidic conditions that can accelerate QD degradation through proton-assisted processes, limiting their effectiveness for intracellular imaging applications.
Current surface passivation strategies, including polymer coatings, silica shells, and zwitterionic ligands, provide only partial solutions to these challenges. While these approaches improve water dispersibility, they often fail to simultaneously address all stability issues. Polymer coatings may increase hydrodynamic size beyond optimal ranges for biological applications, while silica shells can crack under biological conditions. Zwitterionic ligands offer promising charge-neutral surfaces but frequently lack long-term stability in complex biological media.
The trade-off between stability and functionality remains unresolved, as modifications that enhance stability often compromise the quantum yield, increase particle size, or reduce targeting efficiency. This fundamental dilemma continues to drive research toward more sophisticated surface engineering approaches that can simultaneously address multiple stability challenges while maintaining optimal imaging performance.
Current Stabilization Approaches for Water-based QDs
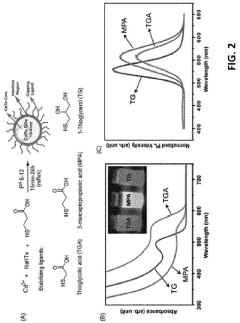
01 Surface modification techniques for quantum dot stabilization
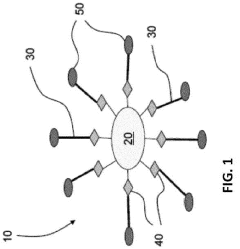
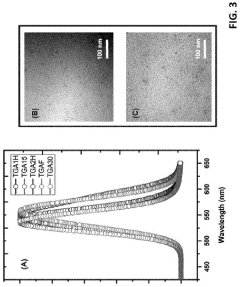
Various surface modification techniques can be employed to enhance the stability of water-based quantum dots. These include ligand exchange processes, coating with hydrophilic polymers, and surface functionalization with specific chemical groups that improve colloidal stability in aqueous environments. These modifications create protective shells around the quantum dots, preventing aggregation and maintaining their optical properties in water-based solutions.- Surface modification techniques for quantum dot stability: Various surface modification techniques can be employed to enhance the stability of water-based quantum dots. These include ligand exchange processes, coating with hydrophilic polymers, and surface functionalization with specific chemical groups. These modifications create a protective layer around the quantum dots, preventing aggregation and oxidation while maintaining their optical properties in aqueous environments. Such techniques significantly improve the colloidal stability and shelf life of water-based quantum dot formulations.
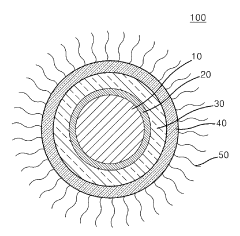
- Core-shell structures for enhanced stability: Core-shell architectures represent an effective approach to improving the stability of water-based quantum dots. By encapsulating the quantum dot core with a shell of another semiconductor material, these structures provide protection against environmental factors that cause degradation. The shell acts as a physical barrier, reducing surface defects and preventing the core from interacting with water molecules and oxygen. This design significantly enhances quantum yield, photostability, and overall durability in aqueous environments.
- Polymer encapsulation methods: Polymer encapsulation offers a robust solution for stabilizing quantum dots in water-based systems. By embedding quantum dots within biocompatible polymers such as PEG, PMMA, or amphiphilic block copolymers, these methods create a protective hydrophilic outer layer while maintaining the hydrophobic environment preferred by the quantum dot surface. This approach prevents aggregation, reduces toxicity, and enhances compatibility with biological systems while preserving the optical properties of the quantum dots in aqueous media.
- pH and ionic strength control strategies: Controlling pH and ionic strength is crucial for maintaining the stability of water-based quantum dots. Buffer systems and electrolyte management techniques can be implemented to create optimal conditions that prevent aggregation and surface degradation. By carefully adjusting these parameters, the electrostatic repulsion between quantum dots can be optimized, enhancing their colloidal stability. Additionally, specific additives can be incorporated to maintain pH homeostasis during storage and application, extending the functional lifetime of quantum dot formulations.
- Antioxidant and stabilizing additives: Various additives can be incorporated into water-based quantum dot formulations to enhance their stability. Antioxidants such as ascorbic acid and tocopherol derivatives can scavenge free radicals that cause oxidative degradation. Stabilizing agents including certain amino acids, thiol compounds, and crown ethers can coordinate with the quantum dot surface, preventing ligand detachment and aggregation. These additives work synergistically to maintain quantum dot integrity, preserving their optical properties and extending shelf life in aqueous environments.
02 Core-shell structures for improved water stability
Core-shell structured quantum dots offer enhanced stability in water-based environments. By encapsulating the quantum dot core with an inorganic shell material (such as ZnS, SiO2, or metal oxides), these structures provide protection against oxidation and degradation. The shell acts as a barrier between the core and the aqueous environment, significantly improving quantum dot stability while maintaining their optical and electronic properties.Expand Specific Solutions03 Polymer encapsulation methods for quantum dot stabilization
Polymer encapsulation represents an effective approach for stabilizing quantum dots in water-based systems. By embedding quantum dots within amphiphilic polymers, dendrimers, or hydrogels, these methods create a protective environment that shields the nanoparticles from water while allowing them to remain dispersed in aqueous media. The polymer shell prevents aggregation and oxidation, extending the shelf life and maintaining the optical properties of water-dispersible quantum dots.Expand Specific Solutions04 pH and ionic strength control for enhanced stability
Controlling pH and ionic strength of the aqueous medium is crucial for maintaining quantum dot stability. Buffer systems and electrolyte management can prevent aggregation and precipitation of quantum dots in water-based environments. Optimization of these parameters helps to maintain the surface charge of quantum dots, creating electrostatic repulsion between particles and preventing their agglomeration, thereby enhancing their colloidal stability in aqueous solutions.Expand Specific Solutions05 Antioxidant additives and storage conditions
Incorporating antioxidant additives and optimizing storage conditions significantly improves the long-term stability of water-based quantum dots. Additives such as ascorbic acid, tocopherol derivatives, and specific radical scavengers can protect quantum dots from oxidative degradation. Additionally, controlling storage parameters such as temperature, light exposure, and container materials helps preserve quantum dot properties in aqueous environments, extending their functional lifetime and maintaining consistent performance.Expand Specific Solutions
Leading Companies and Research Institutions
The quantum dot stability in water-based systems for bioimaging is currently in a growth phase, with the market expected to expand significantly due to increasing applications in medical diagnostics and research. The global market for quantum dots in bioimaging is projected to reach several billion dollars by 2030, driven by advancements in biocompatibility and stability. Technologically, the field is progressing from early-stage development toward commercial maturity, with key players demonstrating varied levels of expertise. Academic institutions like Drexel University, Wuhan University, and Texas A&M are pioneering fundamental research, while companies including Samsung Electronics, Mojo Vision, and Life Technologies are developing commercial applications. Research organizations such as the Agency for Science, Technology & Research and National Research Council of Canada are bridging the gap between academic innovation and industrial implementation, focusing on enhancing quantum dot stability in aqueous environments for improved bioimaging performance.
Life Technologies Corp.
Technical Solution: Life Technologies (now part of Thermo Fisher Scientific) has developed the Qdot® nanocrystal technology, representing one of the most commercially successful water-soluble quantum dot platforms for bioimaging. Their approach utilizes a CdSe/ZnS core-shell structure with a proprietary amphiphilic polymer coating that provides excellent colloidal stability in biological buffers[1]. The technology incorporates a unique surface modification strategy that maintains high quantum yields (typically 50-80%) after transfer to aqueous environments, addressing one of the major challenges in quantum dot bioapplications[2]. Life Technologies has pioneered conjugation chemistries that enable precise targeting of cellular structures through antibody, peptide, or small molecule functionalization while maintaining the optical properties of the quantum dots. Their QDs feature exceptional resistance to photobleaching, allowing continuous imaging for hours compared to minutes with conventional fluorophores[3]. The company has also developed specialized formulations with reduced hydrodynamic diameter (<25 nm) to improve tissue penetration and cellular uptake efficiency. Additionally, their technology includes surface treatments that minimize non-specific binding in complex biological samples, enhancing signal-to-noise ratios in challenging imaging scenarios.
Strengths: Exceptional brightness (10-20 times brighter than organic dyes) and resistance to photobleaching, enabling long-term tracking studies. Their established bioconjugation protocols allow for versatile targeting strategies. Weaknesses: The core materials contain cadmium, raising toxicity concerns for certain applications, particularly in vivo studies. The relatively large size of the complete nanoparticle assembly may limit applications in crowded cellular environments or when studying smaller cellular structures.
The Georgia Tech Research Corp.
Technical Solution: Georgia Tech Research Corporation has developed innovative approaches to quantum dot stability in aqueous environments through their pioneering work on "Green QDs" - environmentally friendly quantum dots specifically designed for biological applications. Their technology utilizes a unique hydrothermal synthesis method that directly produces water-soluble QDs without the need for post-synthetic ligand exchange, which typically causes degradation in optical properties[1]. The research team has engineered multifunctional surface ligands containing both anchoring groups (thiols, phosphines) for strong binding to the QD surface and hydrophilic moieties (carboxyl, hydroxyl, or PEG chains) that provide water solubility and colloidal stability[2]. Their approach incorporates a gradient alloy structure at the core-shell interface that minimizes lattice strain and surface defects, resulting in enhanced photostability in biological environments. Georgia Tech has also developed specialized silica encapsulation methods that provide a protective barrier against oxidation while maintaining the superior optical properties of the QDs. Additionally, their technology includes the integration of pH-responsive polymers that enhance endosomal escape following cellular uptake, improving intracellular imaging capabilities[3].
Strengths: Direct synthesis in aqueous conditions preserves optical properties better than traditional ligand exchange methods. Their environmentally friendly formulations address toxicity concerns for in vivo applications. Weaknesses: The water-based synthesis approach may result in broader size distributions compared to organic-phase methods, potentially affecting spectral purity. The technology may also face challenges with achieving the same quantum yield levels as traditional organic-synthesized QDs.
Key Patents and Breakthroughs in QD Stability
Quantum dot and method for producing same
PatentWO2017116013A1
Innovation
- The development of quantum dots with a core-shell structure and additional stability layers, along with a ligand layer, enhances stability and efficiency, featuring a stability index of 90% or more and quantum efficiency of 80% or more, and allowing for conversion efficiency of 100% or more when switching from lipid-soluble to water-soluble ligands.
Photo-emissive nanoclusters encapsulated in nano-cavities of amphiphilic block copolymer vesicles and methods of their preparation
PatentInactiveEP3597721A1
Innovation
- The use of amphiphilic block copolymers like polystyrene-B-polymethacrylic acid (PS-b-PMAA) to create micelle or vesicle structures with compartmentalized reactive cavities that encapsulate CdTe quantum dots, allowing them to be transferred into an organic phase and maintaining stability, thereby preserving their optical and electronic transport properties.
Biocompatibility and Toxicity Considerations
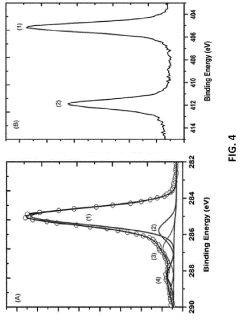
The integration of quantum dots (QDs) into biological systems necessitates rigorous assessment of their biocompatibility and toxicity profiles. Traditional QDs often contain heavy metals such as cadmium, lead, or mercury, which pose significant toxicological concerns when introduced into biological environments. The core-shell structure of QDs can potentially release these toxic elements through degradation processes, particularly in the acidic conditions found in cellular lysosomes.
Water-based quantum dots represent a promising advancement for bioimaging applications, yet their interaction with biological systems remains a critical consideration. Studies have demonstrated that surface chemistry significantly influences the biocompatibility of QDs. Appropriately functionalized surfaces can reduce cytotoxicity and enhance cellular uptake efficiency. For instance, QDs coated with polyethylene glycol (PEG) or zwitterionic ligands have shown reduced non-specific binding to biomolecules and improved biocompatibility profiles.
The size of quantum dots also plays a crucial role in their toxicological impact. Particles below 5-6 nm can potentially cross biological barriers, including the blood-brain barrier, raising concerns about unintended accumulation in sensitive tissues. Furthermore, the renal clearance threshold (typically around 5.5 nm) determines whether QDs can be effectively eliminated from the body or remain for extended periods, potentially causing long-term toxicity.
Recent advancements have focused on developing cadmium-free quantum dots, such as indium phosphide (InP) or zinc sulfide (ZnS) based QDs, which demonstrate significantly reduced toxicity while maintaining desirable optical properties. However, comprehensive long-term toxicity studies remain limited, particularly regarding the potential for these materials to induce oxidative stress, inflammatory responses, or genotoxicity in biological systems.
The biodistribution and clearance pathways of water-based QDs represent another critical aspect of their biocompatibility assessment. Studies indicate that QDs primarily accumulate in the reticuloendothelial system, particularly in the liver and spleen. The residence time of these nanoparticles can extend from days to months, depending on their composition, size, and surface properties, raising concerns about potential chronic toxicity.
Standardized toxicity assessment protocols specifically designed for quantum dots remain underdeveloped, complicating direct comparisons between different formulations. The scientific community has called for the establishment of comprehensive testing frameworks that address both acute and chronic toxicity, immunogenicity, and potential developmental effects of these nanomaterials. Such standardization would facilitate more reliable safety evaluations and accelerate clinical translation of QD-based bioimaging technologies.
Water-based quantum dots represent a promising advancement for bioimaging applications, yet their interaction with biological systems remains a critical consideration. Studies have demonstrated that surface chemistry significantly influences the biocompatibility of QDs. Appropriately functionalized surfaces can reduce cytotoxicity and enhance cellular uptake efficiency. For instance, QDs coated with polyethylene glycol (PEG) or zwitterionic ligands have shown reduced non-specific binding to biomolecules and improved biocompatibility profiles.
The size of quantum dots also plays a crucial role in their toxicological impact. Particles below 5-6 nm can potentially cross biological barriers, including the blood-brain barrier, raising concerns about unintended accumulation in sensitive tissues. Furthermore, the renal clearance threshold (typically around 5.5 nm) determines whether QDs can be effectively eliminated from the body or remain for extended periods, potentially causing long-term toxicity.
Recent advancements have focused on developing cadmium-free quantum dots, such as indium phosphide (InP) or zinc sulfide (ZnS) based QDs, which demonstrate significantly reduced toxicity while maintaining desirable optical properties. However, comprehensive long-term toxicity studies remain limited, particularly regarding the potential for these materials to induce oxidative stress, inflammatory responses, or genotoxicity in biological systems.
The biodistribution and clearance pathways of water-based QDs represent another critical aspect of their biocompatibility assessment. Studies indicate that QDs primarily accumulate in the reticuloendothelial system, particularly in the liver and spleen. The residence time of these nanoparticles can extend from days to months, depending on their composition, size, and surface properties, raising concerns about potential chronic toxicity.
Standardized toxicity assessment protocols specifically designed for quantum dots remain underdeveloped, complicating direct comparisons between different formulations. The scientific community has called for the establishment of comprehensive testing frameworks that address both acute and chronic toxicity, immunogenicity, and potential developmental effects of these nanomaterials. Such standardization would facilitate more reliable safety evaluations and accelerate clinical translation of QD-based bioimaging technologies.
Regulatory Framework for In-vivo Quantum Dot Applications
The regulatory landscape for in-vivo quantum dot applications represents a complex and evolving framework that significantly impacts the development and commercialization of water-based quantum dots for bioimaging. Currently, the FDA and EMA have established stringent guidelines for nanomaterials used in biological applications, with particular emphasis on toxicity assessment, biodistribution studies, and long-term safety profiles.
Quantum dots face specific regulatory challenges due to their unique physicochemical properties and potential toxicity concerns. Heavy metal content, particularly cadmium and lead in traditional quantum dots, triggers heightened scrutiny under regulations such as the European Union's Restriction of Hazardous Substances (RoHS) directive. Manufacturers must demonstrate that quantum dot formulations for in-vivo applications meet acceptable safety thresholds through comprehensive toxicological studies.
The regulatory pathway for quantum dot-based bioimaging agents typically involves a multi-tiered assessment process. Initial evaluations focus on material characterization, including size distribution, surface chemistry, and stability in biological media. Subsequent phases require in-vitro cytotoxicity studies, followed by in-vivo biodistribution and clearance studies in animal models. For clinical translation, manufacturers must provide extensive documentation on manufacturing consistency, sterility, and shelf-life stability.
International harmonization efforts are underway to standardize regulatory approaches to nanomaterials in biomedical applications. The International Organization for Standardization (ISO) has developed several standards specific to nanotechnology, including ISO/TR 13014 for physicochemical characterization and ISO/TS 12901 for risk management. These standards provide valuable frameworks for quantum dot developers seeking regulatory approval across multiple jurisdictions.
Recent regulatory trends indicate increasing focus on the stability of water-based quantum dots under physiological conditions. Regulatory bodies now require robust data demonstrating minimal leaching of core materials, consistent optical properties in biological environments, and predictable degradation pathways. The FDA's guidance on drug-device combination products has particular relevance for quantum dot-based imaging agents that may incorporate targeting moieties or therapeutic components.
Emerging regulatory considerations include the environmental impact of quantum dot disposal and the potential for secondary exposure pathways. Several jurisdictions now require environmental risk assessments as part of the approval process for nanomaterials with potential environmental persistence. Companies developing water-based quantum dots for bioimaging must therefore adopt a holistic approach to regulatory compliance that addresses both patient safety and environmental considerations throughout the product lifecycle.
Quantum dots face specific regulatory challenges due to their unique physicochemical properties and potential toxicity concerns. Heavy metal content, particularly cadmium and lead in traditional quantum dots, triggers heightened scrutiny under regulations such as the European Union's Restriction of Hazardous Substances (RoHS) directive. Manufacturers must demonstrate that quantum dot formulations for in-vivo applications meet acceptable safety thresholds through comprehensive toxicological studies.
The regulatory pathway for quantum dot-based bioimaging agents typically involves a multi-tiered assessment process. Initial evaluations focus on material characterization, including size distribution, surface chemistry, and stability in biological media. Subsequent phases require in-vitro cytotoxicity studies, followed by in-vivo biodistribution and clearance studies in animal models. For clinical translation, manufacturers must provide extensive documentation on manufacturing consistency, sterility, and shelf-life stability.
International harmonization efforts are underway to standardize regulatory approaches to nanomaterials in biomedical applications. The International Organization for Standardization (ISO) has developed several standards specific to nanotechnology, including ISO/TR 13014 for physicochemical characterization and ISO/TS 12901 for risk management. These standards provide valuable frameworks for quantum dot developers seeking regulatory approval across multiple jurisdictions.
Recent regulatory trends indicate increasing focus on the stability of water-based quantum dots under physiological conditions. Regulatory bodies now require robust data demonstrating minimal leaching of core materials, consistent optical properties in biological environments, and predictable degradation pathways. The FDA's guidance on drug-device combination products has particular relevance for quantum dot-based imaging agents that may incorporate targeting moieties or therapeutic components.
Emerging regulatory considerations include the environmental impact of quantum dot disposal and the potential for secondary exposure pathways. Several jurisdictions now require environmental risk assessments as part of the approval process for nanomaterials with potential environmental persistence. Companies developing water-based quantum dots for bioimaging must therefore adopt a holistic approach to regulatory compliance that addresses both patient safety and environmental considerations throughout the product lifecycle.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!