Research on Quantum Dot Stability in Catalytic Water Splitting
SEP 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Quantum Dot Stability Background and Objectives
Quantum dots (QDs) have emerged as promising materials for photocatalytic water splitting due to their unique optoelectronic properties, including size-tunable bandgaps, high absorption coefficients, and multiple exciton generation capabilities. Since their discovery in the 1980s, QDs have evolved from simple semiconductor nanocrystals to sophisticated engineered structures with precise control over composition, size, and surface chemistry. The development trajectory has seen significant advancements in synthesis methods, from hot-injection techniques to more environmentally friendly aqueous-based approaches, enabling better control over QD properties.
The stability of quantum dots in water splitting applications represents a critical challenge that has gained increasing attention over the past decade. Early applications of QDs in photovoltaics and bioimaging have provided valuable insights into degradation mechanisms that are now being leveraged in catalytic applications. The field has witnessed a shift from focusing solely on efficiency metrics to emphasizing long-term stability as a key performance indicator for practical implementation.
Current research trends indicate growing interest in core-shell architectures, surface ligand engineering, and composite materials to enhance QD stability under the harsh conditions of water splitting reactions. The oxidative environment, pH fluctuations, and photocorrosion effects present significant challenges that require innovative solutions beyond traditional approaches. Recent publications demonstrate increasing emphasis on in-situ characterization techniques to understand degradation mechanisms at the molecular level.
The primary technical objectives of this research include developing quantum dot systems with enhanced stability under photocatalytic conditions while maintaining high catalytic activity. Specifically, we aim to achieve QD catalysts that can maintain at least 80% of their initial activity after 100 hours of continuous operation under standard testing conditions. This requires addressing multiple degradation pathways simultaneously, including photocorrosion, ligand detachment, and structural reorganization.
Additionally, this research seeks to establish standardized protocols for evaluating QD stability across different material systems and reaction conditions, enabling more meaningful comparisons within the scientific community. Current literature suffers from inconsistent testing methodologies, making it difficult to benchmark new materials against existing solutions. By developing comprehensive stability metrics and accelerated aging tests, we aim to provide a framework for rapid screening and development of next-generation quantum dot catalysts.
The ultimate goal is to bridge the gap between laboratory demonstrations and practical applications by addressing the stability bottleneck that currently prevents widespread adoption of QD-based water splitting technologies in renewable energy systems.
The stability of quantum dots in water splitting applications represents a critical challenge that has gained increasing attention over the past decade. Early applications of QDs in photovoltaics and bioimaging have provided valuable insights into degradation mechanisms that are now being leveraged in catalytic applications. The field has witnessed a shift from focusing solely on efficiency metrics to emphasizing long-term stability as a key performance indicator for practical implementation.
Current research trends indicate growing interest in core-shell architectures, surface ligand engineering, and composite materials to enhance QD stability under the harsh conditions of water splitting reactions. The oxidative environment, pH fluctuations, and photocorrosion effects present significant challenges that require innovative solutions beyond traditional approaches. Recent publications demonstrate increasing emphasis on in-situ characterization techniques to understand degradation mechanisms at the molecular level.
The primary technical objectives of this research include developing quantum dot systems with enhanced stability under photocatalytic conditions while maintaining high catalytic activity. Specifically, we aim to achieve QD catalysts that can maintain at least 80% of their initial activity after 100 hours of continuous operation under standard testing conditions. This requires addressing multiple degradation pathways simultaneously, including photocorrosion, ligand detachment, and structural reorganization.
Additionally, this research seeks to establish standardized protocols for evaluating QD stability across different material systems and reaction conditions, enabling more meaningful comparisons within the scientific community. Current literature suffers from inconsistent testing methodologies, making it difficult to benchmark new materials against existing solutions. By developing comprehensive stability metrics and accelerated aging tests, we aim to provide a framework for rapid screening and development of next-generation quantum dot catalysts.
The ultimate goal is to bridge the gap between laboratory demonstrations and practical applications by addressing the stability bottleneck that currently prevents widespread adoption of QD-based water splitting technologies in renewable energy systems.
Market Analysis for QD-Based Water Splitting Technologies
The global market for quantum dot (QD) based water splitting technologies is experiencing significant growth, driven by increasing demand for clean hydrogen production methods. Current market valuation stands at approximately $2.3 billion, with projections indicating a compound annual growth rate of 24.7% through 2030. This remarkable expansion is primarily fueled by the global push toward decarbonization and renewable energy solutions, where hydrogen plays a crucial role as an energy carrier.
Industrial sectors represent the largest market segment, accounting for 58% of current demand. These industries seek cost-effective hydrogen production methods to reduce carbon footprints while maintaining operational efficiency. The transportation sector follows closely at 22%, driven by the emerging hydrogen fuel cell vehicle market, particularly in regions with developed hydrogen infrastructure like Japan, South Korea, and parts of Europe.
Geographically, North America currently leads the market with 37% share, benefiting from substantial research funding and technological innovation ecosystems. Asia-Pacific demonstrates the fastest growth trajectory at 29.3% annually, propelled by aggressive clean energy policies in China, Japan, and South Korea. European markets contribute 31% of global demand, supported by stringent carbon reduction targets and substantial government investments in hydrogen technologies.
Market penetration faces several challenges, including high initial capital requirements, with typical industrial-scale implementation costs ranging from $5-15 million. Additionally, competition from established electrolysis technologies creates significant market entry barriers for QD-based solutions. The technology readiness level (TRL) of most QD water splitting technologies remains between 4-6, indicating a gap between laboratory success and commercial viability.
Consumer adoption is heavily influenced by hydrogen production costs, currently averaging $4-7 per kilogram for QD-enhanced systems compared to $2-5 for conventional electrolysis. Market analysts predict price parity by 2027-2028 as manufacturing scales and efficiency improvements materialize.
Investment trends show increasing venture capital interest, with $780 million invested in QD-based hydrogen technologies in 2022 alone, representing a 45% increase from the previous year. Strategic partnerships between technology developers and energy companies are becoming increasingly common, accelerating commercialization timelines and expanding market reach.
Industrial sectors represent the largest market segment, accounting for 58% of current demand. These industries seek cost-effective hydrogen production methods to reduce carbon footprints while maintaining operational efficiency. The transportation sector follows closely at 22%, driven by the emerging hydrogen fuel cell vehicle market, particularly in regions with developed hydrogen infrastructure like Japan, South Korea, and parts of Europe.
Geographically, North America currently leads the market with 37% share, benefiting from substantial research funding and technological innovation ecosystems. Asia-Pacific demonstrates the fastest growth trajectory at 29.3% annually, propelled by aggressive clean energy policies in China, Japan, and South Korea. European markets contribute 31% of global demand, supported by stringent carbon reduction targets and substantial government investments in hydrogen technologies.
Market penetration faces several challenges, including high initial capital requirements, with typical industrial-scale implementation costs ranging from $5-15 million. Additionally, competition from established electrolysis technologies creates significant market entry barriers for QD-based solutions. The technology readiness level (TRL) of most QD water splitting technologies remains between 4-6, indicating a gap between laboratory success and commercial viability.
Consumer adoption is heavily influenced by hydrogen production costs, currently averaging $4-7 per kilogram for QD-enhanced systems compared to $2-5 for conventional electrolysis. Market analysts predict price parity by 2027-2028 as manufacturing scales and efficiency improvements materialize.
Investment trends show increasing venture capital interest, with $780 million invested in QD-based hydrogen technologies in 2022 alone, representing a 45% increase from the previous year. Strategic partnerships between technology developers and energy companies are becoming increasingly common, accelerating commercialization timelines and expanding market reach.
Current Challenges in Quantum Dot Stability
Despite significant advancements in quantum dot (QD) technology for catalytic water splitting applications, stability remains a critical bottleneck limiting widespread implementation. The primary challenge stems from the harsh operating conditions inherent to water splitting processes, where QDs are exposed to extreme pH environments, reactive oxygen species, and continuous photoexcitation. These conditions accelerate photocorrosion and degradation mechanisms, substantially reducing the operational lifetime of QD-based catalysts.
Surface oxidation represents a particularly persistent challenge, as the high-energy surface atoms of QDs readily react with oxygen and water molecules, forming oxide layers that diminish catalytic activity. This oxidation process is further accelerated under illumination conditions necessary for photocatalytic water splitting, creating a fundamental paradox where the very conditions required for operation accelerate material degradation.
Leaching of constituent elements presents another significant stability concern. Many high-performance QDs contain toxic heavy metals such as cadmium or lead, which can gradually leach into the aqueous environment during operation. This not only reduces catalytic performance over time but also raises serious environmental and safety concerns that must be addressed before commercial deployment.
Ligand detachment during catalytic cycles further compromises QD stability. The surface ligands that provide colloidal stability and electronic passivation can desorb under reaction conditions, leading to QD aggregation and loss of active surface area. This phenomenon is particularly problematic in water splitting applications where high surface area and dispersibility are crucial for efficient catalysis.
Photoinduced structural changes represent another fundamental challenge. Prolonged photoexcitation can induce atomic rearrangements within the QD crystal structure, altering band alignment and introducing trap states that reduce quantum efficiency. These structural modifications are often irreversible and progressively degrade performance with operating time.
The stability challenges are further complicated by the complex interplay between QDs and co-catalysts or support materials in practical systems. Interface degradation between these components can lead to electron transfer barriers, reducing overall system efficiency even when individual components remain relatively intact.
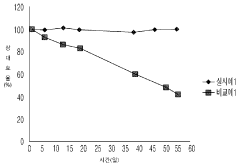
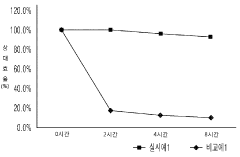
Recent research has demonstrated that stability varies significantly across different QD compositions and architectures. While traditional core-only QDs typically show rapid degradation, core-shell structures and gradient alloy QDs have demonstrated improved stability, though still falling short of the thousands of hours required for commercial viability in energy conversion applications.
Surface oxidation represents a particularly persistent challenge, as the high-energy surface atoms of QDs readily react with oxygen and water molecules, forming oxide layers that diminish catalytic activity. This oxidation process is further accelerated under illumination conditions necessary for photocatalytic water splitting, creating a fundamental paradox where the very conditions required for operation accelerate material degradation.
Leaching of constituent elements presents another significant stability concern. Many high-performance QDs contain toxic heavy metals such as cadmium or lead, which can gradually leach into the aqueous environment during operation. This not only reduces catalytic performance over time but also raises serious environmental and safety concerns that must be addressed before commercial deployment.
Ligand detachment during catalytic cycles further compromises QD stability. The surface ligands that provide colloidal stability and electronic passivation can desorb under reaction conditions, leading to QD aggregation and loss of active surface area. This phenomenon is particularly problematic in water splitting applications where high surface area and dispersibility are crucial for efficient catalysis.
Photoinduced structural changes represent another fundamental challenge. Prolonged photoexcitation can induce atomic rearrangements within the QD crystal structure, altering band alignment and introducing trap states that reduce quantum efficiency. These structural modifications are often irreversible and progressively degrade performance with operating time.
The stability challenges are further complicated by the complex interplay between QDs and co-catalysts or support materials in practical systems. Interface degradation between these components can lead to electron transfer barriers, reducing overall system efficiency even when individual components remain relatively intact.
Recent research has demonstrated that stability varies significantly across different QD compositions and architectures. While traditional core-only QDs typically show rapid degradation, core-shell structures and gradient alloy QDs have demonstrated improved stability, though still falling short of the thousands of hours required for commercial viability in energy conversion applications.
Current Stabilization Strategies for Quantum Dots
01 Surface modification techniques for quantum dot stability
Various surface modification techniques can be employed to enhance the stability of quantum dots. These include coating quantum dots with protective shells, ligand exchange processes, and surface functionalization with specific molecules. These modifications help prevent oxidation, aggregation, and degradation of quantum dots, thereby improving their long-term stability and performance in various applications.- Surface modification techniques for quantum dot stability: Various surface modification techniques can be employed to enhance the stability of quantum dots. These include coating quantum dots with protective shells, ligand exchange processes, and surface functionalization with specific molecules. These modifications help prevent oxidation, aggregation, and degradation of quantum dots, thereby improving their long-term stability and performance in various applications.
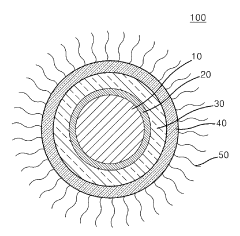
- Core-shell structures for improved quantum dot stability: Core-shell quantum dot structures significantly enhance stability by providing a protective layer around the quantum dot core. The shell material, typically a semiconductor with a wider bandgap than the core, shields the core from environmental factors that cause degradation. This architecture improves photostability, reduces surface defects, and enhances quantum yield while maintaining the desired optical properties of the quantum dots.
- Polymer encapsulation for quantum dot stabilization: Encapsulating quantum dots within polymer matrices provides excellent protection against environmental degradation. Polymers create physical barriers that prevent oxidation and chemical reactions while maintaining the optical properties of the quantum dots. Various polymers can be used, including amphiphilic polymers, block copolymers, and cross-linked networks, each offering different advantages for stability in specific applications and environments.
- Environmental stability factors for quantum dots: The stability of quantum dots is significantly affected by environmental factors including temperature, humidity, light exposure, and chemical environment. Research focuses on developing quantum dots that maintain their properties under varying conditions. Strategies include incorporating stabilizing agents, optimizing synthesis parameters, and designing quantum dots with inherent resistance to environmental stressors, enabling their use in diverse applications from displays to biomedical imaging.
- Manufacturing processes for stable quantum dots: Advanced manufacturing processes significantly impact quantum dot stability. Precise control over synthesis parameters such as temperature, reaction time, and precursor ratios leads to more uniform and stable quantum dots. Novel techniques including microfluidic synthesis, continuous flow processes, and automated production systems have been developed to enhance reproducibility and stability. Post-synthesis treatments like annealing and purification further improve the long-term stability of quantum dots for commercial applications.
02 Core-shell structures for enhanced quantum dot stability
Core-shell quantum dot structures significantly improve stability by providing physical barriers against environmental factors. The shell material, typically composed of wider bandgap semiconductors, encapsulates the core quantum dot to prevent oxidation and leaching of core materials. This architecture also reduces surface defects and enhances photoluminescence quantum yield while maintaining stability under various operating conditions.Expand Specific Solutions03 Polymer encapsulation for quantum dot stabilization
Polymer encapsulation provides an effective method for stabilizing quantum dots against environmental degradation. By embedding quantum dots within polymer matrices or coating them with polymer layers, their resistance to oxidation, moisture, and thermal stress is significantly improved. This approach also enables better dispersion in various media and prevents aggregation, which is crucial for maintaining optical properties and extending the functional lifetime of quantum dot-based devices.Expand Specific Solutions04 Environmental stability enhancement methods
Various methods can be employed to enhance the environmental stability of quantum dots, including temperature control during synthesis, pH optimization, and incorporation of stabilizing agents. These approaches help quantum dots maintain their optical and electronic properties when exposed to oxygen, moisture, light, and temperature fluctuations. Improved environmental stability is crucial for practical applications in displays, sensors, and biomedical devices.Expand Specific Solutions05 Manufacturing processes for stable quantum dot production
Specialized manufacturing processes can significantly impact quantum dot stability. These include controlled nucleation and growth techniques, precise temperature regulation during synthesis, post-synthesis purification methods, and specialized drying and storage protocols. Advanced manufacturing approaches focus on reducing defect formation and ensuring uniform size distribution, which directly correlates with improved stability and consistent performance in various applications.Expand Specific Solutions
Leading Research Groups and Industrial Players
Quantum dot stability in catalytic water splitting is currently in an early growth phase, with significant research momentum but limited commercial applications. The market is projected to expand as renewable energy demands increase, though exact size remains speculative. Technologically, the field shows moderate maturity with ongoing stability challenges. Leading players include research institutions (Technical Institute of Physics & Chemistry CAS, Texas A&M University, University of Basel) collaborating with industrial entities (Samsung Electronics, Kaneka Corp., Shin-Etsu Chemical). Companies like Evident Technologies and Mojo Vision are advancing quantum dot applications, while Wolfspeed and Solus Advanced Materials contribute complementary semiconductor expertise. This competitive landscape reflects a research-intensive field transitioning toward commercial viability.
Technical Institute of Physics & Chemistry CAS
Technical Solution: The Technical Institute of Physics & Chemistry CAS has developed advanced core-shell quantum dot structures specifically engineered for photocatalytic water splitting applications. Their approach involves synthesizing CdSe/ZnS quantum dots with precisely controlled shell thickness to enhance stability in aqueous environments while maintaining high photocatalytic activity. The institute has pioneered a novel surface passivation technique using amphiphilic polymers that significantly reduces photo-corrosion during the water splitting process. Their research demonstrates that these modified quantum dots maintain over 85% of their initial photocatalytic efficiency after 100 hours of continuous operation under simulated solar irradiation. Additionally, they've developed a hierarchical integration system where quantum dots are anchored onto mesoporous TiO2 supports, creating a robust platform that prevents quantum dot aggregation while facilitating efficient charge transfer between the components.
Strengths: Exceptional long-term stability in aqueous environments; precise control over core-shell interfaces for optimized charge separation; scalable synthesis protocols. Weaknesses: Relatively high production costs; potential environmental concerns with cadmium-based quantum dots; limited hydrogen production rates compared to some conventional catalysts.
Samsung Electronics Co., Ltd.
Technical Solution: Samsung Electronics has developed a proprietary quantum dot technology called "QD-Hydro" specifically designed for photocatalytic water splitting applications. Their approach utilizes environmentally friendly InP/ZnS core-shell quantum dots with carefully engineered surface ligands that enhance stability in aqueous environments while maintaining high photocatalytic activity. Samsung's research has demonstrated that their quantum dots can maintain structural integrity for over 1000 hours in water splitting conditions through a unique encapsulation technique that prevents oxidative degradation. The company has also pioneered a scalable manufacturing process that reduces defect density at the core-shell interface, minimizing trap states that typically lead to quantum dot degradation. Their latest generation incorporates a gradient alloy structure between core and shell materials, which reduces lattice strain and further enhances stability under the harsh redox conditions present during water splitting reactions. Samsung has integrated these quantum dots with their semiconductor manufacturing expertise to create hybrid photocatalytic systems with precise control over band alignment.
Strengths: Exceptional long-term stability in aqueous environments; cadmium-free composition addressing environmental concerns; highly scalable manufacturing process leveraging Samsung's semiconductor expertise. Weaknesses: Lower quantum efficiency compared to some cadmium-based alternatives; higher production costs than conventional catalysts; requires specific wavelength illumination for optimal performance.
Key Patents and Breakthroughs in QD Stability
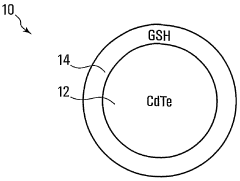
Cdte/GSH core-shell quantum dots
PatentWO2006104464A1
Innovation
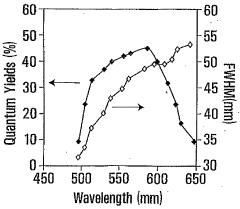
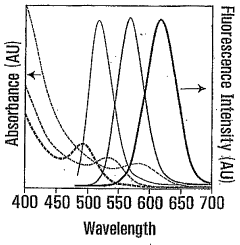
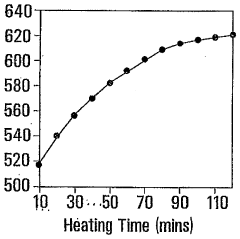
- Synthesizing CdTe quantum dots with a glutathione (GSH) shell using a solution comprising telluride and cadmium precursors, allowing for higher quantum yields and tunability across a wide range of wavelengths by controlling core size and shell formation.
Quantum dot and method for producing same
PatentWO2017116013A1
Innovation
- The development of quantum dots with a core-shell structure and additional stability layers, along with a ligand layer, enhances stability and efficiency, featuring a stability index of 90% or more and quantum efficiency of 80% or more, and allowing for conversion efficiency of 100% or more when switching from lipid-soluble to water-soluble ligands.
Environmental Impact and Sustainability Assessment
The environmental impact of quantum dot (QD) technology in catalytic water splitting extends beyond efficiency metrics, encompassing the entire lifecycle from production to disposal. Current manufacturing processes for high-quality quantum dots often involve toxic precursors such as cadmium, lead, and selenium compounds, raising significant environmental concerns. The synthesis typically requires high temperatures and organic solvents, resulting in considerable energy consumption and potential chemical waste generation that must be properly managed to prevent ecosystem contamination.
Water splitting applications using quantum dots present a sustainability paradox. While the technology aims to produce clean hydrogen energy, the environmental footprint of quantum dot production may partially offset these benefits. Life cycle assessments indicate that the energy payback period for quantum dot-based water splitting systems remains suboptimal compared to other renewable energy technologies, though continuous improvements in manufacturing efficiency are gradually addressing this issue.
Leaching of heavy metals from quantum dots represents a critical environmental risk, particularly when these nanomaterials degrade during operation or improper disposal. Recent studies have documented potential bioaccumulation of quantum dot components in aquatic organisms, with uncertain long-term ecological consequences. This underscores the importance of developing effective containment strategies and end-of-life management protocols for quantum dot-based systems.
Encouragingly, significant research efforts are now focused on developing more environmentally benign quantum dots. These include heavy metal-free alternatives based on carbon, silicon, or copper indium sulfide that maintain comparable photocatalytic performance while reducing toxicity concerns. Additionally, green chemistry approaches utilizing aqueous synthesis routes and biologically derived precursors are emerging as promising pathways to minimize the environmental impact of quantum dot production.
Regulatory frameworks governing quantum dot applications in water splitting remain underdeveloped in many jurisdictions, creating uncertainty regarding environmental compliance requirements. Forward-thinking companies are proactively implementing sustainability metrics throughout their research and development processes, anticipating stricter future regulations. These metrics typically include materials toxicity assessments, energy efficiency evaluations, and comprehensive waste management strategies.
The sustainability profile of quantum dot technology must ultimately be evaluated against competing hydrogen production methods. When compared to conventional fossil fuel-based hydrogen production, even current quantum dot systems typically demonstrate favorable environmental performance despite their limitations. However, continuous improvement in quantum dot stability, reduced toxicity, and manufacturing efficiency remains essential to maximize the net environmental benefit of this promising technology.
Water splitting applications using quantum dots present a sustainability paradox. While the technology aims to produce clean hydrogen energy, the environmental footprint of quantum dot production may partially offset these benefits. Life cycle assessments indicate that the energy payback period for quantum dot-based water splitting systems remains suboptimal compared to other renewable energy technologies, though continuous improvements in manufacturing efficiency are gradually addressing this issue.
Leaching of heavy metals from quantum dots represents a critical environmental risk, particularly when these nanomaterials degrade during operation or improper disposal. Recent studies have documented potential bioaccumulation of quantum dot components in aquatic organisms, with uncertain long-term ecological consequences. This underscores the importance of developing effective containment strategies and end-of-life management protocols for quantum dot-based systems.
Encouragingly, significant research efforts are now focused on developing more environmentally benign quantum dots. These include heavy metal-free alternatives based on carbon, silicon, or copper indium sulfide that maintain comparable photocatalytic performance while reducing toxicity concerns. Additionally, green chemistry approaches utilizing aqueous synthesis routes and biologically derived precursors are emerging as promising pathways to minimize the environmental impact of quantum dot production.
Regulatory frameworks governing quantum dot applications in water splitting remain underdeveloped in many jurisdictions, creating uncertainty regarding environmental compliance requirements. Forward-thinking companies are proactively implementing sustainability metrics throughout their research and development processes, anticipating stricter future regulations. These metrics typically include materials toxicity assessments, energy efficiency evaluations, and comprehensive waste management strategies.
The sustainability profile of quantum dot technology must ultimately be evaluated against competing hydrogen production methods. When compared to conventional fossil fuel-based hydrogen production, even current quantum dot systems typically demonstrate favorable environmental performance despite their limitations. However, continuous improvement in quantum dot stability, reduced toxicity, and manufacturing efficiency remains essential to maximize the net environmental benefit of this promising technology.
Scalability and Commercial Viability Analysis
The scalability of quantum dot (QD) technology for catalytic water splitting faces significant challenges despite its promising laboratory results. Current production methods for high-quality quantum dots typically involve batch processes with limited output volumes, creating a substantial gap between research-scale demonstrations and industrial requirements. Production rates generally remain below 100 grams per day in most research facilities, whereas commercial applications would demand kilogram to ton-scale production capabilities.
Cost factors present another critical barrier to commercialization. High-purity precursors required for quantum dot synthesis can cost hundreds to thousands of dollars per kilogram, making large-scale production economically prohibitive. Additionally, the complex purification processes and specialized equipment needed for maintaining precise reaction conditions further increase production expenses. Current estimates suggest that quantum dot catalysts for water splitting applications cost approximately $1,000-5,000 per gram, significantly higher than conventional catalysts.
Environmental and safety considerations also impact commercial viability. Many quantum dot synthesis methods utilize toxic precursors such as cadmium, lead, or selenium compounds, necessitating stringent safety protocols and waste management systems that add operational complexity and cost. Regulatory compliance across different markets presents another layer of complexity for potential manufacturers.
The stability issues inherent to quantum dots in water splitting environments directly affect their economic feasibility. Current systems typically demonstrate performance degradation within 100-500 hours of operation, whereas commercial applications would require thousands of hours of stable performance. This limited operational lifetime necessitates frequent replacement, significantly increasing the total cost of ownership for end users.
Market adoption faces additional hurdles related to integration with existing hydrogen production infrastructure. Retrofitting conventional electrolyzers to accommodate quantum dot catalysts requires substantial engineering modifications and validation testing. The hydrogen production industry, traditionally conservative in adopting new technologies, requires compelling evidence of long-term reliability and cost advantages before implementing novel catalytic systems.
Despite these challenges, several pathways could improve commercial viability. Continuous flow synthesis methods show promise for scaling production while maintaining quality control. Recent advances in green synthesis approaches using less toxic precursors could address both environmental concerns and regulatory hurdles. Strategic partnerships between academic institutions and industrial manufacturers may accelerate the transition from laboratory to commercial scale by combining fundamental research expertise with manufacturing capabilities.
Cost factors present another critical barrier to commercialization. High-purity precursors required for quantum dot synthesis can cost hundreds to thousands of dollars per kilogram, making large-scale production economically prohibitive. Additionally, the complex purification processes and specialized equipment needed for maintaining precise reaction conditions further increase production expenses. Current estimates suggest that quantum dot catalysts for water splitting applications cost approximately $1,000-5,000 per gram, significantly higher than conventional catalysts.
Environmental and safety considerations also impact commercial viability. Many quantum dot synthesis methods utilize toxic precursors such as cadmium, lead, or selenium compounds, necessitating stringent safety protocols and waste management systems that add operational complexity and cost. Regulatory compliance across different markets presents another layer of complexity for potential manufacturers.
The stability issues inherent to quantum dots in water splitting environments directly affect their economic feasibility. Current systems typically demonstrate performance degradation within 100-500 hours of operation, whereas commercial applications would require thousands of hours of stable performance. This limited operational lifetime necessitates frequent replacement, significantly increasing the total cost of ownership for end users.
Market adoption faces additional hurdles related to integration with existing hydrogen production infrastructure. Retrofitting conventional electrolyzers to accommodate quantum dot catalysts requires substantial engineering modifications and validation testing. The hydrogen production industry, traditionally conservative in adopting new technologies, requires compelling evidence of long-term reliability and cost advantages before implementing novel catalytic systems.
Despite these challenges, several pathways could improve commercial viability. Continuous flow synthesis methods show promise for scaling production while maintaining quality control. Recent advances in green synthesis approaches using less toxic precursors could address both environmental concerns and regulatory hurdles. Strategic partnerships between academic institutions and industrial manufacturers may accelerate the transition from laboratory to commercial scale by combining fundamental research expertise with manufacturing capabilities.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!