Elucidating Molecular Dynamics with High-Field NMR Analysis
SEP 22, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
High-Field NMR Technology Evolution and Objectives
Nuclear Magnetic Resonance (NMR) spectroscopy has evolved significantly since its discovery in the 1940s, transforming from a physics curiosity into an indispensable analytical tool across multiple scientific disciplines. The progression toward high-field NMR represents one of the most significant technological advancements in molecular analysis, enabling unprecedented insights into molecular structures and dynamics at atomic resolution.
The development of high-field NMR technology has been driven by the fundamental relationship between magnetic field strength and spectral resolution. Early NMR instruments operated at field strengths below 2 Tesla, while contemporary systems routinely exceed 23 Tesla (1 GHz proton frequency), with ultra-high field systems pushing beyond 28 Tesla. This exponential increase in field strength has been enabled by breakthroughs in superconducting magnet technology, cryogenics, and advanced electronics.
High-field NMR offers several critical advantages that directly impact molecular dynamics studies. The enhanced spectral dispersion reduces signal overlap, allowing for the resolution of complex molecular structures. The improved signal-to-noise ratio enables detection of low-abundance species and transient interactions. Additionally, the increased sensitivity to chemical shift anisotropy and quadrupolar interactions provides deeper insights into molecular orientation and motion.
The primary objective of high-field NMR in molecular dynamics research is to elucidate the relationship between molecular structure, motion, and function across multiple timescales. This includes characterizing conformational changes, identifying interaction interfaces, quantifying exchange rates, and determining activation energies for molecular processes. These capabilities are particularly valuable for understanding intrinsically disordered proteins, membrane proteins, and complex biomolecular assemblies that resist conventional structural analysis.
Recent technological innovations have expanded the scope of high-field NMR applications. Dynamic Nuclear Polarization (DNP) has dramatically enhanced sensitivity, while advances in pulse sequence design have enabled multi-dimensional experiments that correlate different nuclear interactions. Cryoprobe technology has further improved signal detection, and parallel developments in computational methods have streamlined data analysis and interpretation.
Looking forward, the field aims to achieve even higher magnetic fields, potentially reaching the 30-35 Tesla range through novel magnet designs. Simultaneously, there is a push toward more accessible benchtop systems that maintain adequate resolution for routine applications. The integration of NMR with complementary techniques such as cryo-electron microscopy and mass spectrometry represents another frontier, promising more comprehensive molecular characterization.
The ultimate goal of high-field NMR technology development is to bridge the gap between static structural information and dynamic functional understanding, providing a more complete picture of molecular behavior in biological systems, materials science, and chemical processes.
The development of high-field NMR technology has been driven by the fundamental relationship between magnetic field strength and spectral resolution. Early NMR instruments operated at field strengths below 2 Tesla, while contemporary systems routinely exceed 23 Tesla (1 GHz proton frequency), with ultra-high field systems pushing beyond 28 Tesla. This exponential increase in field strength has been enabled by breakthroughs in superconducting magnet technology, cryogenics, and advanced electronics.
High-field NMR offers several critical advantages that directly impact molecular dynamics studies. The enhanced spectral dispersion reduces signal overlap, allowing for the resolution of complex molecular structures. The improved signal-to-noise ratio enables detection of low-abundance species and transient interactions. Additionally, the increased sensitivity to chemical shift anisotropy and quadrupolar interactions provides deeper insights into molecular orientation and motion.
The primary objective of high-field NMR in molecular dynamics research is to elucidate the relationship between molecular structure, motion, and function across multiple timescales. This includes characterizing conformational changes, identifying interaction interfaces, quantifying exchange rates, and determining activation energies for molecular processes. These capabilities are particularly valuable for understanding intrinsically disordered proteins, membrane proteins, and complex biomolecular assemblies that resist conventional structural analysis.
Recent technological innovations have expanded the scope of high-field NMR applications. Dynamic Nuclear Polarization (DNP) has dramatically enhanced sensitivity, while advances in pulse sequence design have enabled multi-dimensional experiments that correlate different nuclear interactions. Cryoprobe technology has further improved signal detection, and parallel developments in computational methods have streamlined data analysis and interpretation.
Looking forward, the field aims to achieve even higher magnetic fields, potentially reaching the 30-35 Tesla range through novel magnet designs. Simultaneously, there is a push toward more accessible benchtop systems that maintain adequate resolution for routine applications. The integration of NMR with complementary techniques such as cryo-electron microscopy and mass spectrometry represents another frontier, promising more comprehensive molecular characterization.
The ultimate goal of high-field NMR technology development is to bridge the gap between static structural information and dynamic functional understanding, providing a more complete picture of molecular behavior in biological systems, materials science, and chemical processes.
Market Applications and Demand for Advanced NMR Analysis
The global market for advanced Nuclear Magnetic Resonance (NMR) analysis continues to expand significantly, driven by increasing demand across multiple sectors. Pharmaceutical and biotechnology industries represent the largest market segment, where high-field NMR analysis serves as a critical tool for drug discovery, development, and quality control processes. These industries rely on NMR technology to elucidate molecular structures, analyze protein-ligand interactions, and monitor chemical reactions in real-time, accelerating the drug development pipeline and reducing costs associated with failed candidates.
Academic and research institutions form another substantial market segment, utilizing high-field NMR for fundamental research in chemistry, biochemistry, and materials science. The ability to observe molecular dynamics at unprecedented resolution has revolutionized our understanding of complex biological systems and material properties, driving continued investment in advanced NMR capabilities within research settings.
The chemical industry represents a growing application area, employing NMR analysis for quality control, reaction monitoring, and new material development. Particularly in polymer science and petrochemical sectors, the demand for high-resolution structural analysis has intensified as companies seek competitive advantages through product innovation and process optimization.
Food science and agriculture sectors have emerged as expanding markets for NMR technology, using it for food authentication, quality assessment, and metabolomic studies. The ability to detect adulterants, verify product origins, and assess nutritional content non-destructively provides significant value in addressing food safety concerns and regulatory requirements.
Environmental monitoring applications have gained traction, with NMR analysis being deployed to identify pollutants, study environmental degradation processes, and monitor remediation efforts. The non-destructive nature of NMR makes it particularly valuable for analyzing complex environmental samples without extensive preparation.
Market trends indicate growing demand for more accessible, user-friendly NMR systems that maintain high analytical power while reducing operational complexity. This has spurred development of benchtop and portable NMR solutions, expanding the potential user base beyond traditional research settings into industrial quality control and field applications.
Geographically, North America and Europe currently dominate the high-field NMR market, but Asia-Pacific regions, particularly China, Japan, and India, show the fastest growth rates. This expansion correlates with increasing R&D investments in these regions and the establishment of advanced research facilities supporting pharmaceutical and materials science development.
Academic and research institutions form another substantial market segment, utilizing high-field NMR for fundamental research in chemistry, biochemistry, and materials science. The ability to observe molecular dynamics at unprecedented resolution has revolutionized our understanding of complex biological systems and material properties, driving continued investment in advanced NMR capabilities within research settings.
The chemical industry represents a growing application area, employing NMR analysis for quality control, reaction monitoring, and new material development. Particularly in polymer science and petrochemical sectors, the demand for high-resolution structural analysis has intensified as companies seek competitive advantages through product innovation and process optimization.
Food science and agriculture sectors have emerged as expanding markets for NMR technology, using it for food authentication, quality assessment, and metabolomic studies. The ability to detect adulterants, verify product origins, and assess nutritional content non-destructively provides significant value in addressing food safety concerns and regulatory requirements.
Environmental monitoring applications have gained traction, with NMR analysis being deployed to identify pollutants, study environmental degradation processes, and monitor remediation efforts. The non-destructive nature of NMR makes it particularly valuable for analyzing complex environmental samples without extensive preparation.
Market trends indicate growing demand for more accessible, user-friendly NMR systems that maintain high analytical power while reducing operational complexity. This has spurred development of benchtop and portable NMR solutions, expanding the potential user base beyond traditional research settings into industrial quality control and field applications.
Geographically, North America and Europe currently dominate the high-field NMR market, but Asia-Pacific regions, particularly China, Japan, and India, show the fastest growth rates. This expansion correlates with increasing R&D investments in these regions and the establishment of advanced research facilities supporting pharmaceutical and materials science development.
Current Capabilities and Limitations in Molecular Dynamics NMR
High-field Nuclear Magnetic Resonance (NMR) spectroscopy has emerged as a powerful analytical technique for elucidating molecular dynamics, offering unprecedented insights into molecular structure, interactions, and behavior. Current high-field NMR systems typically operate at magnetic field strengths ranging from 500 MHz to 1.2 GHz, enabling researchers to probe molecular phenomena with remarkable resolution and sensitivity.
The capabilities of high-field NMR in molecular dynamics studies are substantial. These systems excel at characterizing protein folding pathways, enzyme-substrate interactions, and conformational changes in biomolecules with temporal resolution reaching microsecond to millisecond timescales. Advanced pulse sequences such as CPMG relaxation dispersion and NOESY experiments have significantly enhanced our ability to detect transient states and intermediate conformations that are critical in understanding biological processes.
Multidimensional NMR techniques (2D, 3D, and 4D) have revolutionized the field by allowing scientists to untangle complex spectral overlaps and extract comprehensive structural information from macromolecules. These approaches have been particularly valuable for investigating intrinsically disordered proteins and dynamic protein complexes that resist traditional structural biology methods.
Despite these advances, high-field NMR faces several limitations in molecular dynamics studies. Sample preparation remains challenging, often requiring isotopic labeling (13C, 15N, 2H) to achieve sufficient sensitivity, which can be expensive and time-consuming. Additionally, the technique struggles with size limitations, as molecules exceeding 50-70 kDa typically yield spectra that are difficult to interpret due to line broadening and signal overlap.
Temporal resolution presents another significant constraint. While NMR can detect dynamics across multiple timescales, processes occurring faster than microseconds often remain inaccessible through conventional methods. This creates a "blind spot" in our understanding of rapid molecular events that may be crucial to biological function.
Data processing and interpretation pose substantial computational challenges. The complexity of NMR data requires sophisticated algorithms and significant computational resources, particularly for large biomolecular systems. Current software solutions often struggle with automated analysis of highly dynamic systems, necessitating extensive manual intervention by skilled spectroscopists.
Accessibility remains a barrier to widespread adoption, as high-field NMR instruments require substantial financial investment, specialized infrastructure, and technical expertise. The most advanced systems (>800 MHz) are typically available only at specialized facilities, limiting routine access for many researchers.
Recent technological developments, including dynamic nuclear polarization (DNP) and non-uniform sampling (NUS), are addressing some of these limitations by enhancing sensitivity and reducing acquisition times. However, significant challenges remain in fully capturing the complexity of molecular dynamics across all relevant timescales and molecular sizes.
The capabilities of high-field NMR in molecular dynamics studies are substantial. These systems excel at characterizing protein folding pathways, enzyme-substrate interactions, and conformational changes in biomolecules with temporal resolution reaching microsecond to millisecond timescales. Advanced pulse sequences such as CPMG relaxation dispersion and NOESY experiments have significantly enhanced our ability to detect transient states and intermediate conformations that are critical in understanding biological processes.
Multidimensional NMR techniques (2D, 3D, and 4D) have revolutionized the field by allowing scientists to untangle complex spectral overlaps and extract comprehensive structural information from macromolecules. These approaches have been particularly valuable for investigating intrinsically disordered proteins and dynamic protein complexes that resist traditional structural biology methods.
Despite these advances, high-field NMR faces several limitations in molecular dynamics studies. Sample preparation remains challenging, often requiring isotopic labeling (13C, 15N, 2H) to achieve sufficient sensitivity, which can be expensive and time-consuming. Additionally, the technique struggles with size limitations, as molecules exceeding 50-70 kDa typically yield spectra that are difficult to interpret due to line broadening and signal overlap.
Temporal resolution presents another significant constraint. While NMR can detect dynamics across multiple timescales, processes occurring faster than microseconds often remain inaccessible through conventional methods. This creates a "blind spot" in our understanding of rapid molecular events that may be crucial to biological function.
Data processing and interpretation pose substantial computational challenges. The complexity of NMR data requires sophisticated algorithms and significant computational resources, particularly for large biomolecular systems. Current software solutions often struggle with automated analysis of highly dynamic systems, necessitating extensive manual intervention by skilled spectroscopists.
Accessibility remains a barrier to widespread adoption, as high-field NMR instruments require substantial financial investment, specialized infrastructure, and technical expertise. The most advanced systems (>800 MHz) are typically available only at specialized facilities, limiting routine access for many researchers.
Recent technological developments, including dynamic nuclear polarization (DNP) and non-uniform sampling (NUS), are addressing some of these limitations by enhancing sensitivity and reducing acquisition times. However, significant challenges remain in fully capturing the complexity of molecular dynamics across all relevant timescales and molecular sizes.
Contemporary Methodologies for Molecular Dynamics Elucidation
01 High-field NMR techniques for molecular structure analysis
High-field Nuclear Magnetic Resonance (NMR) spectroscopy provides enhanced resolution and sensitivity for analyzing molecular structures. These techniques utilize strong magnetic fields to improve signal-to-noise ratios and spectral resolution, allowing for more detailed structural information of complex molecules. Advanced pulse sequences and detection methods enable researchers to determine molecular conformations, identify functional groups, and elucidate three-dimensional structures with high precision.- High-field NMR spectroscopy techniques for molecular structure analysis: High-field nuclear magnetic resonance (NMR) spectroscopy provides enhanced resolution and sensitivity for analyzing molecular structures. These techniques utilize strong magnetic fields to improve signal-to-noise ratios and spectral resolution, allowing for detailed structural elucidation of complex molecules. Advanced pulse sequences and detection methods enable researchers to obtain precise information about molecular conformations, bond angles, and atomic interactions, which is crucial for understanding molecular behavior in various environments.
- Molecular dynamics studies using time-resolved NMR methods: Time-resolved NMR methods enable the study of molecular dynamics by capturing changes in molecular behavior over time. These techniques involve specialized pulse sequences and data acquisition strategies that can detect conformational changes, chemical reactions, and intermolecular interactions as they occur. By analyzing the temporal evolution of NMR signals, researchers can investigate dynamic processes such as protein folding, ligand binding, and chemical exchange phenomena, providing insights into the kinetics and mechanisms of molecular transformations.
- Advanced hardware configurations for high-field NMR analysis: Specialized hardware configurations enhance the capabilities of high-field NMR systems for molecular dynamics studies. These include superconducting magnets capable of generating extremely strong and homogeneous magnetic fields, cryogenically cooled probes that reduce thermal noise, and advanced gradient systems that enable spatial encoding of NMR signals. Such hardware innovations improve sensitivity, resolution, and data acquisition speed, allowing researchers to detect subtle molecular interactions and conformational changes that would be invisible with conventional NMR equipment.
- Data processing and computational methods for NMR-based molecular dynamics: Sophisticated data processing and computational methods are essential for extracting meaningful information from high-field NMR experiments focused on molecular dynamics. These approaches include advanced signal processing algorithms, multidimensional spectral analysis techniques, and computational modeling that correlate experimental NMR data with molecular structures and behaviors. Machine learning and artificial intelligence methods are increasingly being applied to interpret complex NMR datasets, enabling researchers to identify patterns and relationships that reveal the underlying dynamics of molecular systems.
- Applications of high-field NMR in biomolecular dynamics research: High-field NMR analysis has significant applications in studying the dynamics of biomolecules such as proteins, nucleic acids, and membrane components. These techniques provide atomic-level insights into biomolecular flexibility, conformational changes, and interaction mechanisms that are crucial for biological function. Researchers can investigate enzyme catalysis, protein-ligand interactions, allosteric regulation, and other dynamic processes that underlie biological activities. The non-invasive nature of NMR makes it particularly valuable for studying biomolecules under near-physiological conditions, offering insights that complement other structural biology methods.
02 Real-time monitoring of molecular dynamics using NMR
NMR spectroscopy can be used to monitor molecular dynamics in real-time, providing insights into conformational changes, reaction kinetics, and intermolecular interactions. Time-resolved NMR techniques allow researchers to observe dynamic processes such as protein folding, ligand binding, and chemical reactions as they occur. These methods often employ specialized pulse sequences and data acquisition strategies to capture transient states and measure exchange rates between different molecular conformations.Expand Specific Solutions03 Advanced hardware for high-field NMR applications
Specialized hardware components are essential for high-field NMR analysis of molecular dynamics. These include superconducting magnets capable of generating extremely strong and homogeneous magnetic fields, cryogenically cooled probes to enhance sensitivity, and advanced gradient systems for spatial encoding. Hardware innovations focus on improving field stability, reducing electronic noise, and enabling faster data acquisition to capture dynamic molecular processes with greater temporal resolution.Expand Specific Solutions04 Computational methods for analyzing NMR molecular dynamics data
Sophisticated computational algorithms and software tools are developed to process and interpret complex NMR data related to molecular dynamics. These methods include spectral deconvolution, relaxation analysis, and correlation spectroscopy techniques that extract dynamic information from NMR signals. Machine learning and statistical approaches help identify patterns in multidimensional NMR data, enabling researchers to model molecular motion, calculate energy landscapes, and predict conformational changes with high accuracy.Expand Specific Solutions05 Novel NMR pulse sequences for studying molecular dynamics
Innovative pulse sequence designs enable the investigation of specific aspects of molecular dynamics using high-field NMR. These specialized sequences can selectively probe different types of molecular motion, such as rotational diffusion, internal flexibility, and exchange processes. Advanced techniques include relaxation dispersion experiments, diffusion-ordered spectroscopy, and heteronuclear correlation methods that provide detailed information about molecular behavior across different time scales, from picoseconds to seconds.Expand Specific Solutions
Leading Research Institutions and Equipment Manufacturers
High-Field NMR Analysis for molecular dynamics research is currently in a growth phase, with the market expanding due to increasing applications in pharmaceutical development, materials science, and biochemistry. The global market size for advanced NMR technologies is estimated to reach several billion dollars by 2025, driven by demand for higher resolution molecular characterization. Technologically, the field shows varying maturity levels across different applications, with companies like Bruker Switzerland, Agilent Technologies, and Hitachi leading commercial instrumentation development. Academic institutions including Stanford University, The Scripps Research Institute, and École Polytechnique Fédérale de Lausanne are advancing fundamental research, while pharmaceutical entities such as Momenta Pharmaceuticals and Wyeth LLC are implementing these technologies in drug discovery workflows. The competitive landscape features established instrumentation manufacturers collaborating with research institutions to develop next-generation capabilities for increasingly complex molecular systems.
Hitachi Ltd.
Technical Solution: Hitachi has developed compact high-field NMR systems utilizing proprietary superconducting magnet technology that achieves remarkable field homogeneity while requiring significantly less laboratory space. Their ECHELON platform incorporates advanced pulse sequence capabilities specifically designed for molecular dynamics studies, including specialized gradient-enhanced experiments for measuring diffusion coefficients with high precision. Hitachi's systems feature integrated molecular dynamics simulation software that correlates experimental NMR data with computational models, providing deeper insights into molecular behavior. Their unique probe design optimizes signal detection for various nuclei simultaneously, enabling multi-dimensional experiments that reveal complex intramolecular interactions and conformational changes critical for understanding dynamic molecular processes in biological and materials systems.
Strengths: Excellent field stability; compact system footprint; integrated computational analysis tools. Weaknesses: More limited global service network; fewer specialized accessories compared to dedicated NMR manufacturers.
Bruker Switzerland
Technical Solution: Bruker Switzerland has pioneered advanced high-field NMR technology with their AVANCE NEO platform, which operates at field strengths up to 1.2 GHz for unprecedented molecular resolution. Their systems incorporate Dynamic Nuclear Polarization (DNP) enhancement technology that increases sensitivity by up to 100-fold, enabling the observation of previously undetectable molecular interactions. Bruker's CryoProbe technology reduces thermal noise significantly, allowing researchers to elucidate molecular dynamics of proteins and complex biomolecules with exceptional signal-to-noise ratios. Their TopSpin software suite provides sophisticated pulse sequence programming and data analysis tools specifically designed for molecular dynamics studies, including relaxation measurements and diffusion experiments that reveal biomolecular motion across multiple timescales.
Strengths: Industry-leading sensitivity and resolution; comprehensive software ecosystem for data analysis; extensive application support network. Weaknesses: High acquisition and maintenance costs; significant technical expertise required for operation; physical space and infrastructure requirements for ultra-high field systems.
Breakthrough Innovations in High-Field NMR Resolution
Method and apparatus for determining molecular dynamics of materials
PatentInactiveUS5122745A
Innovation
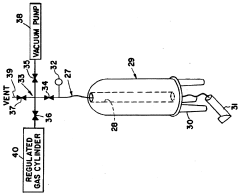
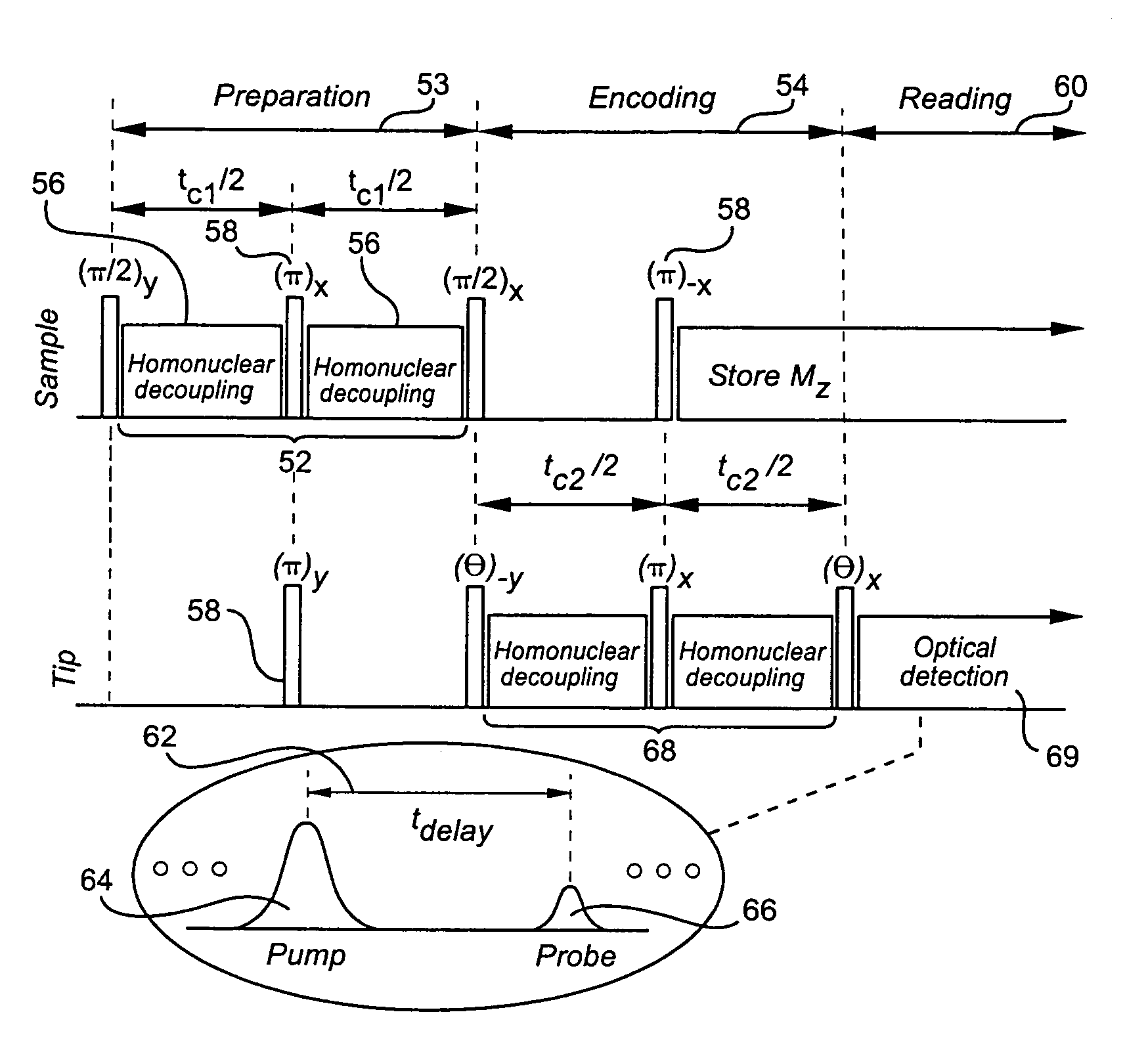
- A method and apparatus using wide line solid state NMR spectroscopy with a sample cell integrated into an evacuation and pressurization system, allowing continuous variable pressure operation up to higher pressures, enabling the analysis of molecular dynamics and structure under varying pressure conditions, and utilizing NMR active diffusion gases to probe the effects of pressure and dissolved gases on polymers.
Method and apparatus for high resolution nuclear magnetic resonance imaging and spectroscopy
PatentInactiveUS7199584B2
Innovation
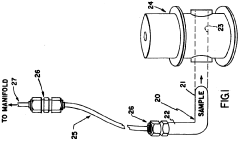
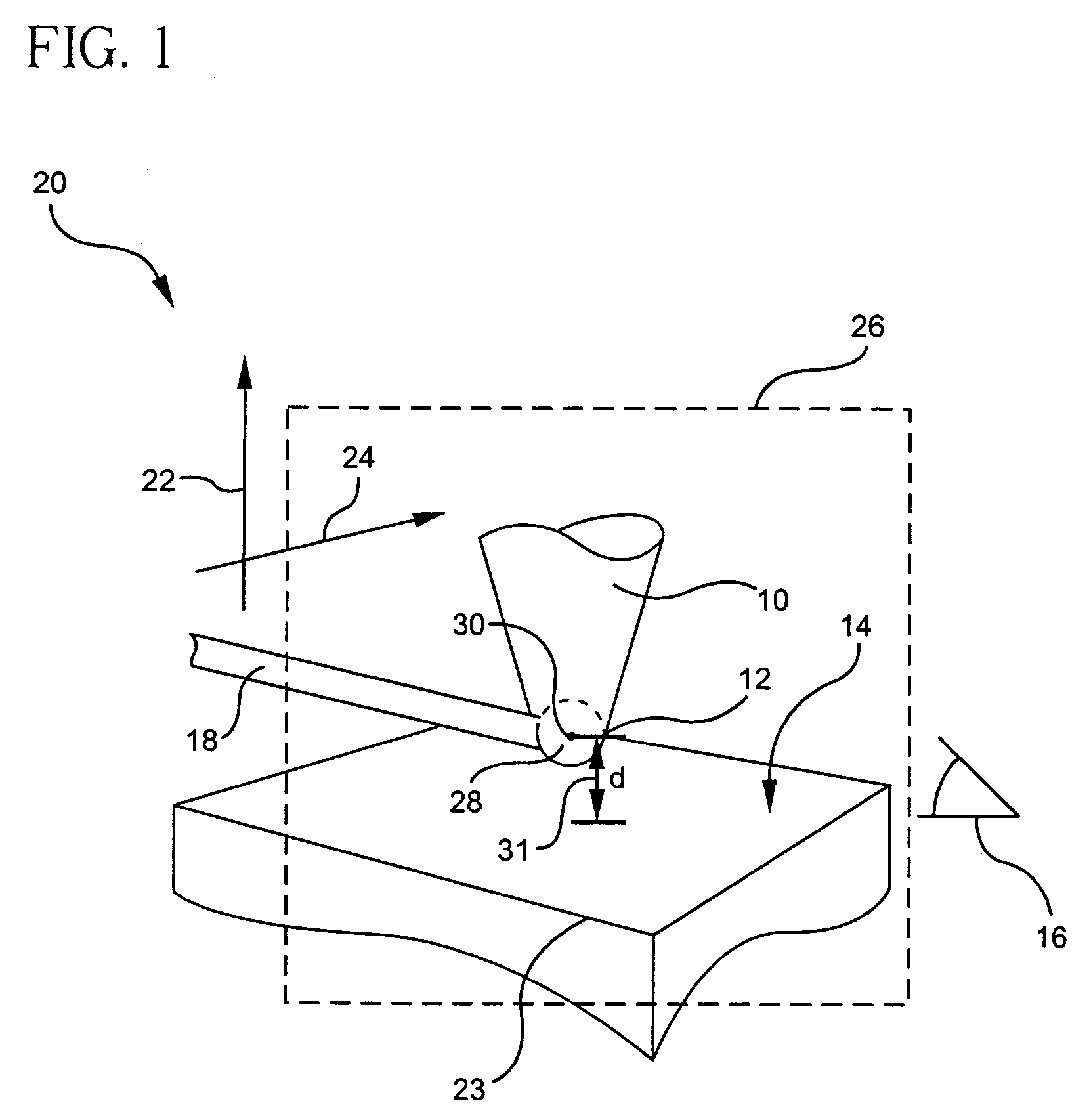
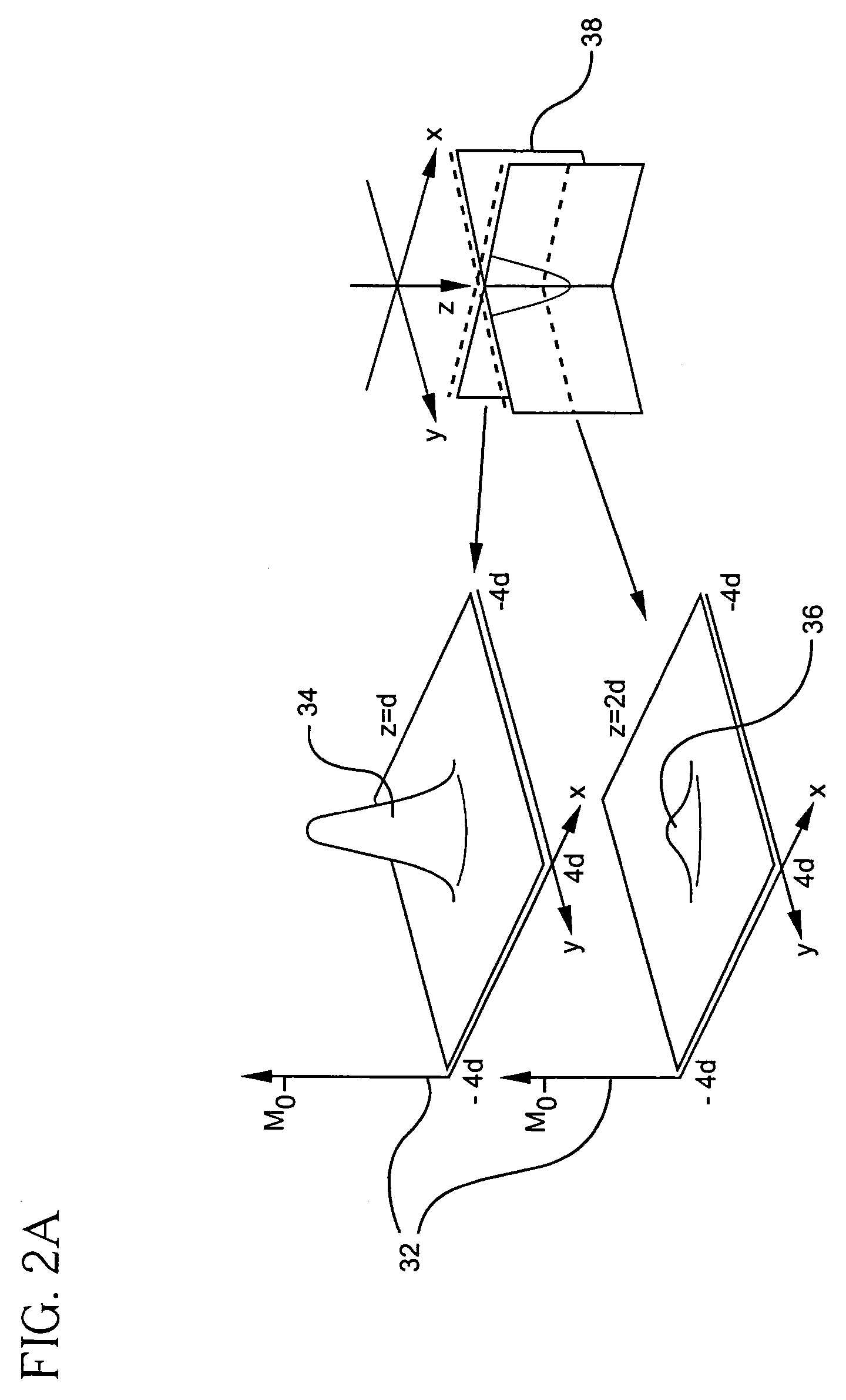
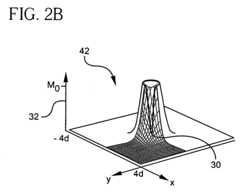
- A hyperpolarized probe tip is used to induce dipolar interactions with the sample, allowing for the modulation of tip magnetization proportional to local sample magnetization, enabling high-resolution and high-sensitivity NMR measurements through a sequence of radio-frequency pulses and detection methods, such as optical or electrical means.
Interdisciplinary Applications in Structural Biology
High-field NMR spectroscopy has revolutionized structural biology by providing unprecedented insights into biomolecular structures and dynamics. The integration of NMR techniques with other disciplines has created powerful synergies that expand our understanding of complex biological systems at the molecular level.
Structural biology represents one of the most significant application domains for high-field NMR analysis, where it complements X-ray crystallography and cryo-electron microscopy. The unique advantage of NMR lies in its ability to study proteins in solution states that closely mimic physiological conditions, capturing dynamic processes that static methods cannot reveal. This capability has proven invaluable for understanding intrinsically disordered proteins (IDPs), which constitute approximately 30% of eukaryotic proteomes and play crucial roles in cellular signaling and regulation.
The convergence of NMR with computational biology has dramatically enhanced structure determination workflows. Modern molecular dynamics simulations, informed by NMR-derived constraints, generate ensemble representations that better reflect the conformational heterogeneity of biomolecules. This hybrid approach has been particularly successful in characterizing transient protein-protein interactions and allosteric mechanisms that underlie many biological processes.
In drug discovery, high-field NMR techniques such as STD-NMR (Saturation Transfer Difference) and TROSY (Transverse Relaxation Optimized Spectroscopy) have become essential tools for fragment-based screening and hit validation. These methods can detect weak binding interactions (KD in the millimolar range) that might be missed by other biophysical techniques, providing valuable starting points for medicinal chemistry optimization.
The application of NMR to membrane protein structural biology represents another frontier where interdisciplinary approaches yield significant advances. By combining solution NMR with solid-state NMR techniques, researchers can now characterize both the structure and dynamics of membrane proteins within lipid bilayers, offering insights into ion channel function, receptor signaling, and membrane transport mechanisms.
Recent developments in metabolomics have leveraged high-field NMR for systems biology applications, enabling the simultaneous detection and quantification of hundreds of metabolites in biological samples. This capability, when integrated with proteomics and genomics data, provides a comprehensive view of cellular responses to environmental changes, disease states, or therapeutic interventions.
The intersection of NMR with synthetic biology has facilitated the rational design of novel enzymes and biomaterials with tailored properties. By elucidating structure-function relationships at atomic resolution, NMR guides protein engineering efforts toward desired catalytic activities or material characteristics, accelerating the development of biotechnological applications.
Structural biology represents one of the most significant application domains for high-field NMR analysis, where it complements X-ray crystallography and cryo-electron microscopy. The unique advantage of NMR lies in its ability to study proteins in solution states that closely mimic physiological conditions, capturing dynamic processes that static methods cannot reveal. This capability has proven invaluable for understanding intrinsically disordered proteins (IDPs), which constitute approximately 30% of eukaryotic proteomes and play crucial roles in cellular signaling and regulation.
The convergence of NMR with computational biology has dramatically enhanced structure determination workflows. Modern molecular dynamics simulations, informed by NMR-derived constraints, generate ensemble representations that better reflect the conformational heterogeneity of biomolecules. This hybrid approach has been particularly successful in characterizing transient protein-protein interactions and allosteric mechanisms that underlie many biological processes.
In drug discovery, high-field NMR techniques such as STD-NMR (Saturation Transfer Difference) and TROSY (Transverse Relaxation Optimized Spectroscopy) have become essential tools for fragment-based screening and hit validation. These methods can detect weak binding interactions (KD in the millimolar range) that might be missed by other biophysical techniques, providing valuable starting points for medicinal chemistry optimization.
The application of NMR to membrane protein structural biology represents another frontier where interdisciplinary approaches yield significant advances. By combining solution NMR with solid-state NMR techniques, researchers can now characterize both the structure and dynamics of membrane proteins within lipid bilayers, offering insights into ion channel function, receptor signaling, and membrane transport mechanisms.
Recent developments in metabolomics have leveraged high-field NMR for systems biology applications, enabling the simultaneous detection and quantification of hundreds of metabolites in biological samples. This capability, when integrated with proteomics and genomics data, provides a comprehensive view of cellular responses to environmental changes, disease states, or therapeutic interventions.
The intersection of NMR with synthetic biology has facilitated the rational design of novel enzymes and biomaterials with tailored properties. By elucidating structure-function relationships at atomic resolution, NMR guides protein engineering efforts toward desired catalytic activities or material characteristics, accelerating the development of biotechnological applications.
Data Processing Algorithms and Computational Challenges
High-field NMR analysis generates massive datasets that require sophisticated computational approaches for effective interpretation. Current data processing algorithms face significant challenges in handling the complexity and volume of spectral information produced during molecular dynamics studies. Traditional Fourier Transform methods, while foundational, often struggle with the resolution requirements of modern high-field experiments, particularly when analyzing complex biomolecular systems.
Advanced signal processing techniques such as Maximum Entropy Method (MEM) and Linear Prediction (LP) have emerged as valuable alternatives, offering improved spectral resolution and noise reduction capabilities. However, these methods demand substantial computational resources, creating bottlenecks in real-time analysis workflows. The implementation of parallel computing architectures has partially addressed this limitation, enabling more efficient processing of multi-dimensional NMR data.
Machine learning approaches represent the cutting edge in NMR data processing, with convolutional neural networks demonstrating particular promise for pattern recognition in complex spectra. These algorithms can identify subtle spectral features that might be overlooked by conventional methods, especially in crowded regions characteristic of large biomolecules. Despite their potential, ML algorithms require extensive training datasets and face challenges in generalizability across different experimental conditions.
Computational challenges extend beyond processing to data storage and management. A typical high-field NMR experiment can generate terabytes of raw data, necessitating robust database architectures and efficient compression algorithms. Cloud-based solutions have gained traction, offering scalable resources for both storage and computation, though concerns regarding data security and transfer speeds persist.
Quantitative analysis of molecular dynamics presents additional computational hurdles. Model-free approaches for analyzing relaxation data require complex fitting algorithms that must balance accuracy with computational efficiency. Similarly, the extraction of structural parameters from NOE data involves solving ill-posed inverse problems, often requiring regularization techniques to achieve stable solutions.
Integration of NMR data with molecular dynamics simulations represents perhaps the most computationally intensive challenge. Bridging experimental observations with theoretical models demands sophisticated algorithms capable of handling multiple time scales and conformational ensembles. Recent developments in Markov State Models and Bayesian statistical methods show promise in this domain, though their implementation remains computationally expensive.
Future algorithm development will likely focus on real-time processing capabilities, enabling dynamic adjustment of experimental parameters based on emerging spectral features. This adaptive approach would significantly enhance the efficiency of high-field NMR investigations into molecular dynamics, particularly for time-sensitive applications in structural biology and drug discovery.
Advanced signal processing techniques such as Maximum Entropy Method (MEM) and Linear Prediction (LP) have emerged as valuable alternatives, offering improved spectral resolution and noise reduction capabilities. However, these methods demand substantial computational resources, creating bottlenecks in real-time analysis workflows. The implementation of parallel computing architectures has partially addressed this limitation, enabling more efficient processing of multi-dimensional NMR data.
Machine learning approaches represent the cutting edge in NMR data processing, with convolutional neural networks demonstrating particular promise for pattern recognition in complex spectra. These algorithms can identify subtle spectral features that might be overlooked by conventional methods, especially in crowded regions characteristic of large biomolecules. Despite their potential, ML algorithms require extensive training datasets and face challenges in generalizability across different experimental conditions.
Computational challenges extend beyond processing to data storage and management. A typical high-field NMR experiment can generate terabytes of raw data, necessitating robust database architectures and efficient compression algorithms. Cloud-based solutions have gained traction, offering scalable resources for both storage and computation, though concerns regarding data security and transfer speeds persist.
Quantitative analysis of molecular dynamics presents additional computational hurdles. Model-free approaches for analyzing relaxation data require complex fitting algorithms that must balance accuracy with computational efficiency. Similarly, the extraction of structural parameters from NOE data involves solving ill-posed inverse problems, often requiring regularization techniques to achieve stable solutions.
Integration of NMR data with molecular dynamics simulations represents perhaps the most computationally intensive challenge. Bridging experimental observations with theoretical models demands sophisticated algorithms capable of handling multiple time scales and conformational ensembles. Recent developments in Markov State Models and Bayesian statistical methods show promise in this domain, though their implementation remains computationally expensive.
Future algorithm development will likely focus on real-time processing capabilities, enabling dynamic adjustment of experimental parameters based on emerging spectral features. This adaptive approach would significantly enhance the efficiency of high-field NMR investigations into molecular dynamics, particularly for time-sensitive applications in structural biology and drug discovery.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!