Enhance High-Throughput NMR to Meet Industrial Standards
SEP 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
NMR Technology Evolution and Industrial Objectives
Nuclear Magnetic Resonance (NMR) spectroscopy has evolved significantly since its discovery in the 1940s, transforming from a physics curiosity into an indispensable analytical tool across multiple industries. The technology leverages the magnetic properties of atomic nuclei to provide detailed molecular structure information, offering unparalleled insights into chemical composition and molecular dynamics without destroying samples.
The evolution of NMR technology has been marked by several breakthrough developments. Early NMR systems operated at low magnetic field strengths with limited resolution and sensitivity. The introduction of superconducting magnets in the 1960s dramatically improved field strength capabilities, enabling higher resolution spectra. The 1970s saw the development of Fourier Transform NMR (FT-NMR), revolutionizing data acquisition speed and sensitivity. Subsequently, multi-dimensional NMR techniques emerged in the 1980s, allowing for more complex structural elucidation of biomolecules.
Despite these advances, traditional NMR systems remain characterized by low throughput, with typical analysis times ranging from minutes to hours per sample. This limitation has restricted NMR's application in industrial settings where rapid, high-volume analysis is essential. The industrial objective now centers on enhancing NMR throughput while maintaining the technique's inherent advantages of non-destructive analysis and detailed structural information.
High-throughput NMR (HT-NMR) represents the next evolutionary step, aiming to meet industrial standards for efficiency and scalability. Key industrial objectives include reducing analysis time to seconds per sample, automating sample handling and data processing, and developing more compact, cost-effective instrumentation suitable for production environments. These improvements would position NMR as a viable option for real-time process monitoring and quality control in pharmaceutical manufacturing, food production, and petrochemical processing.
Another critical industrial objective is the integration of HT-NMR with existing analytical workflows and data management systems. This requires standardized data formats, improved software interfaces, and robust calibration methods to ensure consistent results across different instruments and operating conditions. The development of specialized pulse sequences and data acquisition protocols optimized for specific industrial applications represents another important goal.
Machine learning and artificial intelligence are increasingly viewed as essential components for achieving these industrial objectives, offering potential solutions for automated spectral interpretation, anomaly detection, and predictive analytics. The convergence of these computational approaches with hardware innovations in magnet design, probe technology, and electronics is expected to drive the next generation of HT-NMR systems capable of meeting demanding industrial requirements.
The evolution of NMR technology has been marked by several breakthrough developments. Early NMR systems operated at low magnetic field strengths with limited resolution and sensitivity. The introduction of superconducting magnets in the 1960s dramatically improved field strength capabilities, enabling higher resolution spectra. The 1970s saw the development of Fourier Transform NMR (FT-NMR), revolutionizing data acquisition speed and sensitivity. Subsequently, multi-dimensional NMR techniques emerged in the 1980s, allowing for more complex structural elucidation of biomolecules.
Despite these advances, traditional NMR systems remain characterized by low throughput, with typical analysis times ranging from minutes to hours per sample. This limitation has restricted NMR's application in industrial settings where rapid, high-volume analysis is essential. The industrial objective now centers on enhancing NMR throughput while maintaining the technique's inherent advantages of non-destructive analysis and detailed structural information.
High-throughput NMR (HT-NMR) represents the next evolutionary step, aiming to meet industrial standards for efficiency and scalability. Key industrial objectives include reducing analysis time to seconds per sample, automating sample handling and data processing, and developing more compact, cost-effective instrumentation suitable for production environments. These improvements would position NMR as a viable option for real-time process monitoring and quality control in pharmaceutical manufacturing, food production, and petrochemical processing.
Another critical industrial objective is the integration of HT-NMR with existing analytical workflows and data management systems. This requires standardized data formats, improved software interfaces, and robust calibration methods to ensure consistent results across different instruments and operating conditions. The development of specialized pulse sequences and data acquisition protocols optimized for specific industrial applications represents another important goal.
Machine learning and artificial intelligence are increasingly viewed as essential components for achieving these industrial objectives, offering potential solutions for automated spectral interpretation, anomaly detection, and predictive analytics. The convergence of these computational approaches with hardware innovations in magnet design, probe technology, and electronics is expected to drive the next generation of HT-NMR systems capable of meeting demanding industrial requirements.
Market Analysis for High-Throughput NMR Applications
The global market for high-throughput Nuclear Magnetic Resonance (NMR) technology is experiencing significant growth, driven by increasing demand across pharmaceutical, biotechnology, food science, and materials research sectors. Current market valuation stands at approximately 1.2 billion USD, with projections indicating a compound annual growth rate of 5.7% through 2028, potentially reaching 1.6 billion USD by that time.
Pharmaceutical and biotechnology industries represent the largest market segments, collectively accounting for over 60% of high-throughput NMR applications. These sectors utilize the technology primarily for drug discovery, metabolomics research, and quality control processes. The ability to rapidly analyze multiple samples with minimal preparation has become increasingly valuable as drug development timelines compress and competition intensifies.
Academic and research institutions constitute another significant market segment, representing approximately 20% of the total market share. These organizations typically employ high-throughput NMR for fundamental research in structural biology, materials science, and chemical analysis. Government funding for research infrastructure has been a key driver in this segment, particularly in North America and Europe.
Regional analysis reveals North America as the dominant market (38% share), followed by Europe (32%) and Asia-Pacific (24%). The Asia-Pacific region, particularly China and India, demonstrates the fastest growth trajectory with increasing investments in pharmaceutical research infrastructure and manufacturing capabilities. Latin America and Middle East regions show emerging potential but currently represent smaller market shares.
Key market drivers include the growing emphasis on precision medicine requiring detailed molecular analysis, increasing automation in analytical laboratories, and rising demand for rapid quality control methods in manufacturing. The push toward higher sample throughput while maintaining analytical precision has become a critical competitive factor across industries.
Market challenges primarily revolve around the high capital investment required for advanced NMR systems, technical expertise needed for operation and data interpretation, and competition from alternative analytical technologies such as mass spectrometry. The total cost of ownership, including maintenance and specialized staff, remains a significant barrier to wider adoption in smaller organizations and emerging markets.
Customer demand increasingly focuses on integrated solutions that combine hardware improvements with advanced software for automated data processing and interpretation. The market shows strong preference for systems offering higher magnetic field strengths, improved probe technology, and sophisticated pulse sequences that enable faster acquisition times while maintaining spectral quality necessary for industrial applications.
Pharmaceutical and biotechnology industries represent the largest market segments, collectively accounting for over 60% of high-throughput NMR applications. These sectors utilize the technology primarily for drug discovery, metabolomics research, and quality control processes. The ability to rapidly analyze multiple samples with minimal preparation has become increasingly valuable as drug development timelines compress and competition intensifies.
Academic and research institutions constitute another significant market segment, representing approximately 20% of the total market share. These organizations typically employ high-throughput NMR for fundamental research in structural biology, materials science, and chemical analysis. Government funding for research infrastructure has been a key driver in this segment, particularly in North America and Europe.
Regional analysis reveals North America as the dominant market (38% share), followed by Europe (32%) and Asia-Pacific (24%). The Asia-Pacific region, particularly China and India, demonstrates the fastest growth trajectory with increasing investments in pharmaceutical research infrastructure and manufacturing capabilities. Latin America and Middle East regions show emerging potential but currently represent smaller market shares.
Key market drivers include the growing emphasis on precision medicine requiring detailed molecular analysis, increasing automation in analytical laboratories, and rising demand for rapid quality control methods in manufacturing. The push toward higher sample throughput while maintaining analytical precision has become a critical competitive factor across industries.
Market challenges primarily revolve around the high capital investment required for advanced NMR systems, technical expertise needed for operation and data interpretation, and competition from alternative analytical technologies such as mass spectrometry. The total cost of ownership, including maintenance and specialized staff, remains a significant barrier to wider adoption in smaller organizations and emerging markets.
Customer demand increasingly focuses on integrated solutions that combine hardware improvements with advanced software for automated data processing and interpretation. The market shows strong preference for systems offering higher magnetic field strengths, improved probe technology, and sophisticated pulse sequences that enable faster acquisition times while maintaining spectral quality necessary for industrial applications.
Current Limitations and Technical Barriers in Industrial NMR
Despite significant advancements in Nuclear Magnetic Resonance (NMR) technology, several critical limitations continue to impede its widespread adoption in industrial settings. The primary challenge remains the inherently low sensitivity of NMR spectroscopy, which necessitates either concentrated samples or extended acquisition times. This fundamental limitation creates a significant bottleneck for high-throughput applications where rapid analysis of dilute samples is essential for quality control and process monitoring.
Sample preparation represents another substantial barrier to industrial implementation. Current protocols often require complex preparation steps including precise solvent selection, pH adjustment, and concentration optimization. These requirements not only increase operational complexity but also introduce potential sources of error and variability that can compromise measurement reproducibility in production environments.
The physical footprint and infrastructure requirements of conventional NMR systems present significant obstacles for integration into industrial workflows. Traditional high-field NMR spectrometers require specialized facilities with controlled environments, substantial space allocation, and regular cryogen replenishment. These requirements make on-line or at-line implementation prohibitively complex for many manufacturing settings.
Data processing and interpretation remain highly specialized skills, requiring significant expertise that is often unavailable in industrial settings. The complexity of spectral analysis, peak assignment, and quantification procedures creates a technical barrier that limits accessibility to non-specialists. This expertise gap significantly restricts the practical utility of NMR in routine industrial applications.
Cost considerations further compound these challenges. The high capital expenditure for NMR instrumentation, coupled with substantial operational costs including maintenance, cryogens, and specialized personnel, creates an unfavorable economic proposition for many industrial applications where alternative analytical techniques may offer sufficient performance at lower cost points.
Throughput limitations represent perhaps the most significant barrier to industrial adoption. Conventional NMR protocols typically require minutes to hours per sample, making them incompatible with the rapid analysis requirements of modern production environments. This temporal mismatch between analytical capabilities and industrial needs creates a fundamental barrier to implementation.
Standardization and method validation challenges also persist across different instrument platforms and manufacturers. The lack of universally accepted protocols for calibration, data processing, and reporting creates significant obstacles for regulatory compliance and cross-facility comparability, particularly in highly regulated industries such as pharmaceuticals and food production.
Sample preparation represents another substantial barrier to industrial implementation. Current protocols often require complex preparation steps including precise solvent selection, pH adjustment, and concentration optimization. These requirements not only increase operational complexity but also introduce potential sources of error and variability that can compromise measurement reproducibility in production environments.
The physical footprint and infrastructure requirements of conventional NMR systems present significant obstacles for integration into industrial workflows. Traditional high-field NMR spectrometers require specialized facilities with controlled environments, substantial space allocation, and regular cryogen replenishment. These requirements make on-line or at-line implementation prohibitively complex for many manufacturing settings.
Data processing and interpretation remain highly specialized skills, requiring significant expertise that is often unavailable in industrial settings. The complexity of spectral analysis, peak assignment, and quantification procedures creates a technical barrier that limits accessibility to non-specialists. This expertise gap significantly restricts the practical utility of NMR in routine industrial applications.
Cost considerations further compound these challenges. The high capital expenditure for NMR instrumentation, coupled with substantial operational costs including maintenance, cryogens, and specialized personnel, creates an unfavorable economic proposition for many industrial applications where alternative analytical techniques may offer sufficient performance at lower cost points.
Throughput limitations represent perhaps the most significant barrier to industrial adoption. Conventional NMR protocols typically require minutes to hours per sample, making them incompatible with the rapid analysis requirements of modern production environments. This temporal mismatch between analytical capabilities and industrial needs creates a fundamental barrier to implementation.
Standardization and method validation challenges also persist across different instrument platforms and manufacturers. The lack of universally accepted protocols for calibration, data processing, and reporting creates significant obstacles for regulatory compliance and cross-facility comparability, particularly in highly regulated industries such as pharmaceuticals and food production.
Current High-Throughput NMR Implementation Strategies
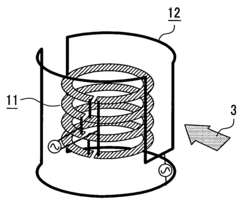
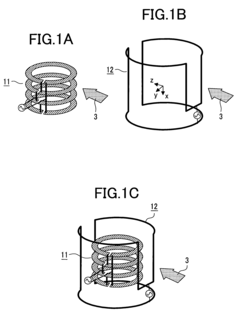
01 Advanced NMR hardware configurations for high-throughput analysis
Specialized hardware configurations in NMR systems can significantly increase throughput capabilities. These include optimized probe designs, automated sample handling systems, and parallel processing capabilities that allow multiple samples to be analyzed simultaneously. Such hardware innovations reduce analysis time while maintaining spectral quality and resolution, enabling more efficient screening of large sample sets.- Advanced NMR hardware configurations for high-throughput analysis: Specialized hardware configurations in NMR systems can significantly increase throughput capabilities. These include optimized probe designs, automated sample handling systems, and parallel detection mechanisms that allow for simultaneous analysis of multiple samples. Such hardware innovations reduce experiment time while maintaining analytical precision, enabling more efficient screening of large sample sets.
- Software and data processing methods for rapid NMR analysis: Advanced software solutions and data processing algorithms are essential for high-throughput NMR applications. These include automated spectral analysis, pattern recognition techniques, and machine learning approaches that can rapidly interpret complex NMR data. Such computational methods enable faster data processing, reducing the bottlenecks in analysis workflows and allowing researchers to handle larger datasets efficiently.
- Sample preparation and handling techniques for NMR throughput optimization: Innovative sample preparation and handling methods significantly impact NMR throughput. Automated sample loading systems, microfluidic devices, and specialized sample containers allow for rapid exchange and positioning of samples within the magnetic field. These approaches minimize downtime between measurements and reduce manual intervention, enabling continuous operation and maximizing the number of samples that can be analyzed in a given timeframe.
- Pulse sequence optimization for accelerated NMR measurements: Specialized pulse sequences designed for rapid data acquisition can dramatically increase NMR throughput. These include fast acquisition techniques, reduced dimensionality experiments, and non-uniform sampling methods that collect essential spectral information in shortened timeframes. By optimizing the timing and nature of radiofrequency pulses, these methods reduce experiment duration while preserving the analytical information needed for accurate sample characterization.
- Integration of NMR with other analytical techniques for comprehensive high-throughput screening: Combining NMR with complementary analytical methods creates powerful high-throughput screening platforms. These integrated systems may incorporate mass spectrometry, optical spectroscopy, or chromatographic techniques alongside NMR to provide multi-modal analysis. Such approaches enable more comprehensive sample characterization while maintaining high throughput, particularly valuable in drug discovery, metabolomics, and quality control applications.
02 Software and data processing methods for rapid NMR analysis
Advanced software solutions and data processing algorithms are essential for high-throughput NMR applications. These include automated spectral analysis, pattern recognition techniques, and machine learning approaches that can rapidly interpret complex NMR data. Real-time data processing capabilities and specialized algorithms for signal enhancement and noise reduction allow for faster acquisition times and more efficient analysis workflows.Expand Specific Solutions03 Microfluidic and miniaturized NMR systems
Miniaturized NMR systems and microfluidic platforms enable high-throughput capabilities by reducing sample volume requirements and analysis time. These systems integrate sample preparation, handling, and analysis into compact devices that can process multiple samples in parallel. The reduced dimensions of these systems allow for faster magnetic field equilibration and more efficient use of laboratory space while maintaining analytical performance.Expand Specific Solutions04 Hyperpolarization techniques for sensitivity enhancement
Hyperpolarization methods significantly enhance NMR signal intensity, enabling faster acquisition times and improved throughput. These techniques temporarily increase the polarization of nuclear spins beyond thermal equilibrium levels, resulting in dramatically enhanced sensitivity. This allows for reduced measurement times, smaller sample volumes, and the ability to detect low-concentration analytes, all contributing to higher throughput capabilities.Expand Specific Solutions05 Integrated sample preparation and automation systems
Automated sample preparation and handling systems integrated with NMR instruments significantly increase throughput by reducing manual intervention. These systems include robotic sample changers, automated dissolution and mixing stations, and integrated quality control mechanisms. Continuous flow systems allow for real-time monitoring and analysis of reactions or processes, further enhancing the throughput capabilities of NMR analysis.Expand Specific Solutions
Leading Companies and Research Institutions in NMR Technology
High-throughput NMR technology is currently in a transitional phase, evolving from research applications to industrial standards. The market is experiencing moderate growth, estimated at $1.5-2 billion annually, driven by increasing demand in pharmaceutical, chemical, and materials science industries. Technologically, the field shows varying maturity levels across players. Established instrumentation companies like Bruker Switzerland, Oxford Instruments, and Agilent Technologies lead with advanced commercial solutions, while academic institutions (Harvard College, Kyoto University, Zhejiang University) contribute fundamental research innovations. United Imaging Healthcare and Hitachi Ltd. are leveraging their medical imaging expertise to develop industrial NMR applications. Recent advancements by Schlumberger Technologies and Halliburton in field-deployable systems indicate growing industrial adoption, though challenges in sensitivity, throughput, and standardization remain significant barriers to widespread implementation.
Hitachi Ltd.
Technical Solution: Hitachi has developed a comprehensive approach to high-throughput NMR that focuses on integration with industrial processes. Their solution centers around the concept of "connected NMR" where instruments are fully incorporated into production workflows. Hitachi's system features compact permanent magnet designs that can be installed directly in production environments, bringing analysis closer to the point of need rather than requiring samples to be sent to a central laboratory[1]. Their technology incorporates rapid pulse sequence optimization that automatically adjusts parameters based on sample type, reducing setup time between different product analyses. For industrial applications, Hitachi has implemented real-time monitoring capabilities that allow continuous sampling from production streams with automated data analysis to detect deviations from quality parameters. Their systems include specialized probes designed for specific industrial matrices that optimize signal-to-noise ratio for challenging samples. Hitachi's software platform provides automated decision support, translating NMR results into process control recommendations without requiring operator interpretation[2]. The company has also developed miniaturized NMR technology that enables multiple units to be deployed throughout a production facility, creating distributed analysis networks that significantly increase overall throughput while providing spatial resolution of process variations.
Strengths: Excellent integration with industrial production systems; compact form factor allowing point-of-need deployment; automated decision support reducing need for specialist interpretation; distributed analysis capability. Weaknesses: Lower spectral resolution compared to high-field systems; more limited range of nuclei that can be effectively analyzed; requires careful calibration to maintain consistency between multiple units.
Bruker Switzerland
Technical Solution: Bruker Switzerland has developed advanced high-throughput NMR systems that meet industrial standards through their innovative AVANCE NEO platform. This platform incorporates parallel acquisition technology allowing simultaneous measurement of multiple samples, significantly increasing throughput capacity[1]. Their systems feature automated sample handling with robotic sample changers capable of managing hundreds of samples continuously without human intervention. Bruker's CryoProbe technology dramatically enhances sensitivity (up to 4x for 13C and 16x for 1H)[2], enabling faster acquisition times and detection of low-concentration analytes. Their TopSpin software suite provides automated data processing workflows specifically designed for industrial applications, with batch processing capabilities and integration with LIMS (Laboratory Information Management Systems). Bruker has also implemented standardized validation protocols that ensure compliance with regulatory requirements such as GMP, GLP, and FDA 21 CFR Part 11, making their systems suitable for pharmaceutical quality control and other regulated industries[3].
Strengths: Industry-leading sensitivity with CryoProbe technology; comprehensive automation solutions; robust validation and compliance features; extensive software integration capabilities. Weaknesses: Higher initial investment costs compared to conventional NMR systems; requires specialized maintenance for cryogenic components; complex system setup may require dedicated technical expertise.
Key Patents and Innovations in NMR Throughput Enhancement
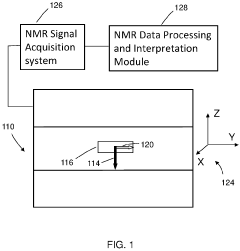
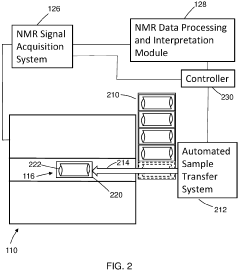
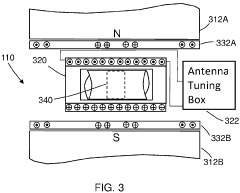
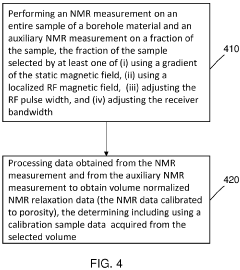
Method and apparatus for high-throughput nuclear magnetic resonance measurements on borehole materials
PatentActiveUS11815482B2
Innovation
- A high-throughput automated low field NMR relaxometer system with a controllable static magnetic field gradient and reconfigurable NMR antenna allows for measurements on irregularly shaped samples by selecting a sensitivity volume within the sample using a switchable gradient, enabling calibration to porosity without independent volume measurement, and processing NMR signals from both the entire and a fraction of the sample to determine petrophysical properties.
Nuclear magnetic resonance system
PatentInactiveUS7141975B2
Innovation
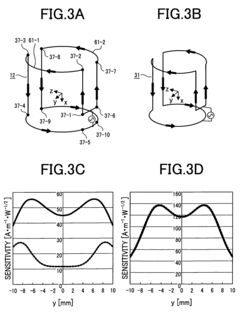
- The implementation of a nuclear magnetic resonance system comprising a superconductivity reception coil, a transmission coil, and an additional coil with four electric current loops where the directions of currents in the inner and outer loops are opposite, arranged to ensure that the high-frequency magnetic fields developed in these coils are orthogonal, thereby preventing electromagnetic interference and maintaining sensitivity.
Cost-Benefit Analysis of High-Throughput NMR Solutions
The implementation of High-Throughput NMR (HT-NMR) systems requires substantial initial investment, necessitating a comprehensive cost-benefit analysis to justify adoption in industrial settings. Initial capital expenditures for HT-NMR systems typically range from $500,000 to $2 million, depending on specifications, automation level, and sample handling capabilities. This includes costs for the spectrometer, automation equipment, sample preparation systems, and specialized software for data analysis.
Operational costs must also be considered, including maintenance contracts (approximately 10-15% of initial equipment cost annually), consumables, specialized staff training, and potential facility modifications to accommodate the equipment. Energy consumption represents another significant ongoing expense, as high-field NMR systems require continuous cryogen supply and substantial power.
Against these costs, organizations must weigh quantifiable benefits. Sample throughput increases of 3-10 times compared to conventional NMR systems translate to significant labor cost reductions and enhanced laboratory efficiency. Studies indicate that automated HT-NMR systems can process 200-500 samples daily versus 30-50 samples with traditional methods.
Quality improvements represent another substantial benefit, with HT-NMR systems demonstrating 30-40% reduction in analytical errors through standardized procedures and reduced human intervention. This improved reliability directly impacts product quality and regulatory compliance, potentially avoiding costly product recalls or regulatory penalties.
Time-to-market acceleration constitutes a critical advantage, particularly in pharmaceutical and materials science industries. HT-NMR can reduce analytical bottlenecks by 40-60%, allowing faster decision-making in R&D pipelines and accelerating product development cycles by weeks or months.
Return on investment calculations typically show break-even periods of 2-4 years for organizations with high sample volumes. Companies processing over 10,000 samples annually generally achieve faster ROI, while those with lower volumes may require longer periods to realize financial benefits.
The scalability of HT-NMR solutions offers additional long-term value. Modern systems can be upgraded incrementally, allowing organizations to expand capabilities without complete system replacement. This modular approach provides flexibility to adapt to changing analytical needs while protecting the initial investment.
Operational costs must also be considered, including maintenance contracts (approximately 10-15% of initial equipment cost annually), consumables, specialized staff training, and potential facility modifications to accommodate the equipment. Energy consumption represents another significant ongoing expense, as high-field NMR systems require continuous cryogen supply and substantial power.
Against these costs, organizations must weigh quantifiable benefits. Sample throughput increases of 3-10 times compared to conventional NMR systems translate to significant labor cost reductions and enhanced laboratory efficiency. Studies indicate that automated HT-NMR systems can process 200-500 samples daily versus 30-50 samples with traditional methods.
Quality improvements represent another substantial benefit, with HT-NMR systems demonstrating 30-40% reduction in analytical errors through standardized procedures and reduced human intervention. This improved reliability directly impacts product quality and regulatory compliance, potentially avoiding costly product recalls or regulatory penalties.
Time-to-market acceleration constitutes a critical advantage, particularly in pharmaceutical and materials science industries. HT-NMR can reduce analytical bottlenecks by 40-60%, allowing faster decision-making in R&D pipelines and accelerating product development cycles by weeks or months.
Return on investment calculations typically show break-even periods of 2-4 years for organizations with high sample volumes. Companies processing over 10,000 samples annually generally achieve faster ROI, while those with lower volumes may require longer periods to realize financial benefits.
The scalability of HT-NMR solutions offers additional long-term value. Modern systems can be upgraded incrementally, allowing organizations to expand capabilities without complete system replacement. This modular approach provides flexibility to adapt to changing analytical needs while protecting the initial investment.
Quality Control and Validation Protocols for Industrial NMR
Quality control and validation protocols are essential components for the successful integration of high-throughput NMR systems into industrial environments. These protocols must be rigorously designed to ensure that NMR data meets the stringent requirements of industrial applications, particularly in sectors such as pharmaceuticals, food safety, and materials manufacturing.
The foundation of effective quality control for industrial NMR begins with the establishment of standardized operating procedures (SOPs) that detail sample preparation, instrument calibration, data acquisition, and analysis methodologies. These SOPs must be validated through collaborative testing across multiple laboratories to ensure reproducibility and reliability of results regardless of operator or specific instrument configuration.
Validation protocols for high-throughput NMR systems should incorporate both system suitability tests and performance qualification procedures. System suitability tests evaluate the instrument's performance against predetermined specifications before sample analysis, including parameters such as spectral resolution, sensitivity, and chemical shift accuracy. Performance qualification procedures verify that the complete analytical method consistently produces results within acceptable limits under normal operating conditions.
Statistical process control methods play a crucial role in maintaining quality standards for industrial NMR applications. Implementation of control charts to monitor critical quality attributes over time enables early detection of system drift or performance degradation. Establishing appropriate warning and action limits based on statistical analysis of historical data provides objective criteria for intervention when necessary.
Reference standards and certified reference materials (CRMs) are indispensable for validating NMR methodologies in industrial settings. These standards must be traceable to recognized metrological authorities and should closely match the matrix composition of routine samples. Regular analysis of these reference materials serves as an ongoing verification of system performance and analytical accuracy.
Automated quality checks embedded within high-throughput workflows represent an advanced approach to quality control. These automated systems can evaluate spectral quality metrics in real-time, flagging problematic data for review and potentially triggering corrective actions without human intervention. Such automation is particularly valuable in high-volume industrial environments where manual review of each spectrum would create significant bottlenecks.
Documentation and audit trails form the final critical element of industrial NMR quality control. Complete records of instrument maintenance, calibration history, method validation studies, and quality control results must be maintained in compliance with relevant regulatory requirements. Electronic systems for data management should incorporate appropriate security features to prevent unauthorized modifications while ensuring data integrity throughout the analytical lifecycle.
The foundation of effective quality control for industrial NMR begins with the establishment of standardized operating procedures (SOPs) that detail sample preparation, instrument calibration, data acquisition, and analysis methodologies. These SOPs must be validated through collaborative testing across multiple laboratories to ensure reproducibility and reliability of results regardless of operator or specific instrument configuration.
Validation protocols for high-throughput NMR systems should incorporate both system suitability tests and performance qualification procedures. System suitability tests evaluate the instrument's performance against predetermined specifications before sample analysis, including parameters such as spectral resolution, sensitivity, and chemical shift accuracy. Performance qualification procedures verify that the complete analytical method consistently produces results within acceptable limits under normal operating conditions.
Statistical process control methods play a crucial role in maintaining quality standards for industrial NMR applications. Implementation of control charts to monitor critical quality attributes over time enables early detection of system drift or performance degradation. Establishing appropriate warning and action limits based on statistical analysis of historical data provides objective criteria for intervention when necessary.
Reference standards and certified reference materials (CRMs) are indispensable for validating NMR methodologies in industrial settings. These standards must be traceable to recognized metrological authorities and should closely match the matrix composition of routine samples. Regular analysis of these reference materials serves as an ongoing verification of system performance and analytical accuracy.
Automated quality checks embedded within high-throughput workflows represent an advanced approach to quality control. These automated systems can evaluate spectral quality metrics in real-time, flagging problematic data for review and potentially triggering corrective actions without human intervention. Such automation is particularly valuable in high-volume industrial environments where manual review of each spectrum would create significant bottlenecks.
Documentation and audit trails form the final critical element of industrial NMR quality control. Complete records of instrument maintenance, calibration history, method validation studies, and quality control results must be maintained in compliance with relevant regulatory requirements. Electronic systems for data management should incorporate appropriate security features to prevent unauthorized modifications while ensuring data integrity throughout the analytical lifecycle.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!