NMR Impact on Allosteric Control Mechanisms: Dynamic Examination
SEP 22, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
NMR Technology Background and Objectives
Nuclear Magnetic Resonance (NMR) spectroscopy has evolved significantly since its discovery in the 1940s, transforming from a physics curiosity into an indispensable tool for molecular structure determination and dynamic analysis. The technology leverages the magnetic properties of atomic nuclei to provide detailed information about molecular structure, dynamics, and interactions at atomic resolution. Over the decades, NMR has undergone revolutionary advancements in hardware capabilities, pulse sequence development, and data analysis methodologies, enabling increasingly sophisticated applications across multiple scientific disciplines.
The evolution of NMR technology has been marked by several pivotal developments, including the introduction of Fourier Transform NMR in the 1970s, which dramatically improved sensitivity, and the development of multidimensional NMR techniques in the 1980s, which expanded the technology's applicability to larger biomolecules. Recent years have witnessed significant improvements in magnet technology, with ultra-high field instruments (>1 GHz) becoming available, alongside advances in cryoprobe technology that have substantially enhanced signal-to-noise ratios.
In the context of allosteric control mechanisms, NMR offers unique capabilities for examining dynamic molecular processes that are often inaccessible to other structural biology techniques. Allostery—the phenomenon where binding at one site affects function at a distant site—fundamentally involves changes in protein dynamics and conformational equilibria. Traditional structural techniques like X-ray crystallography provide static snapshots but cannot fully capture the dynamic nature of allosteric regulation.
The primary objective of applying NMR to allosteric control mechanisms is to elucidate the dynamic networks of communication between distal sites in proteins and other macromolecules. This includes mapping conformational changes, identifying allosteric pathways, quantifying thermodynamic and kinetic parameters of allosteric transitions, and understanding how these processes are perturbed by ligands, mutations, or environmental factors.
Current technological trends in this field include the development of advanced relaxation dispersion methods to characterize microsecond-to-millisecond timescale motions relevant to allostery, paramagnetic relaxation enhancement techniques to measure long-range distances, and residual dipolar coupling measurements to assess changes in molecular alignment. Additionally, real-time NMR methods are emerging to monitor allosteric events as they occur, while integrated computational approaches are enhancing the interpretation of complex NMR datasets.
The ultimate goal of this technology is to establish a comprehensive, dynamic understanding of allosteric mechanisms at atomic resolution, enabling rational design of allosteric modulators for therapeutic applications and providing fundamental insights into the relationship between protein dynamics and function in biological systems.
The evolution of NMR technology has been marked by several pivotal developments, including the introduction of Fourier Transform NMR in the 1970s, which dramatically improved sensitivity, and the development of multidimensional NMR techniques in the 1980s, which expanded the technology's applicability to larger biomolecules. Recent years have witnessed significant improvements in magnet technology, with ultra-high field instruments (>1 GHz) becoming available, alongside advances in cryoprobe technology that have substantially enhanced signal-to-noise ratios.
In the context of allosteric control mechanisms, NMR offers unique capabilities for examining dynamic molecular processes that are often inaccessible to other structural biology techniques. Allostery—the phenomenon where binding at one site affects function at a distant site—fundamentally involves changes in protein dynamics and conformational equilibria. Traditional structural techniques like X-ray crystallography provide static snapshots but cannot fully capture the dynamic nature of allosteric regulation.
The primary objective of applying NMR to allosteric control mechanisms is to elucidate the dynamic networks of communication between distal sites in proteins and other macromolecules. This includes mapping conformational changes, identifying allosteric pathways, quantifying thermodynamic and kinetic parameters of allosteric transitions, and understanding how these processes are perturbed by ligands, mutations, or environmental factors.
Current technological trends in this field include the development of advanced relaxation dispersion methods to characterize microsecond-to-millisecond timescale motions relevant to allostery, paramagnetic relaxation enhancement techniques to measure long-range distances, and residual dipolar coupling measurements to assess changes in molecular alignment. Additionally, real-time NMR methods are emerging to monitor allosteric events as they occur, while integrated computational approaches are enhancing the interpretation of complex NMR datasets.
The ultimate goal of this technology is to establish a comprehensive, dynamic understanding of allosteric mechanisms at atomic resolution, enabling rational design of allosteric modulators for therapeutic applications and providing fundamental insights into the relationship between protein dynamics and function in biological systems.
Market Applications of NMR in Allosteric Control Research
The application of Nuclear Magnetic Resonance (NMR) spectroscopy in allosteric control research has expanded significantly across multiple market sectors, creating substantial commercial opportunities. The pharmaceutical industry represents the largest market segment, where NMR techniques enable detailed mapping of protein-ligand interactions and allosteric binding sites. This capability has revolutionized drug discovery processes by allowing researchers to design more selective allosteric modulators with fewer side effects, addressing a market valued at over $180 billion globally for targeted therapeutics.
Biotechnology companies have increasingly incorporated NMR-based allosteric research into their R&D pipelines, particularly for developing enzyme inhibitors and protein-based therapeutics. The ability to observe real-time conformational changes has created a specialized market for NMR equipment manufacturers who now offer dedicated systems optimized for allosteric studies, with enhanced sensitivity and resolution capabilities tailored to detect subtle structural changes.
The agricultural sector has emerged as a growing application area, where NMR techniques help develop allosteric inhibitors targeting pest-specific enzymes. This approach enables the creation of more environmentally friendly pesticides with reduced ecological impact, addressing increasing regulatory pressure and consumer demand for sustainable agricultural solutions.
Academic and research institutions constitute another significant market segment, driving demand for advanced NMR technologies and methodologies. This has fostered a specialized consulting and service industry where expertise in NMR-based allosteric studies is offered to organizations lacking in-house capabilities, creating a knowledge-transfer economy estimated to be growing at 12% annually.
The food and beverage industry has begun exploring NMR applications for studying allosteric regulation in food enzymes, potentially leading to improved food processing techniques and novel ingredient development. Companies in this sector are investing in collaborative research with academic institutions to leverage NMR expertise for competitive advantage.
Medical diagnostics represents an emerging application area where NMR-based allosteric studies are being explored for developing novel biomarkers and diagnostic tools. The ability to detect disease-specific allosteric changes in proteins shows promise for early disease detection, particularly in neurological and metabolic disorders.
The market for computational tools and software specifically designed for analyzing NMR data in allosteric studies has also expanded, with specialized algorithms for interpreting complex conformational dynamics. This software market segment serves as a complementary ecosystem to hardware providers, creating integrated solutions for researchers across multiple industries.
Biotechnology companies have increasingly incorporated NMR-based allosteric research into their R&D pipelines, particularly for developing enzyme inhibitors and protein-based therapeutics. The ability to observe real-time conformational changes has created a specialized market for NMR equipment manufacturers who now offer dedicated systems optimized for allosteric studies, with enhanced sensitivity and resolution capabilities tailored to detect subtle structural changes.
The agricultural sector has emerged as a growing application area, where NMR techniques help develop allosteric inhibitors targeting pest-specific enzymes. This approach enables the creation of more environmentally friendly pesticides with reduced ecological impact, addressing increasing regulatory pressure and consumer demand for sustainable agricultural solutions.
Academic and research institutions constitute another significant market segment, driving demand for advanced NMR technologies and methodologies. This has fostered a specialized consulting and service industry where expertise in NMR-based allosteric studies is offered to organizations lacking in-house capabilities, creating a knowledge-transfer economy estimated to be growing at 12% annually.
The food and beverage industry has begun exploring NMR applications for studying allosteric regulation in food enzymes, potentially leading to improved food processing techniques and novel ingredient development. Companies in this sector are investing in collaborative research with academic institutions to leverage NMR expertise for competitive advantage.
Medical diagnostics represents an emerging application area where NMR-based allosteric studies are being explored for developing novel biomarkers and diagnostic tools. The ability to detect disease-specific allosteric changes in proteins shows promise for early disease detection, particularly in neurological and metabolic disorders.
The market for computational tools and software specifically designed for analyzing NMR data in allosteric studies has also expanded, with specialized algorithms for interpreting complex conformational dynamics. This software market segment serves as a complementary ecosystem to hardware providers, creating integrated solutions for researchers across multiple industries.
Current Challenges in NMR-Based Allosteric Mechanism Studies
Nuclear Magnetic Resonance (NMR) spectroscopy has emerged as a powerful tool for investigating allosteric control mechanisms in biological systems. However, despite significant advancements, several critical challenges continue to impede comprehensive NMR-based studies of allosteric mechanisms. These challenges span technical limitations, sample preparation complexities, and data interpretation hurdles.
One of the primary technical challenges involves the inherent sensitivity limitations of NMR spectroscopy. Allosteric events often involve subtle conformational changes that may fall below detection thresholds, particularly when examining large protein complexes or membrane-bound systems. This sensitivity issue becomes more pronounced when investigating low-populated conformational states that are crucial for understanding the complete allosteric landscape.
Sample preparation presents another significant obstacle. Many allosteric systems require specific conditions to maintain their native functional states, and these conditions may not always be compatible with optimal NMR experimental parameters. The requirement for isotopic labeling further complicates sample preparation, especially for large multi-domain proteins or protein complexes where selective labeling strategies become necessary but technically demanding.
Time resolution constraints represent a substantial challenge when studying dynamic allosteric processes. Many allosteric transitions occur on microsecond to millisecond timescales, which can be difficult to capture with traditional NMR experiments. While relaxation dispersion and CPMG techniques have improved time resolution, they still struggle to provide comprehensive dynamic information across all relevant timescales simultaneously.
Data interpretation and integration pose additional challenges. The complexity of NMR data from allosteric systems often necessitates sophisticated computational approaches for meaningful analysis. Current methods for integrating NMR data with other structural and computational techniques remain underdeveloped, limiting the holistic understanding of allosteric networks.
The size limitation of NMR spectroscopy presents a persistent challenge. Many physiologically relevant allosteric systems involve large protein assemblies that exceed the practical size limits of solution NMR. While solid-state NMR offers some advantages for larger systems, it introduces its own set of technical difficulties and resolution limitations.
Signal overlap in complex spectra further complicates the analysis of allosteric systems. As protein size increases, spectral crowding becomes more problematic, making it difficult to track specific residues involved in allosteric communication pathways. Advanced multidimensional experiments partially address this issue but at the cost of increased experimental time and technical complexity.
Finally, there remains a significant gap in standardized methodologies for NMR-based allosteric studies. The field lacks consensus on optimal experimental protocols and data analysis pipelines, leading to challenges in comparing results across different research groups and establishing reliable benchmarks for allosteric mechanism characterization.
One of the primary technical challenges involves the inherent sensitivity limitations of NMR spectroscopy. Allosteric events often involve subtle conformational changes that may fall below detection thresholds, particularly when examining large protein complexes or membrane-bound systems. This sensitivity issue becomes more pronounced when investigating low-populated conformational states that are crucial for understanding the complete allosteric landscape.
Sample preparation presents another significant obstacle. Many allosteric systems require specific conditions to maintain their native functional states, and these conditions may not always be compatible with optimal NMR experimental parameters. The requirement for isotopic labeling further complicates sample preparation, especially for large multi-domain proteins or protein complexes where selective labeling strategies become necessary but technically demanding.
Time resolution constraints represent a substantial challenge when studying dynamic allosteric processes. Many allosteric transitions occur on microsecond to millisecond timescales, which can be difficult to capture with traditional NMR experiments. While relaxation dispersion and CPMG techniques have improved time resolution, they still struggle to provide comprehensive dynamic information across all relevant timescales simultaneously.
Data interpretation and integration pose additional challenges. The complexity of NMR data from allosteric systems often necessitates sophisticated computational approaches for meaningful analysis. Current methods for integrating NMR data with other structural and computational techniques remain underdeveloped, limiting the holistic understanding of allosteric networks.
The size limitation of NMR spectroscopy presents a persistent challenge. Many physiologically relevant allosteric systems involve large protein assemblies that exceed the practical size limits of solution NMR. While solid-state NMR offers some advantages for larger systems, it introduces its own set of technical difficulties and resolution limitations.
Signal overlap in complex spectra further complicates the analysis of allosteric systems. As protein size increases, spectral crowding becomes more problematic, making it difficult to track specific residues involved in allosteric communication pathways. Advanced multidimensional experiments partially address this issue but at the cost of increased experimental time and technical complexity.
Finally, there remains a significant gap in standardized methodologies for NMR-based allosteric studies. The field lacks consensus on optimal experimental protocols and data analysis pipelines, leading to challenges in comparing results across different research groups and establishing reliable benchmarks for allosteric mechanism characterization.
Established NMR Methodologies for Allosteric Control Investigation
01 NMR pulse sequence techniques for dynamic examination
Various pulse sequence techniques are employed in NMR for dynamic examination of samples. These techniques include specialized timing and frequency manipulation to capture temporal changes in the sample properties. Dynamic NMR examinations can reveal molecular motion, chemical exchange processes, and structural transitions that are not observable in static measurements. These methods enhance the sensitivity and specificity of NMR analysis for studying time-dependent phenomena.- NMR pulse sequence techniques for dynamic examination: Various pulse sequence techniques are employed in Nuclear Magnetic Resonance (NMR) for dynamic examination of samples. These techniques include specialized timing and frequency manipulation to capture dynamic processes in materials or biological tissues. Advanced pulse sequences allow for real-time monitoring of chemical reactions, molecular motion, and physiological changes, providing valuable information about dynamic systems that static measurements cannot reveal.
- Hardware configurations for dynamic NMR measurements: Specialized hardware configurations are designed for dynamic NMR measurements, including modified magnets, gradient coils, and RF systems. These hardware setups enable rapid data acquisition necessary for capturing dynamic processes. Some systems incorporate mobile or portable components for field measurements, while others feature high-field magnets with enhanced stability for precise dynamic measurements over extended periods.
- Applications of dynamic NMR in medical diagnostics: Dynamic NMR examination techniques are widely applied in medical diagnostics, particularly for functional imaging of organs and tissues. These methods can detect and monitor physiological processes such as blood flow, tissue perfusion, and metabolic activity in real-time. Dynamic contrast-enhanced NMR provides valuable information about vascular permeability and tissue characterization, aiding in the diagnosis and treatment monitoring of various diseases including cancer and cardiovascular disorders.
- Dynamic NMR for material characterization and industrial applications: Dynamic NMR examination is utilized for characterizing materials and monitoring industrial processes. This technique enables the study of polymer dynamics, crystallization processes, and phase transitions in various materials. In industrial settings, dynamic NMR can monitor chemical reactions in real-time, assess product quality, and optimize manufacturing processes. The ability to observe molecular mobility and structural changes makes it valuable for research and quality control in industries such as petrochemicals, polymers, and pharmaceuticals.
- Data processing and analysis methods for dynamic NMR: Advanced data processing and analysis methods are essential for interpreting dynamic NMR examination results. These include specialized algorithms for signal processing, noise reduction, and multidimensional data analysis. Machine learning and artificial intelligence approaches are increasingly applied to extract meaningful patterns from complex dynamic NMR datasets. Time-resolved spectroscopy techniques allow for tracking changes in molecular structure and interactions over time, providing insights into reaction kinetics and dynamic molecular processes.
02 Hardware configurations for dynamic NMR measurements
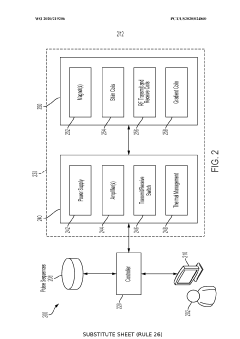
Specialized hardware configurations are designed for dynamic NMR measurements, including modified magnet arrangements, gradient coil systems, and RF transmitter/receiver architectures. These hardware innovations enable faster data acquisition, improved signal-to-noise ratios, and enhanced spatial resolution for dynamic studies. Advanced hardware components allow for real-time monitoring of dynamic processes across various timescales, from milliseconds to hours, expanding the application range of NMR in both research and industrial settings.Expand Specific Solutions03 Data processing methods for dynamic NMR signals
Sophisticated data processing methods are essential for analyzing dynamic NMR signals. These include advanced algorithms for signal filtering, noise reduction, and spectral analysis that can extract meaningful information from time-varying NMR data. Computational approaches such as Fourier transformation, wavelet analysis, and machine learning techniques are applied to process complex dynamic NMR datasets. These methods enable the quantification of dynamic parameters, identification of transient species, and visualization of dynamic processes at the molecular level.Expand Specific Solutions04 Applications of dynamic NMR in material characterization
Dynamic NMR examination is widely applied in material characterization to study properties such as porosity, diffusion, and phase transitions. This technique provides insights into material behavior under varying conditions like temperature, pressure, or mechanical stress. Dynamic NMR methods can probe molecular mobility, structural reorganization, and chemical reactions within materials, making them valuable for research in polymers, ceramics, composites, and other advanced materials. These applications help in optimizing material properties and developing new materials with tailored characteristics.Expand Specific Solutions05 Dynamic NMR for biomedical and pharmaceutical applications
Dynamic NMR examination plays a crucial role in biomedical and pharmaceutical applications, including drug discovery, metabolism studies, and disease diagnosis. This technique can monitor biochemical processes in real-time, track drug-target interactions, and assess metabolic changes in tissues. In clinical settings, dynamic NMR methods provide functional information about organs and tissues, complementing structural imaging techniques. These applications leverage the non-invasive nature of NMR to study dynamic biological processes without disrupting the system under investigation.Expand Specific Solutions
Leading Research Groups and Instrument Manufacturers
The NMR impact on allosteric control mechanisms market is in a growth phase, with increasing applications in drug discovery and structural biology. The global market is expanding as NMR technology becomes essential for understanding protein dynamics and allosteric regulation. Technologically, the field shows moderate maturity with established players like Bruker BioSpin leading instrumentation development, while pharmaceutical companies (AstraZeneca, Bristol Myers Squibb, Boehringer Ingelheim) leverage NMR for drug development. Academic institutions (MIT, Max Planck, University of California) drive fundamental research, while research organizations (National Research Council, CNRS) bridge the gap between theoretical advances and practical applications. The ecosystem demonstrates a collaborative environment between technology providers, pharmaceutical industry, and research institutions advancing dynamic examination of allosteric mechanisms.
Bruker BioSpin MRI GmbH
Technical Solution: Bruker BioSpin has developed advanced NMR spectroscopy platforms specifically designed for studying protein dynamics and allosteric mechanisms. Their technology combines high-field NMR systems with specialized pulse sequences to detect subtle conformational changes in proteins during allosteric events. Their Dynamic Nuclear Polarization (DNP) enhanced NMR technology significantly improves sensitivity by transferring polarization from electrons to nuclei, allowing detection of previously unobservable allosteric interactions. Bruker's time-resolved NMR methodologies enable researchers to capture transient states during allosteric transitions with millisecond resolution, providing insights into the kinetics of conformational changes. Their integrated software solutions offer sophisticated data analysis tools specifically designed for interpreting complex allosteric networks from NMR data, including chemical shift perturbation mapping and relaxation dispersion analysis.
Strengths: Industry-leading sensitivity and resolution in NMR instrumentation; comprehensive software ecosystem for allosteric mechanism analysis; extensive experience in biomolecular NMR applications. Weaknesses: High cost of equipment limits accessibility; requires significant technical expertise to operate effectively; data interpretation remains challenging despite software advances.
Swiss Federal Institute of Technology
Technical Solution: The Swiss Federal Institute of Technology (ETH Zurich) has pioneered innovative NMR methodologies for investigating allosteric control mechanisms in proteins. Their research teams have developed specialized relaxation dispersion techniques that can detect and characterize microsecond-to-millisecond timescale motions critical for allosteric communication. ETH researchers have implemented advanced isotope labeling strategies that enable selective observation of specific protein domains during allosteric transitions. Their work combines solution NMR with computational approaches to map energy landscapes of allosteric proteins, revealing how conformational ensembles shift upon ligand binding. The institute has also developed novel paramagnetic relaxation enhancement (PRE) methods to measure long-range distances within proteins, providing crucial spatial constraints for modeling allosteric networks. Their integrated experimental-computational framework allows for the quantitative description of allosteric communication pathways with unprecedented detail.
Strengths: World-class integration of experimental NMR with computational modeling; innovative pulse sequence development; strong interdisciplinary approach combining structural biology, biophysics, and computational science. Weaknesses: Academic focus sometimes limits translation to industrial applications; research often requires specialized equipment not widely available; methodologies can be technically demanding for broader adoption.
Key NMR Pulse Sequences and Data Analysis Approaches
Methods of dynamic mechanical analysis using nuclear magnetic resonance
PatentPendingUS20240331908A1
Innovation
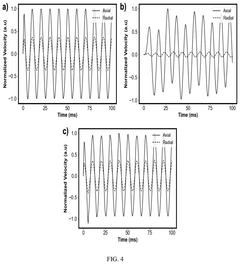
- A method employing a small unilateral three-magnet array with an extended constant gradient to measure the velocity of a vibrating sample by orienting vibrations in the direction of the gradient, using motion-sensitized phase accumulation and delayed pulse sequences to acquire complete velocity waveforms, allowing for characterization of viscoelasticity through dynamic modulus and loss-angle determination.
Techniques for dynamic control of a magnetic resonance imaging system
PatentWO2020219206A1
Innovation
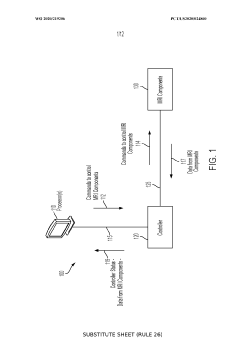
- Implementing a single Field Programmable Gate Array (FPGA) controller that dynamically issues commands while receiving further commands from a processor, using a command prioritization system and waveform compression to reduce the number of commands and increase bandwidth, allowing for control of MRI components with relaxed timing requirements.
Integration with Computational Modeling Platforms
The integration of Nuclear Magnetic Resonance (NMR) spectroscopy with computational modeling platforms represents a significant advancement in the study of allosteric control mechanisms. This synergistic approach combines the experimental precision of NMR with the predictive power of computational models, creating a comprehensive framework for understanding protein dynamics and allosteric regulation at multiple scales.
Current computational platforms such as GROMACS, AMBER, and NAMD have developed specialized modules that can directly incorporate NMR-derived constraints into molecular dynamics simulations. These constraints, including chemical shifts, residual dipolar couplings, and relaxation parameters, serve as critical validation points for computational predictions while simultaneously enhancing the accuracy of simulated protein motions.
Machine learning algorithms have emerged as powerful tools for bridging NMR data with computational models. Neural networks trained on extensive NMR datasets can now predict protein structural changes in response to ligand binding, offering insights into allosteric pathways that would be difficult to discern through either method alone. Platforms like DeepMind's AlphaFold have begun incorporating NMR data to refine predictions of protein dynamics beyond static structures.
Real-time integration systems have been developed that allow for the continuous refinement of computational models as new NMR data becomes available. These systems employ Bayesian statistical frameworks to update model parameters, creating an iterative process that progressively improves the accuracy of allosteric mechanism predictions. Companies like Schrödinger have pioneered commercial solutions that implement this approach for drug discovery applications.
Cloud-based platforms now enable collaborative research by providing shared computational resources for NMR data analysis and modeling. These platforms facilitate the integration of diverse datasets from multiple experimental sources, creating more robust models of allosteric networks. The European Molecular Biology Laboratory's BioExcel Center of Excellence exemplifies this approach, offering integrated tools for biomolecular research that combine NMR data with computational simulations.
Visualization tools have evolved to represent both NMR experimental data and computational predictions simultaneously, allowing researchers to intuitively explore the relationship between local molecular motions and global allosteric effects. Software packages like VMD (Visual Molecular Dynamics) now feature specialized plugins for displaying NMR parameters alongside simulated conformational ensembles, creating a more intuitive understanding of dynamic processes.
The integration of time-resolved NMR techniques with computational kinetic models has enabled researchers to map the energy landscapes of allosteric transitions with unprecedented temporal resolution. This approach reveals intermediate states that are often invisible to static structural methods, providing crucial insights into the mechanisms of signal propagation through protein structures.
Current computational platforms such as GROMACS, AMBER, and NAMD have developed specialized modules that can directly incorporate NMR-derived constraints into molecular dynamics simulations. These constraints, including chemical shifts, residual dipolar couplings, and relaxation parameters, serve as critical validation points for computational predictions while simultaneously enhancing the accuracy of simulated protein motions.
Machine learning algorithms have emerged as powerful tools for bridging NMR data with computational models. Neural networks trained on extensive NMR datasets can now predict protein structural changes in response to ligand binding, offering insights into allosteric pathways that would be difficult to discern through either method alone. Platforms like DeepMind's AlphaFold have begun incorporating NMR data to refine predictions of protein dynamics beyond static structures.
Real-time integration systems have been developed that allow for the continuous refinement of computational models as new NMR data becomes available. These systems employ Bayesian statistical frameworks to update model parameters, creating an iterative process that progressively improves the accuracy of allosteric mechanism predictions. Companies like Schrödinger have pioneered commercial solutions that implement this approach for drug discovery applications.
Cloud-based platforms now enable collaborative research by providing shared computational resources for NMR data analysis and modeling. These platforms facilitate the integration of diverse datasets from multiple experimental sources, creating more robust models of allosteric networks. The European Molecular Biology Laboratory's BioExcel Center of Excellence exemplifies this approach, offering integrated tools for biomolecular research that combine NMR data with computational simulations.
Visualization tools have evolved to represent both NMR experimental data and computational predictions simultaneously, allowing researchers to intuitively explore the relationship between local molecular motions and global allosteric effects. Software packages like VMD (Visual Molecular Dynamics) now feature specialized plugins for displaying NMR parameters alongside simulated conformational ensembles, creating a more intuitive understanding of dynamic processes.
The integration of time-resolved NMR techniques with computational kinetic models has enabled researchers to map the energy landscapes of allosteric transitions with unprecedented temporal resolution. This approach reveals intermediate states that are often invisible to static structural methods, providing crucial insights into the mechanisms of signal propagation through protein structures.
Translational Potential for Drug Discovery Applications
Nuclear Magnetic Resonance (NMR) spectroscopy has emerged as a transformative technology in drug discovery, particularly through its ability to elucidate allosteric control mechanisms at the molecular level. The translational potential of NMR-based dynamic examinations for pharmaceutical development represents a significant opportunity for innovation in targeted therapeutics.
The application of NMR techniques to drug discovery workflows offers unprecedented insights into protein-ligand interactions that traditional screening methods cannot provide. By capturing the dynamic nature of allosteric regulation, researchers can design compounds that specifically modulate protein function through non-competitive binding sites, potentially reducing off-target effects and improving therapeutic indices.
Pharmaceutical companies are increasingly incorporating NMR-based approaches into their early-stage drug discovery pipelines. These methods enable the identification of novel binding pockets and allosteric modulators that might be overlooked by conventional structure-based drug design. The ability to observe conformational changes in real-time provides critical information for optimizing lead compounds and understanding structure-activity relationships.
Fragment-based drug discovery has particularly benefited from NMR techniques, as they allow detection of weak binding interactions that would be missed by other screening technologies. This capability has led to the successful development of several clinical candidates targeting previously "undruggable" proteins through allosteric mechanisms, expanding the pharmaceutical target space considerably.
Translational applications extend beyond small molecule discovery to biologics and protein therapeutics. NMR-derived insights into allosteric networks can guide the engineering of therapeutic antibodies and proteins with enhanced specificity and efficacy. This approach has shown promise in developing modulators for immune checkpoint proteins and other challenging therapeutic targets.
The integration of NMR data with computational methods represents another frontier with significant translational potential. Machine learning algorithms trained on NMR-derived conformational ensembles can predict allosteric binding sites and simulate drug-induced conformational changes, accelerating the hit-to-lead optimization process and reducing experimental costs.
Regulatory agencies have begun recognizing NMR data as valuable supporting evidence for drug approval submissions, particularly for demonstrating mechanism of action and target engagement. This regulatory acceptance further enhances the translational value of NMR-based allosteric studies in the pharmaceutical development pathway.
The application of NMR techniques to drug discovery workflows offers unprecedented insights into protein-ligand interactions that traditional screening methods cannot provide. By capturing the dynamic nature of allosteric regulation, researchers can design compounds that specifically modulate protein function through non-competitive binding sites, potentially reducing off-target effects and improving therapeutic indices.
Pharmaceutical companies are increasingly incorporating NMR-based approaches into their early-stage drug discovery pipelines. These methods enable the identification of novel binding pockets and allosteric modulators that might be overlooked by conventional structure-based drug design. The ability to observe conformational changes in real-time provides critical information for optimizing lead compounds and understanding structure-activity relationships.
Fragment-based drug discovery has particularly benefited from NMR techniques, as they allow detection of weak binding interactions that would be missed by other screening technologies. This capability has led to the successful development of several clinical candidates targeting previously "undruggable" proteins through allosteric mechanisms, expanding the pharmaceutical target space considerably.
Translational applications extend beyond small molecule discovery to biologics and protein therapeutics. NMR-derived insights into allosteric networks can guide the engineering of therapeutic antibodies and proteins with enhanced specificity and efficacy. This approach has shown promise in developing modulators for immune checkpoint proteins and other challenging therapeutic targets.
The integration of NMR data with computational methods represents another frontier with significant translational potential. Machine learning algorithms trained on NMR-derived conformational ensembles can predict allosteric binding sites and simulate drug-induced conformational changes, accelerating the hit-to-lead optimization process and reducing experimental costs.
Regulatory agencies have begun recognizing NMR data as valuable supporting evidence for drug approval submissions, particularly for demonstrating mechanism of action and target engagement. This regulatory acceptance further enhances the translational value of NMR-based allosteric studies in the pharmaceutical development pathway.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!