How Ethyl Acetate Improves Chemical Synthetic Routes?
JUN 27, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ethyl Acetate in Synthesis: Background and Objectives
Ethyl acetate has emerged as a crucial solvent and reagent in modern chemical synthesis, playing a pivotal role in improving synthetic routes across various industries. The evolution of ethyl acetate's application in synthesis can be traced back to the early 20th century, with its importance growing exponentially in recent decades due to its versatile properties and environmentally friendly nature.
The development of ethyl acetate as a key component in chemical synthesis has been driven by the increasing demand for more efficient, cost-effective, and sustainable production methods. Its unique combination of properties, including low toxicity, high solvency power, and moderate boiling point, has made it an attractive alternative to more hazardous solvents traditionally used in organic synthesis.
The trend towards greener chemistry has further accelerated the adoption of ethyl acetate in synthetic processes. As industries strive to reduce their environmental footprint, ethyl acetate's biodegradability and lower toxicity compared to many other organic solvents have positioned it as a preferred choice in numerous applications, from pharmaceutical manufacturing to agrochemical production.
In the context of improving chemical synthetic routes, ethyl acetate serves multiple functions. It acts as an excellent solvent for a wide range of organic compounds, facilitating reactions by providing a homogeneous medium. Additionally, its ability to form azeotropes with water makes it valuable in extraction and purification processes, often simplifying complex synthetic procedures.
The objectives of utilizing ethyl acetate in synthesis extend beyond mere solvent replacement. Researchers and industry professionals aim to leverage its unique properties to develop novel synthetic methodologies, enhance reaction yields, and improve product purity. There is a growing focus on exploring ethyl acetate's potential as a reactant in various transformations, particularly in esterification reactions and as a source of acetyl groups.
Furthermore, the integration of ethyl acetate into continuous flow chemistry and other advanced synthetic techniques represents a frontier in process intensification. These approaches seek to maximize efficiency, reduce waste, and enable more precise control over reaction conditions, aligning with the broader goals of sustainable chemical manufacturing.
As we delve deeper into the role of ethyl acetate in improving chemical synthetic routes, it becomes clear that its impact extends far beyond its traditional uses. The ongoing research and development in this field aim to unlock new applications, optimize existing processes, and contribute to the overall advancement of green chemistry principles in industrial and academic settings.
The development of ethyl acetate as a key component in chemical synthesis has been driven by the increasing demand for more efficient, cost-effective, and sustainable production methods. Its unique combination of properties, including low toxicity, high solvency power, and moderate boiling point, has made it an attractive alternative to more hazardous solvents traditionally used in organic synthesis.
The trend towards greener chemistry has further accelerated the adoption of ethyl acetate in synthetic processes. As industries strive to reduce their environmental footprint, ethyl acetate's biodegradability and lower toxicity compared to many other organic solvents have positioned it as a preferred choice in numerous applications, from pharmaceutical manufacturing to agrochemical production.
In the context of improving chemical synthetic routes, ethyl acetate serves multiple functions. It acts as an excellent solvent for a wide range of organic compounds, facilitating reactions by providing a homogeneous medium. Additionally, its ability to form azeotropes with water makes it valuable in extraction and purification processes, often simplifying complex synthetic procedures.
The objectives of utilizing ethyl acetate in synthesis extend beyond mere solvent replacement. Researchers and industry professionals aim to leverage its unique properties to develop novel synthetic methodologies, enhance reaction yields, and improve product purity. There is a growing focus on exploring ethyl acetate's potential as a reactant in various transformations, particularly in esterification reactions and as a source of acetyl groups.
Furthermore, the integration of ethyl acetate into continuous flow chemistry and other advanced synthetic techniques represents a frontier in process intensification. These approaches seek to maximize efficiency, reduce waste, and enable more precise control over reaction conditions, aligning with the broader goals of sustainable chemical manufacturing.
As we delve deeper into the role of ethyl acetate in improving chemical synthetic routes, it becomes clear that its impact extends far beyond its traditional uses. The ongoing research and development in this field aim to unlock new applications, optimize existing processes, and contribute to the overall advancement of green chemistry principles in industrial and academic settings.
Market Demand Analysis for Ethyl Acetate in Chemical Synthesis
The market demand for ethyl acetate in chemical synthesis has been steadily growing, driven by its versatile applications and advantageous properties. As a key solvent and reagent, ethyl acetate plays a crucial role in various chemical processes, particularly in the pharmaceutical, agrochemical, and fine chemical industries.
In the pharmaceutical sector, ethyl acetate is extensively used in the synthesis of active pharmaceutical ingredients (APIs) and intermediates. Its ability to improve reaction efficiency and yield makes it an indispensable component in many drug manufacturing processes. The global pharmaceutical market's continuous expansion, coupled with the increasing demand for novel drugs, has significantly boosted the consumption of ethyl acetate in this sector.
The agrochemical industry also contributes substantially to the market demand for ethyl acetate. Its use in the production of pesticides, herbicides, and other crop protection chemicals has grown in response to the need for increased agricultural productivity. As global food demand rises and arable land becomes scarcer, the agrochemical sector's reliance on efficient synthetic routes involving ethyl acetate is expected to intensify.
In the fine chemicals industry, ethyl acetate's role in improving synthetic routes has led to its increased adoption in the production of flavors, fragrances, and specialty chemicals. Its low toxicity and high solvency power make it an attractive choice for manufacturers seeking to optimize their processes and meet stringent environmental regulations.
The market demand for ethyl acetate is further bolstered by its eco-friendly profile compared to some alternative solvents. As sustainability becomes a key focus across industries, the preference for greener solvents like ethyl acetate is likely to grow, driving its demand in chemical synthesis applications.
Geographically, Asia-Pacific has emerged as the largest consumer of ethyl acetate in chemical synthesis, primarily due to the rapid industrialization and growth of the pharmaceutical and agrochemical sectors in countries like China and India. North America and Europe follow, with steady demand from their well-established chemical industries.
The market size for ethyl acetate in chemical synthesis is projected to expand further, with analysts predicting a compound annual growth rate (CAGR) of several percentage points over the next five years. This growth is attributed to the increasing adoption of ethyl acetate in various synthetic processes, as well as ongoing research and development efforts to explore new applications and improve existing ones.
However, the market demand is not without challenges. Fluctuations in raw material prices, particularly ethanol and acetic acid, can impact the production costs and, consequently, the market dynamics of ethyl acetate. Additionally, the development of alternative solvents and green chemistry initiatives may pose potential threats to the growth of ethyl acetate demand in certain applications.
In the pharmaceutical sector, ethyl acetate is extensively used in the synthesis of active pharmaceutical ingredients (APIs) and intermediates. Its ability to improve reaction efficiency and yield makes it an indispensable component in many drug manufacturing processes. The global pharmaceutical market's continuous expansion, coupled with the increasing demand for novel drugs, has significantly boosted the consumption of ethyl acetate in this sector.
The agrochemical industry also contributes substantially to the market demand for ethyl acetate. Its use in the production of pesticides, herbicides, and other crop protection chemicals has grown in response to the need for increased agricultural productivity. As global food demand rises and arable land becomes scarcer, the agrochemical sector's reliance on efficient synthetic routes involving ethyl acetate is expected to intensify.
In the fine chemicals industry, ethyl acetate's role in improving synthetic routes has led to its increased adoption in the production of flavors, fragrances, and specialty chemicals. Its low toxicity and high solvency power make it an attractive choice for manufacturers seeking to optimize their processes and meet stringent environmental regulations.
The market demand for ethyl acetate is further bolstered by its eco-friendly profile compared to some alternative solvents. As sustainability becomes a key focus across industries, the preference for greener solvents like ethyl acetate is likely to grow, driving its demand in chemical synthesis applications.
Geographically, Asia-Pacific has emerged as the largest consumer of ethyl acetate in chemical synthesis, primarily due to the rapid industrialization and growth of the pharmaceutical and agrochemical sectors in countries like China and India. North America and Europe follow, with steady demand from their well-established chemical industries.
The market size for ethyl acetate in chemical synthesis is projected to expand further, with analysts predicting a compound annual growth rate (CAGR) of several percentage points over the next five years. This growth is attributed to the increasing adoption of ethyl acetate in various synthetic processes, as well as ongoing research and development efforts to explore new applications and improve existing ones.
However, the market demand is not without challenges. Fluctuations in raw material prices, particularly ethanol and acetic acid, can impact the production costs and, consequently, the market dynamics of ethyl acetate. Additionally, the development of alternative solvents and green chemistry initiatives may pose potential threats to the growth of ethyl acetate demand in certain applications.
Current State and Challenges in Ethyl Acetate Utilization
Ethyl acetate has emerged as a versatile and valuable solvent in chemical synthetic routes, offering significant improvements in various aspects of organic synthesis. Currently, its utilization is widespread across pharmaceutical, agrochemical, and fine chemical industries. The compound's unique properties, including low toxicity, high solvency power, and moderate boiling point, make it an attractive choice for many synthetic processes.
In the pharmaceutical sector, ethyl acetate is extensively used in the synthesis of active pharmaceutical ingredients (APIs) and intermediates. Its ability to dissolve a wide range of organic compounds facilitates efficient extraction and purification processes. Moreover, its relatively low boiling point allows for easy removal from reaction mixtures, reducing the risk of thermal degradation of sensitive products.
The agrochemical industry also benefits significantly from ethyl acetate's properties. It serves as an excellent solvent for pesticide formulations and aids in the synthesis of various crop protection agents. Its low toxicity profile aligns well with the increasing demand for environmentally friendly agricultural solutions.
Despite its widespread use, the utilization of ethyl acetate in chemical synthetic routes faces several challenges. One primary concern is its flammability, which necessitates careful handling and storage procedures. This characteristic limits its use in certain high-temperature reactions and requires specialized equipment for large-scale operations.
Another challenge lies in the recovery and recycling of ethyl acetate. While its low boiling point facilitates easy removal, efficient recycling systems are crucial for maintaining cost-effectiveness and reducing environmental impact. Developing improved distillation and purification techniques remains an ongoing area of research.
The synthesis of ethyl acetate itself presents challenges in terms of sustainability. Traditional production methods often rely on petrochemical feedstocks, raising concerns about long-term viability and environmental impact. There is a growing interest in developing bio-based routes for ethyl acetate production, utilizing renewable resources such as ethanol derived from biomass.
Regulatory constraints also pose challenges to ethyl acetate utilization. Stringent environmental regulations in some regions limit the use of volatile organic compounds (VOCs), including ethyl acetate. This has spurred research into alternative solvents and process modifications to comply with evolving regulatory standards.
Despite these challenges, ongoing research continues to expand the potential applications of ethyl acetate in chemical synthesis. Innovations in reaction engineering, such as continuous flow chemistry and microreactor technology, are enhancing the efficiency and safety of ethyl acetate-based processes. Additionally, the development of novel catalytic systems is opening up new possibilities for selective transformations in ethyl acetate media.
In the pharmaceutical sector, ethyl acetate is extensively used in the synthesis of active pharmaceutical ingredients (APIs) and intermediates. Its ability to dissolve a wide range of organic compounds facilitates efficient extraction and purification processes. Moreover, its relatively low boiling point allows for easy removal from reaction mixtures, reducing the risk of thermal degradation of sensitive products.
The agrochemical industry also benefits significantly from ethyl acetate's properties. It serves as an excellent solvent for pesticide formulations and aids in the synthesis of various crop protection agents. Its low toxicity profile aligns well with the increasing demand for environmentally friendly agricultural solutions.
Despite its widespread use, the utilization of ethyl acetate in chemical synthetic routes faces several challenges. One primary concern is its flammability, which necessitates careful handling and storage procedures. This characteristic limits its use in certain high-temperature reactions and requires specialized equipment for large-scale operations.
Another challenge lies in the recovery and recycling of ethyl acetate. While its low boiling point facilitates easy removal, efficient recycling systems are crucial for maintaining cost-effectiveness and reducing environmental impact. Developing improved distillation and purification techniques remains an ongoing area of research.
The synthesis of ethyl acetate itself presents challenges in terms of sustainability. Traditional production methods often rely on petrochemical feedstocks, raising concerns about long-term viability and environmental impact. There is a growing interest in developing bio-based routes for ethyl acetate production, utilizing renewable resources such as ethanol derived from biomass.
Regulatory constraints also pose challenges to ethyl acetate utilization. Stringent environmental regulations in some regions limit the use of volatile organic compounds (VOCs), including ethyl acetate. This has spurred research into alternative solvents and process modifications to comply with evolving regulatory standards.
Despite these challenges, ongoing research continues to expand the potential applications of ethyl acetate in chemical synthesis. Innovations in reaction engineering, such as continuous flow chemistry and microreactor technology, are enhancing the efficiency and safety of ethyl acetate-based processes. Additionally, the development of novel catalytic systems is opening up new possibilities for selective transformations in ethyl acetate media.
Existing Synthetic Routes Utilizing Ethyl Acetate
01 Esterification of ethanol and acetic acid
One of the primary methods for synthesizing ethyl acetate is through the esterification reaction between ethanol and acetic acid. This process typically involves heating the reactants in the presence of an acid catalyst, such as sulfuric acid, to promote the formation of the ester. The reaction is reversible, so various techniques may be employed to shift the equilibrium towards the product.- Esterification of ethanol and acetic acid: One of the primary methods for synthesizing ethyl acetate is through the esterification reaction between ethanol and acetic acid. This process typically involves heating the reactants in the presence of an acid catalyst, such as sulfuric acid, to promote the formation of the ester bond. The reaction is reversible, so various techniques may be employed to shift the equilibrium towards the product.
- Catalytic dehydrogenation of ethanol: Another route for ethyl acetate synthesis involves the catalytic dehydrogenation of ethanol. This process typically uses metal catalysts, such as copper or noble metals, to remove hydrogen from ethanol molecules, resulting in the formation of ethyl acetate. The reaction often occurs at elevated temperatures and may require specific reactor designs to optimize yield and selectivity.
- Tishchenko reaction of acetaldehyde: The Tishchenko reaction can be used to produce ethyl acetate from acetaldehyde. This process involves the dimerization of acetaldehyde molecules in the presence of an aluminum alkoxide catalyst. The reaction results in the formation of ethyl acetate without the need for external oxidants or reductants, making it an atom-efficient synthetic route.
- Oxidative esterification of ethanol: Ethyl acetate can be synthesized through the oxidative esterification of ethanol. This process involves the partial oxidation of ethanol in the presence of oxygen or air, often using noble metal catalysts. The reaction can be carried out in both liquid and gas phases, with various reactor configurations and process conditions influencing the yield and selectivity of ethyl acetate formation.
- Continuous flow processes for ethyl acetate synthesis: Continuous flow processes have been developed for the efficient production of ethyl acetate. These methods often involve the use of specialized reactors, such as microreactors or fixed-bed reactors, which allow for better control of reaction conditions and improved heat and mass transfer. Continuous processes can offer advantages in terms of productivity, product quality, and energy efficiency compared to batch production methods.
02 Catalytic dehydrogenation of ethanol
Another route for ethyl acetate synthesis involves the catalytic dehydrogenation of ethanol. This process typically uses metal catalysts, such as copper or silver, to facilitate the reaction. The ethanol undergoes oxidation and dehydrogenation to form acetaldehyde, which then reacts with another ethanol molecule to produce ethyl acetate.Expand Specific Solutions03 Tishchenko reaction of acetaldehyde
The Tishchenko reaction can be used to produce ethyl acetate from acetaldehyde. In this process, two molecules of acetaldehyde undergo a disproportionation reaction in the presence of an aluminum alkoxide catalyst. This reaction results in the formation of ethyl acetate without the need for external oxidants or reductants.Expand Specific Solutions04 Continuous flow processes for ethyl acetate production
Continuous flow processes have been developed for the efficient production of ethyl acetate. These methods often involve the use of fixed-bed reactors or microreactors, allowing for better control of reaction conditions and improved yields. Continuous processes can be applied to various synthetic routes, including esterification and dehydrogenation reactions.Expand Specific Solutions05 Green chemistry approaches for ethyl acetate synthesis
Recent research has focused on developing more environmentally friendly methods for ethyl acetate production. These approaches may include the use of biocatalysts, such as enzymes, or the utilization of renewable feedstocks. Green chemistry techniques aim to reduce waste, improve energy efficiency, and minimize the use of harmful chemicals in the synthesis process.Expand Specific Solutions
Key Players in Ethyl Acetate Production and Application
The ethyl acetate market is in a mature growth stage, with a global market size estimated at over $3 billion. The technology for improving chemical synthetic routes using ethyl acetate is well-established but still evolving. Major players like Eastman Chemical, SABIC, and Celanese have advanced capabilities in this area. Universities such as Zhejiang University and South China University of Technology are conducting cutting-edge research to further optimize processes. Emerging companies like Genomatica and ZuvaSyntha are developing novel bio-based routes for ethyl acetate production. The competitive landscape is characterized by a mix of large chemical corporations, specialized manufacturers, and innovative startups exploring greener synthesis methods.
SABIC Global Technologies BV
Technical Solution: SABIC has developed a novel approach to ethyl acetate production that improves chemical synthetic routes. Their method involves the direct esterification of ethanol with acetic acid using advanced heterogeneous catalysts[4]. This process eliminates the need for intermediate steps, reducing overall energy consumption and improving atom economy. SABIC has also implemented a continuous flow reactor system, which allows for better control of reaction conditions and increased product consistency[5]. Furthermore, the company has invested in research on membrane separation technologies to enhance the purification of ethyl acetate, reducing the reliance on traditional distillation methods[6].
Strengths: Simplified production process, improved energy efficiency, potential for continuous production. Weaknesses: Possible limitations in catalyst lifetime, challenges in scaling up new separation technologies.
Eastman Chemical Co.
Technical Solution: Eastman Chemical Co. has developed innovative processes for ethyl acetate production, focusing on improving chemical synthetic routes. Their approach involves a two-step process: first, the carbonylation of methanol to produce acetic acid, followed by the esterification of acetic acid with ethanol to form ethyl acetate[1]. This method utilizes a rhodium-based catalyst system, which allows for high selectivity and yield. The company has also implemented advanced separation techniques, such as reactive distillation, to enhance the efficiency of the ethyl acetate purification process[2]. Additionally, Eastman has explored the use of bio-based feedstocks, such as cellulosic biomass, to produce ethyl acetate, aligning with sustainability goals[3].
Strengths: High selectivity and yield, improved process efficiency, potential for bio-based production. Weaknesses: Reliance on precious metal catalysts, potential high energy requirements for separation processes.
Innovative Applications of Ethyl Acetate in Synthesis
Process improvement for continuous ethyl acetate production
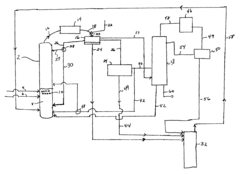
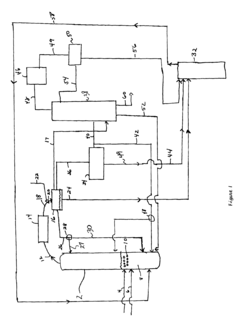
PatentInactiveUS6768021B2
Innovation
- The process involves using a membrane separation unit to remove water from the condensed reaction stream, recycling the dried stream back into the production process, and employing an additional distillation zone to produce purified ethyl acetate with minimal acid content, thereby optimizing water management and increasing process capacity.
Enzymatic method for preparing glyceryl butyrate
PatentActiveUS20190276861A1
Innovation
- An enzymatic method using lipase as a catalyst with ethyl acetate or ethyl formate as additives for esterification between n-butyric acid and glycerol at normal temperature and pressure without a water-carrying agent, optimizing the reaction conditions to enhance catalytic efficiency and conversion rates.
Green Chemistry Aspects of Ethyl Acetate Use
Ethyl acetate plays a significant role in advancing green chemistry principles within chemical synthetic routes. As a relatively low-toxicity solvent with favorable environmental characteristics, ethyl acetate aligns well with the goals of sustainable chemistry practices. Its use contributes to reducing the overall environmental impact of chemical processes while maintaining or improving reaction efficiency.
One of the key green chemistry aspects of ethyl acetate is its biodegradability. Unlike many traditional organic solvents, ethyl acetate readily breaks down in the environment, reducing long-term ecological concerns. This property makes it an attractive option for industries seeking to minimize their environmental footprint and comply with increasingly stringent regulations on chemical waste.
Furthermore, ethyl acetate can be produced from renewable resources, such as ethanol derived from biomass fermentation. This renewable sourcing aligns with the principles of green chemistry by reducing dependence on fossil fuel-based raw materials. The production of ethyl acetate from bio-ethanol represents a more sustainable approach to solvent manufacturing, contributing to the circular economy concept in the chemical industry.
In terms of reaction conditions, ethyl acetate often allows for milder processing parameters compared to more aggressive solvents. This can lead to energy savings in chemical syntheses, as reactions may be carried out at lower temperatures or pressures. Additionally, the lower boiling point of ethyl acetate compared to water facilitates easier solvent recovery and recycling, further enhancing the sustainability of chemical processes.
Ethyl acetate's versatility as a solvent extends to its ability to replace more hazardous alternatives in various synthetic routes. For instance, it can substitute for chlorinated solvents or other volatile organic compounds (VOCs) that pose greater risks to human health and the environment. This substitution not only improves worker safety but also reduces the potential for harmful emissions and the need for extensive pollution control measures.
In the context of green metrics, the use of ethyl acetate can contribute to improved atom economy and reduced E-factor (Environmental factor) in chemical reactions. Its efficient solvation properties often allow for higher yields and selectivities, minimizing waste generation. Moreover, the relatively easy separation of ethyl acetate from reaction mixtures can lead to more efficient purification processes, further reducing the overall environmental impact of chemical manufacturing.
As industries strive to adopt more sustainable practices, the integration of ethyl acetate into chemical synthetic routes represents a tangible step towards greener chemistry. Its favorable properties make it an exemplar of how solvent choice can significantly influence the environmental profile of chemical processes, aligning with the broader goals of sustainability in the chemical industry.
One of the key green chemistry aspects of ethyl acetate is its biodegradability. Unlike many traditional organic solvents, ethyl acetate readily breaks down in the environment, reducing long-term ecological concerns. This property makes it an attractive option for industries seeking to minimize their environmental footprint and comply with increasingly stringent regulations on chemical waste.
Furthermore, ethyl acetate can be produced from renewable resources, such as ethanol derived from biomass fermentation. This renewable sourcing aligns with the principles of green chemistry by reducing dependence on fossil fuel-based raw materials. The production of ethyl acetate from bio-ethanol represents a more sustainable approach to solvent manufacturing, contributing to the circular economy concept in the chemical industry.
In terms of reaction conditions, ethyl acetate often allows for milder processing parameters compared to more aggressive solvents. This can lead to energy savings in chemical syntheses, as reactions may be carried out at lower temperatures or pressures. Additionally, the lower boiling point of ethyl acetate compared to water facilitates easier solvent recovery and recycling, further enhancing the sustainability of chemical processes.
Ethyl acetate's versatility as a solvent extends to its ability to replace more hazardous alternatives in various synthetic routes. For instance, it can substitute for chlorinated solvents or other volatile organic compounds (VOCs) that pose greater risks to human health and the environment. This substitution not only improves worker safety but also reduces the potential for harmful emissions and the need for extensive pollution control measures.
In the context of green metrics, the use of ethyl acetate can contribute to improved atom economy and reduced E-factor (Environmental factor) in chemical reactions. Its efficient solvation properties often allow for higher yields and selectivities, minimizing waste generation. Moreover, the relatively easy separation of ethyl acetate from reaction mixtures can lead to more efficient purification processes, further reducing the overall environmental impact of chemical manufacturing.
As industries strive to adopt more sustainable practices, the integration of ethyl acetate into chemical synthetic routes represents a tangible step towards greener chemistry. Its favorable properties make it an exemplar of how solvent choice can significantly influence the environmental profile of chemical processes, aligning with the broader goals of sustainability in the chemical industry.
Economic Impact of Ethyl Acetate in Chemical Industry
The economic impact of ethyl acetate in the chemical industry is substantial and multifaceted. As a versatile solvent and reagent, ethyl acetate plays a crucial role in various chemical processes, significantly influencing production costs, efficiency, and overall industry dynamics.
In terms of cost-effectiveness, ethyl acetate offers a competitive advantage over many alternative solvents. Its relatively low production cost and high availability make it an attractive option for manufacturers across different sectors of the chemical industry. This cost-efficiency translates into reduced production expenses for a wide range of products, from pharmaceuticals to paints and coatings, ultimately contributing to improved profit margins for companies.
The use of ethyl acetate in chemical synthetic routes has led to enhanced process efficiency in many applications. Its excellent solvency properties and low boiling point facilitate faster reaction times and easier product isolation, thereby increasing production throughput. This improved efficiency not only reduces operational costs but also allows companies to meet market demands more effectively, potentially increasing their market share and revenue.
Environmental regulations have become increasingly stringent in recent years, and ethyl acetate's relatively low toxicity and biodegradability have positioned it as a preferred choice in many industrial processes. This environmental compatibility reduces the costs associated with waste management and regulatory compliance, providing both economic and reputational benefits to companies that utilize it.
The widespread adoption of ethyl acetate has also stimulated growth in its production sector. Increased demand has led to investments in production facilities and technologies, creating jobs and contributing to economic growth in regions where ethyl acetate is manufactured. This ripple effect extends to related industries, such as transportation and packaging, further amplifying its economic impact.
In the realm of innovation, ethyl acetate's versatility has spurred research and development efforts to explore new applications and improve existing processes. This ongoing innovation drives technological advancements in the chemical industry, potentially leading to the creation of new products and markets, and thus fostering economic growth and competitiveness.
The global trade of ethyl acetate also contributes significantly to the chemical industry's economic landscape. As a widely used commodity, it is subject to international market dynamics, influencing trade balances and creating economic opportunities for both producing and consuming countries.
In terms of cost-effectiveness, ethyl acetate offers a competitive advantage over many alternative solvents. Its relatively low production cost and high availability make it an attractive option for manufacturers across different sectors of the chemical industry. This cost-efficiency translates into reduced production expenses for a wide range of products, from pharmaceuticals to paints and coatings, ultimately contributing to improved profit margins for companies.
The use of ethyl acetate in chemical synthetic routes has led to enhanced process efficiency in many applications. Its excellent solvency properties and low boiling point facilitate faster reaction times and easier product isolation, thereby increasing production throughput. This improved efficiency not only reduces operational costs but also allows companies to meet market demands more effectively, potentially increasing their market share and revenue.
Environmental regulations have become increasingly stringent in recent years, and ethyl acetate's relatively low toxicity and biodegradability have positioned it as a preferred choice in many industrial processes. This environmental compatibility reduces the costs associated with waste management and regulatory compliance, providing both economic and reputational benefits to companies that utilize it.
The widespread adoption of ethyl acetate has also stimulated growth in its production sector. Increased demand has led to investments in production facilities and technologies, creating jobs and contributing to economic growth in regions where ethyl acetate is manufactured. This ripple effect extends to related industries, such as transportation and packaging, further amplifying its economic impact.
In the realm of innovation, ethyl acetate's versatility has spurred research and development efforts to explore new applications and improve existing processes. This ongoing innovation drives technological advancements in the chemical industry, potentially leading to the creation of new products and markets, and thus fostering economic growth and competitiveness.
The global trade of ethyl acetate also contributes significantly to the chemical industry's economic landscape. As a widely used commodity, it is subject to international market dynamics, influencing trade balances and creating economic opportunities for both producing and consuming countries.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!