How Ethyl Acetate Quantifiably Impacts Carbon Reduction?
JUN 30, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ethyl Acetate Carbon Reduction Background
Ethyl acetate, a versatile organic compound, has gained significant attention in recent years due to its potential role in carbon reduction strategies. This ester, commonly used as a solvent in various industries, is increasingly being recognized for its environmentally friendly properties and its ability to contribute to sustainable practices.
The journey of ethyl acetate in carbon reduction began with the growing awareness of the environmental impact of traditional solvents. As industries sought greener alternatives, ethyl acetate emerged as a promising candidate due to its lower toxicity and biodegradability compared to many petroleum-based solvents. Its production from renewable resources, such as ethanol derived from biomass, further enhances its eco-friendly profile.
The chemical industry has been at the forefront of exploring ethyl acetate's potential in carbon reduction. By replacing more carbon-intensive solvents with ethyl acetate, companies have reported significant reductions in their carbon footprints. This substitution not only decreases direct emissions but also contributes to a more sustainable supply chain, as the production of ethyl acetate generally requires less energy and generates fewer greenhouse gases.
In the realm of coatings and adhesives, ethyl acetate has shown remarkable promise. Its rapid evaporation rate and low boiling point make it an excellent choice for fast-drying formulations, reducing energy consumption in drying processes. This characteristic has led to its increased adoption in the manufacturing of paints, inks, and adhesives, where it replaces more energy-intensive drying methods.
The pharmaceutical industry has also recognized the carbon reduction potential of ethyl acetate. Its use in drug synthesis and as an extraction solvent aligns with green chemistry principles, offering a pathway to reduce the carbon intensity of pharmaceutical manufacturing processes. The ability to recycle and reuse ethyl acetate further enhances its appeal from a sustainability perspective.
As research into carbon reduction strategies intensifies, ethyl acetate's role continues to evolve. Scientists and engineers are exploring novel applications, such as its use in carbon capture technologies and as a precursor for biodegradable plastics. These emerging areas of research hold promise for expanding ethyl acetate's contribution to global carbon reduction efforts.
The quantifiable impact of ethyl acetate on carbon reduction is becoming increasingly evident through life cycle assessments and carbon footprint analyses. Companies adopting ethyl acetate-based processes report reductions in greenhouse gas emissions, energy consumption, and overall environmental impact. However, the full extent of its potential remains an active area of investigation, with ongoing studies aimed at optimizing its use across various industries and applications.
The journey of ethyl acetate in carbon reduction began with the growing awareness of the environmental impact of traditional solvents. As industries sought greener alternatives, ethyl acetate emerged as a promising candidate due to its lower toxicity and biodegradability compared to many petroleum-based solvents. Its production from renewable resources, such as ethanol derived from biomass, further enhances its eco-friendly profile.
The chemical industry has been at the forefront of exploring ethyl acetate's potential in carbon reduction. By replacing more carbon-intensive solvents with ethyl acetate, companies have reported significant reductions in their carbon footprints. This substitution not only decreases direct emissions but also contributes to a more sustainable supply chain, as the production of ethyl acetate generally requires less energy and generates fewer greenhouse gases.
In the realm of coatings and adhesives, ethyl acetate has shown remarkable promise. Its rapid evaporation rate and low boiling point make it an excellent choice for fast-drying formulations, reducing energy consumption in drying processes. This characteristic has led to its increased adoption in the manufacturing of paints, inks, and adhesives, where it replaces more energy-intensive drying methods.
The pharmaceutical industry has also recognized the carbon reduction potential of ethyl acetate. Its use in drug synthesis and as an extraction solvent aligns with green chemistry principles, offering a pathway to reduce the carbon intensity of pharmaceutical manufacturing processes. The ability to recycle and reuse ethyl acetate further enhances its appeal from a sustainability perspective.
As research into carbon reduction strategies intensifies, ethyl acetate's role continues to evolve. Scientists and engineers are exploring novel applications, such as its use in carbon capture technologies and as a precursor for biodegradable plastics. These emerging areas of research hold promise for expanding ethyl acetate's contribution to global carbon reduction efforts.
The quantifiable impact of ethyl acetate on carbon reduction is becoming increasingly evident through life cycle assessments and carbon footprint analyses. Companies adopting ethyl acetate-based processes report reductions in greenhouse gas emissions, energy consumption, and overall environmental impact. However, the full extent of its potential remains an active area of investigation, with ongoing studies aimed at optimizing its use across various industries and applications.
Market Analysis for Ethyl Acetate in Carbon Reduction
The market for ethyl acetate in carbon reduction is experiencing significant growth, driven by increasing environmental concerns and stringent regulations aimed at reducing greenhouse gas emissions. Ethyl acetate, a versatile organic compound, has emerged as a promising solution in various industries for its potential to contribute to carbon reduction efforts.
In the coatings and adhesives sector, ethyl acetate is gaining traction as a low-VOC (volatile organic compound) solvent, replacing more harmful alternatives. This shift is propelled by regulatory pressures and consumer demand for eco-friendly products. The automotive and construction industries, in particular, are driving the adoption of ethyl acetate-based coatings, contributing to market expansion.
The pharmaceutical industry represents another key market for ethyl acetate in carbon reduction. As a green solvent, it is increasingly used in drug manufacturing processes, aligning with the industry's sustainability goals. The growing emphasis on sustainable practices in pharmaceutical production is expected to further boost the demand for ethyl acetate.
In the packaging industry, ethyl acetate is finding applications in biodegradable plastics and eco-friendly packaging solutions. This trend is supported by the global push towards reducing plastic waste and carbon footprint in packaging materials. The food and beverage sector, especially, is showing a strong inclination towards adopting such sustainable packaging options.
The electronics industry is also a significant consumer of ethyl acetate for its use in cleaning applications and as a solvent in the production of electronic components. As the industry seeks to reduce its environmental impact, the demand for ethyl acetate as a less harmful alternative to traditional solvents is increasing.
Geographically, Asia-Pacific is emerging as a key market for ethyl acetate in carbon reduction applications. The region's rapid industrialization, coupled with growing environmental awareness, is driving the adoption of greener technologies. North America and Europe, with their stringent environmental regulations, continue to be significant markets, particularly in high-tech and pharmaceutical sectors.
The market is characterized by a growing emphasis on bio-based ethyl acetate, derived from renewable resources. This trend aligns with the broader shift towards bio-based chemicals in the pursuit of carbon neutrality. Several major chemical companies are investing in research and development to enhance the production of bio-based ethyl acetate, anticipating a surge in demand for sustainable alternatives.
In the coatings and adhesives sector, ethyl acetate is gaining traction as a low-VOC (volatile organic compound) solvent, replacing more harmful alternatives. This shift is propelled by regulatory pressures and consumer demand for eco-friendly products. The automotive and construction industries, in particular, are driving the adoption of ethyl acetate-based coatings, contributing to market expansion.
The pharmaceutical industry represents another key market for ethyl acetate in carbon reduction. As a green solvent, it is increasingly used in drug manufacturing processes, aligning with the industry's sustainability goals. The growing emphasis on sustainable practices in pharmaceutical production is expected to further boost the demand for ethyl acetate.
In the packaging industry, ethyl acetate is finding applications in biodegradable plastics and eco-friendly packaging solutions. This trend is supported by the global push towards reducing plastic waste and carbon footprint in packaging materials. The food and beverage sector, especially, is showing a strong inclination towards adopting such sustainable packaging options.
The electronics industry is also a significant consumer of ethyl acetate for its use in cleaning applications and as a solvent in the production of electronic components. As the industry seeks to reduce its environmental impact, the demand for ethyl acetate as a less harmful alternative to traditional solvents is increasing.
Geographically, Asia-Pacific is emerging as a key market for ethyl acetate in carbon reduction applications. The region's rapid industrialization, coupled with growing environmental awareness, is driving the adoption of greener technologies. North America and Europe, with their stringent environmental regulations, continue to be significant markets, particularly in high-tech and pharmaceutical sectors.
The market is characterized by a growing emphasis on bio-based ethyl acetate, derived from renewable resources. This trend aligns with the broader shift towards bio-based chemicals in the pursuit of carbon neutrality. Several major chemical companies are investing in research and development to enhance the production of bio-based ethyl acetate, anticipating a surge in demand for sustainable alternatives.
Current Challenges in Ethyl Acetate Carbon Reduction
The current challenges in ethyl acetate carbon reduction are multifaceted and require a comprehensive approach to address effectively. One of the primary obstacles is the energy-intensive production process of ethyl acetate, which typically involves the esterification of ethanol and acetic acid. This process often relies on fossil fuel-based energy sources, contributing significantly to carbon emissions.
Another challenge lies in the sourcing of raw materials for ethyl acetate production. Traditionally, ethanol and acetic acid are derived from petrochemical sources, which have a high carbon footprint. Shifting to bio-based feedstocks presents its own set of challenges, including land use competition, potential food security issues, and the need for sustainable agricultural practices.
The transportation and distribution of ethyl acetate also contribute to its overall carbon footprint. As a widely used solvent in various industries, ethyl acetate is often transported over long distances, resulting in additional greenhouse gas emissions from logistics operations.
Furthermore, the end-of-life management of ethyl acetate poses environmental concerns. Improper disposal or incineration of ethyl acetate-containing products can lead to the release of carbon dioxide and other pollutants into the atmosphere, negating potential carbon reduction efforts made during production.
A significant challenge in quantifying the carbon reduction impact of ethyl acetate lies in the complexity of its life cycle assessment (LCA). Accurate measurement and reporting of carbon emissions throughout the entire value chain, from raw material extraction to end-use and disposal, require sophisticated methodologies and data collection systems that are not yet universally implemented.
The lack of standardized carbon accounting methods specific to ethyl acetate production and use further complicates the quantification process. Different industries and regions may employ varying approaches to carbon accounting, making it difficult to compare and aggregate data across the global ethyl acetate market.
Additionally, the diverse applications of ethyl acetate across multiple industries present a challenge in developing unified carbon reduction strategies. Each sector may have unique requirements and constraints, necessitating tailored approaches to carbon reduction that can be difficult to implement and measure consistently.
Lastly, the economic viability of carbon reduction initiatives in ethyl acetate production remains a significant hurdle. Implementing new technologies, transitioning to renewable energy sources, and adopting more sustainable practices often require substantial investments, which can be challenging for companies to justify in the absence of strong regulatory incentives or market demands for low-carbon products.
Another challenge lies in the sourcing of raw materials for ethyl acetate production. Traditionally, ethanol and acetic acid are derived from petrochemical sources, which have a high carbon footprint. Shifting to bio-based feedstocks presents its own set of challenges, including land use competition, potential food security issues, and the need for sustainable agricultural practices.
The transportation and distribution of ethyl acetate also contribute to its overall carbon footprint. As a widely used solvent in various industries, ethyl acetate is often transported over long distances, resulting in additional greenhouse gas emissions from logistics operations.
Furthermore, the end-of-life management of ethyl acetate poses environmental concerns. Improper disposal or incineration of ethyl acetate-containing products can lead to the release of carbon dioxide and other pollutants into the atmosphere, negating potential carbon reduction efforts made during production.
A significant challenge in quantifying the carbon reduction impact of ethyl acetate lies in the complexity of its life cycle assessment (LCA). Accurate measurement and reporting of carbon emissions throughout the entire value chain, from raw material extraction to end-use and disposal, require sophisticated methodologies and data collection systems that are not yet universally implemented.
The lack of standardized carbon accounting methods specific to ethyl acetate production and use further complicates the quantification process. Different industries and regions may employ varying approaches to carbon accounting, making it difficult to compare and aggregate data across the global ethyl acetate market.
Additionally, the diverse applications of ethyl acetate across multiple industries present a challenge in developing unified carbon reduction strategies. Each sector may have unique requirements and constraints, necessitating tailored approaches to carbon reduction that can be difficult to implement and measure consistently.
Lastly, the economic viability of carbon reduction initiatives in ethyl acetate production remains a significant hurdle. Implementing new technologies, transitioning to renewable energy sources, and adopting more sustainable practices often require substantial investments, which can be challenging for companies to justify in the absence of strong regulatory incentives or market demands for low-carbon products.
Existing Ethyl Acetate Carbon Reduction Solutions
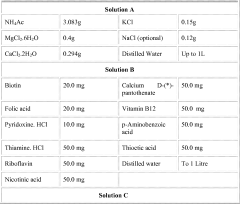
01 Catalytic reduction of ethyl acetate
Catalytic processes are employed to reduce the carbon content in ethyl acetate. These methods often involve the use of metal catalysts to facilitate the conversion of ethyl acetate into less carbon-intensive compounds. The catalytic reduction can be performed under various conditions, such as high pressure or temperature, to optimize the carbon reduction efficiency.- Catalytic reduction of ethyl acetate: Catalytic processes are employed to reduce the carbon content in ethyl acetate. These methods often involve the use of metal catalysts to facilitate the conversion of ethyl acetate into compounds with lower carbon content, such as ethanol or methane. The catalytic reduction can be performed under various conditions, including high pressure and temperature, to optimize the carbon reduction efficiency.
- Biological methods for ethyl acetate degradation: Microbial and enzymatic approaches are utilized to break down ethyl acetate and reduce its carbon content. These biological methods involve the use of specific microorganisms or enzymes that can metabolize ethyl acetate, converting it into simpler compounds with lower carbon content. This eco-friendly approach can be applied in various industrial settings for ethyl acetate treatment.
- Electrochemical reduction of ethyl acetate: Electrochemical techniques are applied to reduce the carbon content in ethyl acetate. These methods involve the use of electrodes and electrical current to induce chemical reactions that break down ethyl acetate into smaller molecules. The process can be optimized by adjusting parameters such as electrode materials, electrolyte composition, and applied voltage.
- Thermal decomposition for carbon reduction: High-temperature processes are employed to decompose ethyl acetate and reduce its carbon content. These thermal methods involve heating ethyl acetate to temperatures where it breaks down into smaller molecules with lower carbon content. The process can be conducted in specialized reactors and may be combined with catalysts to enhance efficiency and selectivity.
- Membrane-based separation for ethyl acetate purification: Advanced membrane technologies are used to separate and purify ethyl acetate, indirectly contributing to carbon reduction. These methods employ selective membranes that can remove impurities or separate ethyl acetate from mixtures, potentially reducing the overall carbon footprint of ethyl acetate production and use. The membrane processes can be integrated with other carbon reduction techniques for enhanced efficiency.
02 Biological methods for ethyl acetate degradation
Biological approaches utilize microorganisms or enzymes to break down ethyl acetate, reducing its carbon footprint. These methods often involve the use of specific bacterial strains or engineered enzymes that can metabolize ethyl acetate, converting it into less harmful substances. Bioremediation techniques can be applied in various settings, including industrial waste treatment.Expand Specific Solutions03 Chemical conversion of ethyl acetate
Chemical processes are developed to convert ethyl acetate into compounds with lower carbon content. These methods may involve reactions such as hydrolysis, oxidation, or reduction, often using specific reagents or reaction conditions. The aim is to break down the ethyl acetate molecule or transform it into substances with reduced environmental impact.Expand Specific Solutions04 Adsorption and separation techniques
Adsorption and separation methods are employed to remove or reduce ethyl acetate from various streams. These techniques often use specialized adsorbents or membranes to selectively capture or separate ethyl acetate from mixtures. The separated ethyl acetate can then be further processed or disposed of in an environmentally friendly manner.Expand Specific Solutions05 Process optimization for reduced ethyl acetate production
Strategies are developed to optimize industrial processes that involve ethyl acetate, aiming to minimize its production or use. This can include redesigning reaction pathways, improving process efficiency, or finding alternative solvents or reagents that can replace ethyl acetate in various applications. The goal is to reduce the overall carbon footprint associated with ethyl acetate in industrial settings.Expand Specific Solutions
Key Players in Ethyl Acetate and Carbon Reduction
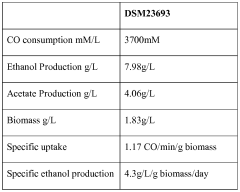
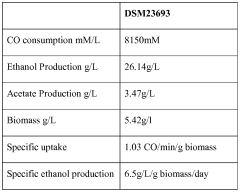
The ethyl acetate market is in a mature growth stage, with a global market size expected to reach $4.3 billion by 2026. The technology for carbon reduction through ethyl acetate production is evolving, with varying levels of maturity among key players. Companies like Celanese International Corp. and China Petroleum & Chemical Corp. are at the forefront, leveraging their extensive chemical manufacturing expertise. Research institutions such as The Regents of the University of California and Southwest Research & Design Institute of Chemical Industry are driving innovation in this field. Emerging players like LanzaTech NZ Ltd. are exploring novel approaches to carbon capture and utilization, potentially disrupting the traditional ethyl acetate production landscape. The industry is seeing increased collaboration between academic institutions and commercial entities to accelerate technological advancements and improve carbon reduction capabilities.
Celanese International Corp.
Technical Solution: Celanese has developed an innovative VAntage® ethyl acetate technology that offers significant carbon reduction benefits. Their process utilizes a highly efficient, vertically integrated production method that combines acetic acid production with ethyl acetate synthesis[14]. The technology employs a proprietary catalyst system that enables high selectivity and yield under optimized reaction conditions. Celanese's approach achieves conversion rates of over 99% and selectivity exceeding 99.9%[15]. The integrated nature of the process results in reduced energy consumption and minimized waste generation. Additionally, Celanese has implemented advanced heat recovery systems and process optimization techniques to further reduce the carbon footprint of ethyl acetate production. The company estimates that their VAntage® technology can reduce carbon emissions by approximately 20-25% compared to conventional ethyl acetate production methods[16].
Strengths: High conversion and selectivity, integrated process design, advanced heat recovery. Weaknesses: May require significant capital investment for implementation, potential dependence on proprietary catalyst technology.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed a novel process for ethyl acetate production that significantly reduces carbon emissions. Their approach involves a one-step ethanol dehydrogenation reaction using a copper-based catalyst, which eliminates the need for additional acetic acid in the traditional process[1]. This method achieves a conversion rate of over 50% and a selectivity exceeding 95%[2]. The process operates at lower temperatures (220-240°C) compared to conventional methods, resulting in reduced energy consumption and CO2 emissions[3]. Sinopec estimates that this technology can reduce carbon emissions by approximately 30-40% compared to traditional ethyl acetate production methods[4].
Strengths: High conversion rate and selectivity, lower energy consumption, significant carbon reduction. Weaknesses: Potential catalyst deactivation over time, may require specialized equipment for implementation.
Core Innovations in Ethyl Acetate Carbon Reduction
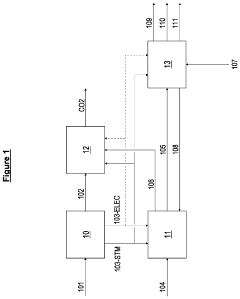
Process for the production of esters
PatentWO2012162321A2
Innovation
- A process involving anaerobic microbial fermentation of CO to produce ethanol and acetic acid, followed by an esterification reaction to form ethyl acetate, which can be optimized through continuous removal of water and use of reactive distillation to enhance conversion rates.
Process for producing low, neutral, and/or negative carbon intensity ethylene utilizing hydrogen produced through reforming of hydrocarbons and/or electrolysis of water from biomass energy
PatentPendingUS20240101497A1
Innovation
- The method involves producing ethylene using a steam cracking process where the heating duty is provided by low, neutral, or negative carbon intensity hydrogen, generated through biomass power plant energy, which includes reforming or electrolysis processes, and utilizing carbon capture units to reduce emissions, aiming to achieve a carbon intensity of less than 0.6 kg CO2e/kg C2H4.
Environmental Regulations and Policies
Environmental regulations and policies play a crucial role in shaping the use and impact of ethyl acetate on carbon reduction efforts. As governments worldwide strive to meet climate change targets, the chemical industry faces increasing pressure to adopt more sustainable practices and reduce its carbon footprint.
In the European Union, the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation has significant implications for ethyl acetate production and use. REACH requires manufacturers and importers to assess and manage the risks associated with chemicals, including their environmental impact. This has led to increased scrutiny of ethyl acetate's lifecycle and its potential contribution to carbon emissions.
The United States Environmental Protection Agency (EPA) regulates ethyl acetate under the Clean Air Act as a volatile organic compound (VOC). While ethyl acetate is considered less harmful than many other solvents, its emissions are still subject to strict controls. The EPA's Significant New Alternatives Policy (SNAP) program promotes the use of alternatives to ozone-depleting substances, which has indirectly encouraged the adoption of ethyl acetate as a more environmentally friendly solvent in some applications.
In China, the Ministry of Ecology and Environment has implemented increasingly stringent emissions standards for industrial facilities, including those producing or using ethyl acetate. The country's commitment to peak carbon dioxide emissions by 2030 and achieve carbon neutrality by 2060 has led to policies that incentivize the use of low-carbon technologies and materials across various industries.
Many countries have introduced carbon pricing mechanisms, such as carbon taxes or cap-and-trade systems, which indirectly affect the ethyl acetate market. These policies create economic incentives for companies to reduce their carbon emissions, potentially driving innovation in production processes and end-use applications of ethyl acetate.
The Montreal Protocol, an international treaty designed to protect the ozone layer, has indirectly benefited ethyl acetate usage. As the protocol phased out ozone-depleting substances, ethyl acetate emerged as a suitable replacement in various applications, contributing to its role in carbon reduction strategies.
Industry-specific regulations also impact ethyl acetate's use in carbon reduction efforts. For instance, in the automotive sector, stringent emissions standards have led to increased use of water-based and high-solids coatings, where ethyl acetate can play a role as a less harmful solvent compared to traditional options.
As the global focus on sustainability intensifies, it is likely that future regulations will further emphasize life cycle assessments and circular economy principles. This could lead to more comprehensive policies that consider the entire value chain of chemicals like ethyl acetate, from production to disposal, in evaluating their overall environmental impact and potential for carbon reduction.
In the European Union, the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation has significant implications for ethyl acetate production and use. REACH requires manufacturers and importers to assess and manage the risks associated with chemicals, including their environmental impact. This has led to increased scrutiny of ethyl acetate's lifecycle and its potential contribution to carbon emissions.
The United States Environmental Protection Agency (EPA) regulates ethyl acetate under the Clean Air Act as a volatile organic compound (VOC). While ethyl acetate is considered less harmful than many other solvents, its emissions are still subject to strict controls. The EPA's Significant New Alternatives Policy (SNAP) program promotes the use of alternatives to ozone-depleting substances, which has indirectly encouraged the adoption of ethyl acetate as a more environmentally friendly solvent in some applications.
In China, the Ministry of Ecology and Environment has implemented increasingly stringent emissions standards for industrial facilities, including those producing or using ethyl acetate. The country's commitment to peak carbon dioxide emissions by 2030 and achieve carbon neutrality by 2060 has led to policies that incentivize the use of low-carbon technologies and materials across various industries.
Many countries have introduced carbon pricing mechanisms, such as carbon taxes or cap-and-trade systems, which indirectly affect the ethyl acetate market. These policies create economic incentives for companies to reduce their carbon emissions, potentially driving innovation in production processes and end-use applications of ethyl acetate.
The Montreal Protocol, an international treaty designed to protect the ozone layer, has indirectly benefited ethyl acetate usage. As the protocol phased out ozone-depleting substances, ethyl acetate emerged as a suitable replacement in various applications, contributing to its role in carbon reduction strategies.
Industry-specific regulations also impact ethyl acetate's use in carbon reduction efforts. For instance, in the automotive sector, stringent emissions standards have led to increased use of water-based and high-solids coatings, where ethyl acetate can play a role as a less harmful solvent compared to traditional options.
As the global focus on sustainability intensifies, it is likely that future regulations will further emphasize life cycle assessments and circular economy principles. This could lead to more comprehensive policies that consider the entire value chain of chemicals like ethyl acetate, from production to disposal, in evaluating their overall environmental impact and potential for carbon reduction.
Life Cycle Assessment of Ethyl Acetate
Life Cycle Assessment (LCA) of ethyl acetate provides a comprehensive evaluation of its environmental impact throughout its entire lifecycle, from raw material extraction to disposal. This assessment is crucial for understanding how ethyl acetate quantifiably impacts carbon reduction. The LCA typically encompasses four main stages: raw material acquisition, manufacturing, use, and end-of-life disposal.
In the raw material acquisition phase, the primary components for ethyl acetate production, ethanol and acetic acid, are sourced. The carbon footprint of this stage largely depends on the production methods of these precursors. Ethanol can be derived from renewable sources like corn or sugarcane, potentially offering a lower carbon footprint compared to petrochemical-based alternatives. Acetic acid production, often through methanol carbonylation, contributes significantly to the overall carbon emissions.
The manufacturing stage involves the esterification reaction between ethanol and acetic acid. This process requires energy input, typically in the form of heat and pressure, which contributes to the carbon footprint. However, modern catalytic processes have improved efficiency, reducing energy consumption and associated emissions. The purification steps, including distillation, also factor into the overall environmental impact of this stage.
During the use phase, ethyl acetate's impact on carbon reduction becomes more apparent. As a volatile organic compound (VOC) with low toxicity, it often replaces more harmful solvents in various applications. Its use in coatings, adhesives, and cleaning products can lead to reduced emissions compared to alternatives with higher global warming potential. Additionally, its biodegradability contributes to a lower long-term environmental impact.
The end-of-life stage considers the disposal or recycling of ethyl acetate. Due to its volatility, much of the compound evaporates during use, but proper handling of waste and recycling can further mitigate environmental impact. Incineration with energy recovery can offset some of the carbon footprint, while advanced recycling techniques can reduce the need for new production.
Quantifying the carbon reduction impact of ethyl acetate requires a holistic view of these lifecycle stages. Comparative LCAs with alternative solvents or processes provide valuable insights into the net carbon benefit. Factors such as energy efficiency in production, renewable feedstock usage, and end-use applications significantly influence the overall carbon footprint. By optimizing each stage of the lifecycle, the carbon reduction potential of ethyl acetate can be maximized, contributing to more sustainable chemical processes and products.
In the raw material acquisition phase, the primary components for ethyl acetate production, ethanol and acetic acid, are sourced. The carbon footprint of this stage largely depends on the production methods of these precursors. Ethanol can be derived from renewable sources like corn or sugarcane, potentially offering a lower carbon footprint compared to petrochemical-based alternatives. Acetic acid production, often through methanol carbonylation, contributes significantly to the overall carbon emissions.
The manufacturing stage involves the esterification reaction between ethanol and acetic acid. This process requires energy input, typically in the form of heat and pressure, which contributes to the carbon footprint. However, modern catalytic processes have improved efficiency, reducing energy consumption and associated emissions. The purification steps, including distillation, also factor into the overall environmental impact of this stage.
During the use phase, ethyl acetate's impact on carbon reduction becomes more apparent. As a volatile organic compound (VOC) with low toxicity, it often replaces more harmful solvents in various applications. Its use in coatings, adhesives, and cleaning products can lead to reduced emissions compared to alternatives with higher global warming potential. Additionally, its biodegradability contributes to a lower long-term environmental impact.
The end-of-life stage considers the disposal or recycling of ethyl acetate. Due to its volatility, much of the compound evaporates during use, but proper handling of waste and recycling can further mitigate environmental impact. Incineration with energy recovery can offset some of the carbon footprint, while advanced recycling techniques can reduce the need for new production.
Quantifying the carbon reduction impact of ethyl acetate requires a holistic view of these lifecycle stages. Comparative LCAs with alternative solvents or processes provide valuable insights into the net carbon benefit. Factors such as energy efficiency in production, renewable feedstock usage, and end-use applications significantly influence the overall carbon footprint. By optimizing each stage of the lifecycle, the carbon reduction potential of ethyl acetate can be maximized, contributing to more sustainable chemical processes and products.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!