How Ethyl Acetate Stimulates Advancement in Chemical Sectors?
Ethyl Acetate Evolution
Ethyl acetate has undergone a remarkable evolution since its discovery in the early 19th century. Initially recognized as a naturally occurring compound in fruits and wines, it quickly gained attention for its potential industrial applications. The journey of ethyl acetate from a laboratory curiosity to a vital industrial chemical spans over two centuries, marked by significant technological advancements and expanding applications.
In the early stages of its industrial production, ethyl acetate was primarily synthesized through the esterification of ethanol and acetic acid. This process, while effective, was limited by the availability of raw materials and the efficiency of catalysts. The mid-20th century saw a shift towards more economical and scalable production methods, including the Tishchenko reaction using acetaldehyde as a starting material.
The 1960s and 1970s brought about a revolution in ethyl acetate production with the development of continuous flow reactors and improved catalytic systems. These advancements significantly increased production efficiency and reduced costs, making ethyl acetate more accessible for various industrial applications. The introduction of heterogeneous catalysts in the 1980s further enhanced the production process, offering better selectivity and longer catalyst lifetimes.
The late 20th and early 21st centuries witnessed a growing emphasis on sustainable production methods. Researchers began exploring bio-based routes for ethyl acetate synthesis, utilizing renewable resources such as bioethanol and biomass-derived acetic acid. This shift aligned with the increasing global focus on reducing carbon footprints and developing greener chemical processes.
Recent years have seen the integration of advanced technologies in ethyl acetate production. Process intensification techniques, such as reactive distillation and membrane reactors, have been implemented to improve energy efficiency and product purity. Additionally, the application of artificial intelligence and machine learning in process optimization has led to more precise control and predictive maintenance in production facilities.
The evolution of ethyl acetate has not been limited to production methods alone. Its applications have expanded significantly over time, driving further innovations in its synthesis and purification. From its initial use as a solvent in the paint and coating industry, ethyl acetate has found its way into diverse sectors including pharmaceuticals, food processing, and electronics manufacturing.
This continuous evolution has positioned ethyl acetate as a critical component in the advancement of various chemical sectors. Its journey reflects the broader trends in chemical engineering and industrial chemistry, showcasing how a single compound can drive innovation across multiple industries and contribute to the development of more sustainable and efficient chemical processes.
Market Demand Analysis
The market demand for ethyl acetate has been steadily increasing across various chemical sectors, driven by its versatile applications and favorable properties. As a key solvent and reagent, ethyl acetate plays a crucial role in numerous industries, including paints and coatings, adhesives, pharmaceuticals, and food processing.
In the paints and coatings industry, ethyl acetate is highly valued for its fast-evaporating properties and ability to dissolve a wide range of resins. This has led to a significant uptick in demand, particularly in emerging economies where construction and automotive sectors are booming. The global paints and coatings market is projected to grow at a compound annual growth rate (CAGR) of 5.4% from 2021 to 2026, directly influencing the demand for ethyl acetate.
The adhesives industry represents another major consumer of ethyl acetate. With the rise of e-commerce and packaging needs, the demand for adhesives has surged, consequently boosting the market for ethyl acetate. The global adhesives market is expected to reach a value of $84.9 billion by 2027, with ethyl acetate playing a pivotal role in this growth.
In the pharmaceutical sector, ethyl acetate is extensively used as a solvent in the production of various drugs and active pharmaceutical ingredients (APIs). The pharmaceutical industry's robust growth, particularly in the wake of the global pandemic, has further amplified the demand for ethyl acetate. The global pharmaceutical market is forecasted to expand at a CAGR of 7.4% from 2021 to 2028, indicating a sustained demand for ethyl acetate in this sector.
The food processing industry also contributes significantly to the market demand for ethyl acetate. Its application as a flavoring agent and in the extraction of caffeine from coffee and tea has led to increased consumption. The global food additives market, which includes ethyl acetate, is projected to grow at a CAGR of 5.5% from 2021 to 2026.
Environmental regulations and sustainability concerns have also shaped the market demand for ethyl acetate. As a more environmentally friendly alternative to some other solvents, ethyl acetate has seen increased adoption in various applications. This trend is expected to continue as industries strive to meet stricter environmental standards and consumer preferences for eco-friendly products.
The Asia-Pacific region, particularly China and India, has emerged as a major consumer and producer of ethyl acetate, driven by rapid industrialization and economic growth. North America and Europe also maintain significant market shares, with steady demand from established chemical and pharmaceutical industries.
Technical Challenges
The advancement of ethyl acetate in chemical sectors faces several technical challenges that require innovative solutions. One of the primary obstacles is the optimization of production processes to enhance efficiency and reduce costs. Traditional methods of ethyl acetate synthesis often involve energy-intensive reactions and generate significant waste, necessitating the development of more sustainable and economical production routes.
Another challenge lies in the purification of ethyl acetate to meet stringent quality standards for various applications. The presence of impurities, such as water and acetic acid, can significantly affect the product's performance and shelf life. Developing advanced separation and purification techniques that can efficiently remove these contaminants while maintaining high product yields remains a critical area of focus for researchers and industry professionals.
The storage and transportation of ethyl acetate pose additional technical hurdles due to its volatile nature and flammability. Ensuring safe handling and minimizing losses during storage and distribution require sophisticated containment systems and careful monitoring of environmental conditions. This challenge extends to the development of improved packaging materials and designs that can withstand the solvent properties of ethyl acetate while maintaining product integrity.
Environmental concerns associated with ethyl acetate production and use present another set of technical challenges. The chemical industry faces increasing pressure to reduce emissions and minimize the environmental footprint of manufacturing processes. This necessitates the exploration of green chemistry principles and the development of bio-based alternatives or recycling methods for ethyl acetate production.
The application of ethyl acetate in emerging technologies, such as advanced materials and nanotechnology, introduces new technical complexities. Researchers must overcome challenges related to the controlled release of ethyl acetate in drug delivery systems, its integration into smart materials, and its role in the synthesis of novel compounds. These applications require a deep understanding of the molecular interactions between ethyl acetate and various substrates.
Scaling up laboratory processes to industrial production levels presents significant engineering challenges. Maintaining reaction efficiency, product quality, and safety standards at larger scales often requires substantial modifications to equipment and process parameters. The design of robust, scalable production systems that can adapt to varying market demands while adhering to regulatory requirements is a continuous challenge for chemical engineers.
Lastly, the integration of digital technologies and automation in ethyl acetate production and application processes introduces new technical hurdles. Implementing real-time monitoring systems, predictive maintenance algorithms, and process optimization through machine learning requires a multidisciplinary approach, combining chemical engineering expertise with advanced data analytics and control systems.
Current Applications
01 Production and purification of ethyl acetate
Various methods and processes for producing and purifying ethyl acetate are described. These include esterification reactions, distillation techniques, and the use of specific catalysts or reactants to improve yield and purity.- Production and purification of ethyl acetate: Various methods for producing and purifying ethyl acetate are described. These include esterification processes, distillation techniques, and the use of specific catalysts to improve yield and purity. The processes aim to optimize the production of ethyl acetate for industrial applications.
- Applications of ethyl acetate in chemical processes: Ethyl acetate is utilized in various chemical processes and industries. It serves as a solvent, reactant, or intermediate in the production of other chemicals, pharmaceuticals, and materials. Its versatility makes it valuable in diverse applications across different sectors.
- Ethyl acetate in extraction and separation processes: Ethyl acetate is employed in extraction and separation processes for various compounds. Its properties make it suitable for liquid-liquid extraction, azeotropic distillation, and other separation techniques. These processes are used in the purification of chemicals and the isolation of specific compounds.
- Environmental and safety considerations for ethyl acetate: The use and handling of ethyl acetate involve environmental and safety considerations. This includes methods for reducing emissions, improving workplace safety, and developing more sustainable production processes. Efforts are made to minimize the environmental impact of ethyl acetate production and use.
- Novel applications and formulations of ethyl acetate: Research into new applications and formulations of ethyl acetate is ongoing. This includes its use in novel materials, coatings, and specialty chemicals. Innovations focus on exploiting the unique properties of ethyl acetate to develop improved products and processes across various industries.
02 Applications of ethyl acetate in industrial processes
Ethyl acetate is utilized in diverse industrial applications, including as a solvent in chemical reactions, a component in coatings and adhesives, and in the production of various materials such as plastics and textiles.Expand Specific Solutions03 Ethyl acetate in pharmaceutical and cosmetic formulations
The use of ethyl acetate in pharmaceutical and cosmetic products is explored, including its role as a solvent for active ingredients, a component in drug delivery systems, and its application in personal care products.Expand Specific Solutions04 Environmental and safety considerations for ethyl acetate
Research and development efforts focus on improving the environmental impact and safety aspects of ethyl acetate production and use. This includes developing greener production methods, reducing emissions, and enhancing handling procedures.Expand Specific Solutions05 Novel synthesis routes and catalysts for ethyl acetate
Innovative approaches to synthesizing ethyl acetate are presented, including the development of new catalysts, alternative feedstocks, and improved reaction conditions to enhance efficiency and selectivity in production.Expand Specific Solutions
Industry Leaders
The ethyl acetate market is in a mature growth stage, with a global market size expected to reach $4.3 billion by 2026. The technology for ethyl acetate production is well-established, with major players like Celanese, China Petroleum & Chemical Corp., and Eastman Chemical Co. leading the industry. These companies have developed advanced catalysts and processes for efficient ethyl acetate synthesis. Emerging players such as LanzaTech and Resonac Corp. are exploring innovative bio-based production methods, indicating potential for future technological advancements. The competitive landscape is characterized by a mix of established chemical giants and specialized manufacturers, with ongoing research at institutions like Nanjing University and Tianjin University contributing to incremental improvements in production efficiency and sustainability.
Celanese International Corp.
Eastman Chemical Co.
Key Patents Review
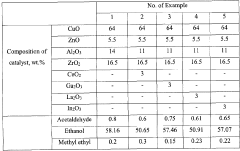
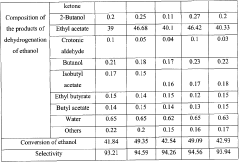
- A catalyst comprising a mixture of CuO, ZnO, ZrO2, and Al2O3 oxides with additional metal oxides like Ce, Ga, La, or In, used for vapor-phase heterogeneous catalytic dehydrogenation at lower temperatures and pressures, achieving high selectivity and conversion of ethyl acetate from technical-grade ethanol with up to 10 wt.% water content.
- A process utilizing citric acid as a natural catalyst, combined with biologically produced ethanol and acetic acid, eliminates the need for mineral acids, employing a reactor system with specific heating and condensation steps to achieve high purity ethyl acetate production.
Environmental Impact
The environmental impact of ethyl acetate production and usage is a critical consideration in the chemical industry's advancement. As a widely used solvent and reagent, ethyl acetate's lifecycle has significant implications for sustainability and ecological balance.
In the manufacturing process, ethyl acetate production traditionally involves the esterification of ethanol and acetic acid. This reaction, while efficient, can generate waste products and consume substantial energy. However, recent advancements in green chemistry have led to more environmentally friendly production methods. Biocatalytic processes using enzymes, for instance, operate at lower temperatures and pressures, reducing energy consumption and minimizing harmful byproducts.
The use of ethyl acetate in various industries also contributes to its environmental footprint. As a volatile organic compound (VOC), it can contribute to air pollution and smog formation if released into the atmosphere. To mitigate this, many chemical sectors have implemented advanced emission control technologies and closed-loop systems to capture and recycle ethyl acetate vapors.
Water pollution is another concern associated with ethyl acetate. Although it has low water solubility, improper disposal or accidental spills can lead to contamination of water bodies. This has prompted the development of more effective wastewater treatment methods and stricter regulations on chemical handling and disposal in many regions.
On a positive note, ethyl acetate's biodegradability offers an advantage over some other solvents. It breaks down relatively quickly in the environment, reducing long-term ecological impact. This property has made it an attractive alternative to more persistent solvents in various applications, including paints, coatings, and pharmaceutical processes.
The chemical industry's focus on sustainability has driven research into bio-based ethyl acetate production. Utilizing renewable feedstocks, such as agricultural waste or algae-derived ethanol, can significantly reduce the carbon footprint of ethyl acetate manufacturing. This approach aligns with circular economy principles and helps decrease dependence on fossil fuel-based raw materials.
As environmental regulations become more stringent globally, the chemical sector is investing in life cycle assessments (LCA) for ethyl acetate. These comprehensive analyses evaluate the environmental impacts from raw material extraction to end-of-life disposal, guiding more sustainable practices throughout the value chain.
In conclusion, while ethyl acetate presents certain environmental challenges, its role in advancing chemical sectors is increasingly aligned with sustainability goals. Through ongoing innovations in green chemistry, emission control, and circular economy practices, the industry is working to minimize the environmental impact of this versatile compound while harnessing its benefits for technological progress.
Regulatory Framework
The regulatory framework surrounding ethyl acetate plays a crucial role in shaping its impact on the chemical industry. As a widely used solvent and intermediate, ethyl acetate is subject to various regulations across different regions and sectors. These regulations primarily focus on ensuring safety, environmental protection, and quality control throughout the production, handling, and application processes.
In the United States, the Environmental Protection Agency (EPA) regulates ethyl acetate under the Toxic Substances Control Act (TSCA). The substance is listed on the TSCA inventory, which means it has been assessed for potential risks to human health and the environment. Additionally, the Occupational Safety and Health Administration (OSHA) sets permissible exposure limits for workers handling ethyl acetate in industrial settings.
The European Union has implemented the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation, which requires manufacturers and importers to register chemicals, including ethyl acetate, and provide safety data. This comprehensive regulatory framework aims to protect human health and the environment while promoting innovation in the chemical industry.
In Asia, countries like China and Japan have their own regulatory systems for chemical substances. China's Measures for Environmental Management of New Chemical Substances (MEP Order 7) and Japan's Chemical Substances Control Law (CSCL) both include provisions for the registration and management of ethyl acetate and similar compounds.
The regulatory landscape also extends to specific applications of ethyl acetate. For instance, in the food industry, ethyl acetate is regulated as a food additive by agencies such as the U.S. Food and Drug Administration (FDA) and the European Food Safety Authority (EFSA). These regulations set limits on its use and ensure its safety in food products.
The pharmaceutical sector faces stringent regulations regarding the use of ethyl acetate in drug manufacturing processes. Good Manufacturing Practice (GMP) guidelines, enforced by regulatory bodies like the FDA and the European Medicines Agency (EMA), dictate the quality standards for solvents used in pharmaceutical production.
As sustainability becomes increasingly important, regulations are evolving to encourage the development of greener alternatives and more environmentally friendly production methods for ethyl acetate. This includes incentives for bio-based production and stricter controls on emissions and waste management in traditional manufacturing processes.
The regulatory framework surrounding ethyl acetate continues to evolve, driven by advancements in scientific understanding, changing environmental priorities, and the need to balance industrial growth with public safety. This dynamic regulatory landscape both challenges and stimulates innovation in the chemical sector, pushing companies to develop safer, more efficient, and sustainable practices in the production and use of ethyl acetate.