How Ethyl Acetate Supports Eco-Conscious Manufacturing Goals?
JUN 27, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ethyl Acetate Evolution
Ethyl acetate has undergone a significant evolution in its production methods and applications, particularly in the context of eco-conscious manufacturing. Initially synthesized in the early 19th century, ethyl acetate was primarily produced through the esterification of ethanol and acetic acid. This traditional method, while effective, often relied on petrochemical feedstocks and energy-intensive processes.
As environmental concerns gained prominence in the late 20th century, the production of ethyl acetate began to shift towards more sustainable practices. The introduction of bio-based feedstocks marked a crucial turning point. Manufacturers started exploring the use of renewable resources such as corn, sugarcane, and other plant-based materials to produce ethanol, a key component in ethyl acetate synthesis.
The early 2000s saw the development of more efficient catalytic processes, reducing energy consumption and improving yield. These advancements not only enhanced the eco-friendliness of ethyl acetate production but also made it more economically viable. Continuous flow reactors and process intensification techniques further optimized production, minimizing waste and reducing the overall environmental footprint.
In recent years, the focus has shifted towards circular economy principles in ethyl acetate manufacturing. Innovative recycling technologies have emerged, allowing for the recovery and reuse of ethyl acetate from various industrial processes. This closed-loop approach significantly reduces the demand for virgin materials and minimizes waste generation.
The latest frontier in ethyl acetate evolution involves the integration of green chemistry principles. Researchers are exploring novel catalysts derived from abundant, non-toxic materials to further enhance the sustainability of the production process. Additionally, there is growing interest in utilizing atmospheric carbon dioxide as a feedstock for ethyl acetate synthesis, potentially transforming a greenhouse gas into a valuable chemical product.
As industries strive for carbon neutrality, the role of ethyl acetate in eco-conscious manufacturing continues to expand. Its versatility as a solvent in low-VOC (Volatile Organic Compound) formulations has made it increasingly popular in the production of environmentally friendly paints, coatings, and adhesives. Furthermore, its biodegradability and low toxicity profile align well with the growing demand for sustainable consumer products.
The evolution of ethyl acetate production and application demonstrates a clear trajectory towards sustainability. From its petrochemical origins to its current status as a key player in green chemistry, ethyl acetate exemplifies the potential for traditional chemicals to be reimagined and repurposed in the context of eco-conscious manufacturing goals.
As environmental concerns gained prominence in the late 20th century, the production of ethyl acetate began to shift towards more sustainable practices. The introduction of bio-based feedstocks marked a crucial turning point. Manufacturers started exploring the use of renewable resources such as corn, sugarcane, and other plant-based materials to produce ethanol, a key component in ethyl acetate synthesis.
The early 2000s saw the development of more efficient catalytic processes, reducing energy consumption and improving yield. These advancements not only enhanced the eco-friendliness of ethyl acetate production but also made it more economically viable. Continuous flow reactors and process intensification techniques further optimized production, minimizing waste and reducing the overall environmental footprint.
In recent years, the focus has shifted towards circular economy principles in ethyl acetate manufacturing. Innovative recycling technologies have emerged, allowing for the recovery and reuse of ethyl acetate from various industrial processes. This closed-loop approach significantly reduces the demand for virgin materials and minimizes waste generation.
The latest frontier in ethyl acetate evolution involves the integration of green chemistry principles. Researchers are exploring novel catalysts derived from abundant, non-toxic materials to further enhance the sustainability of the production process. Additionally, there is growing interest in utilizing atmospheric carbon dioxide as a feedstock for ethyl acetate synthesis, potentially transforming a greenhouse gas into a valuable chemical product.
As industries strive for carbon neutrality, the role of ethyl acetate in eco-conscious manufacturing continues to expand. Its versatility as a solvent in low-VOC (Volatile Organic Compound) formulations has made it increasingly popular in the production of environmentally friendly paints, coatings, and adhesives. Furthermore, its biodegradability and low toxicity profile align well with the growing demand for sustainable consumer products.
The evolution of ethyl acetate production and application demonstrates a clear trajectory towards sustainability. From its petrochemical origins to its current status as a key player in green chemistry, ethyl acetate exemplifies the potential for traditional chemicals to be reimagined and repurposed in the context of eco-conscious manufacturing goals.
Green Solvent Demand
The demand for green solvents has been steadily increasing in recent years, driven by growing environmental concerns and stricter regulations on volatile organic compounds (VOCs) emissions. Ethyl acetate, a biodegradable and low-toxicity solvent, has emerged as a promising alternative to traditional petroleum-based solvents in various manufacturing processes. This shift towards eco-friendly solvents is particularly evident in industries such as paints and coatings, adhesives, pharmaceuticals, and personal care products.
The global green solvents market has experienced significant growth, with projections indicating continued expansion in the coming years. This growth is attributed to the increasing adoption of sustainable practices by manufacturers and the rising consumer preference for environmentally friendly products. Ethyl acetate, being derived from renewable resources such as corn and sugarcane, aligns well with these market trends and sustainability goals.
In the paints and coatings industry, ethyl acetate has gained traction as a replacement for more harmful solvents like toluene and xylene. Its low volatility and excellent solvency properties make it an ideal choice for formulating low-VOC products that meet stringent environmental regulations. Similarly, in the adhesives sector, ethyl acetate is being increasingly used in water-based adhesive formulations, contributing to reduced environmental impact without compromising product performance.
The pharmaceutical industry has also recognized the benefits of ethyl acetate as a green solvent. Its use in drug synthesis and purification processes has increased, driven by the industry's focus on sustainable manufacturing practices and the need to reduce the environmental footprint of pharmaceutical production. The personal care and cosmetics sector is another area where the demand for ethyl acetate is growing, particularly in the formulation of nail polish removers and other beauty products.
The rising demand for ethyl acetate as a green solvent is further supported by its favorable properties, including low toxicity, high solvency power, and rapid evaporation rate. These characteristics make it suitable for a wide range of applications while minimizing environmental and health risks associated with traditional solvents. Additionally, the increasing availability of bio-based ethyl acetate, produced through fermentation processes, is expected to further boost its adoption in eco-conscious manufacturing.
As industries continue to prioritize sustainability and seek ways to reduce their environmental impact, the demand for green solvents like ethyl acetate is expected to grow. This trend is likely to drive innovation in production methods, leading to more efficient and cost-effective manufacturing processes for ethyl acetate. The growing market demand also presents opportunities for companies to differentiate their products and gain a competitive edge by incorporating environmentally friendly solvents into their manufacturing processes.
The global green solvents market has experienced significant growth, with projections indicating continued expansion in the coming years. This growth is attributed to the increasing adoption of sustainable practices by manufacturers and the rising consumer preference for environmentally friendly products. Ethyl acetate, being derived from renewable resources such as corn and sugarcane, aligns well with these market trends and sustainability goals.
In the paints and coatings industry, ethyl acetate has gained traction as a replacement for more harmful solvents like toluene and xylene. Its low volatility and excellent solvency properties make it an ideal choice for formulating low-VOC products that meet stringent environmental regulations. Similarly, in the adhesives sector, ethyl acetate is being increasingly used in water-based adhesive formulations, contributing to reduced environmental impact without compromising product performance.
The pharmaceutical industry has also recognized the benefits of ethyl acetate as a green solvent. Its use in drug synthesis and purification processes has increased, driven by the industry's focus on sustainable manufacturing practices and the need to reduce the environmental footprint of pharmaceutical production. The personal care and cosmetics sector is another area where the demand for ethyl acetate is growing, particularly in the formulation of nail polish removers and other beauty products.
The rising demand for ethyl acetate as a green solvent is further supported by its favorable properties, including low toxicity, high solvency power, and rapid evaporation rate. These characteristics make it suitable for a wide range of applications while minimizing environmental and health risks associated with traditional solvents. Additionally, the increasing availability of bio-based ethyl acetate, produced through fermentation processes, is expected to further boost its adoption in eco-conscious manufacturing.
As industries continue to prioritize sustainability and seek ways to reduce their environmental impact, the demand for green solvents like ethyl acetate is expected to grow. This trend is likely to drive innovation in production methods, leading to more efficient and cost-effective manufacturing processes for ethyl acetate. The growing market demand also presents opportunities for companies to differentiate their products and gain a competitive edge by incorporating environmentally friendly solvents into their manufacturing processes.
Eco-Manufacturing Hurdles
The transition towards eco-conscious manufacturing faces numerous challenges, particularly in the adoption of sustainable solvents like ethyl acetate. One of the primary hurdles is the entrenched use of traditional, often petroleum-based solvents in established manufacturing processes. Many industries have long-standing practices and equipment optimized for these conventional solvents, making the switch to greener alternatives a complex and potentially costly endeavor.
Financial considerations pose another significant barrier. While ethyl acetate offers environmental benefits, its production and implementation may initially require substantial investments in new equipment, process modifications, and staff training. This upfront cost can be a deterrent for companies, especially small and medium-sized enterprises operating on tight budgets.
Technical challenges also abound in the integration of ethyl acetate into existing manufacturing systems. The solvent's properties, such as its lower boiling point compared to some traditional solvents, may necessitate adjustments in process parameters, safety protocols, and quality control measures. Ensuring consistent product quality and maintaining production efficiency during the transition can be a formidable task for manufacturers.
Regulatory compliance presents another hurdle. While ethyl acetate is generally considered environmentally friendly, manufacturers must navigate a complex landscape of environmental regulations, safety standards, and product certifications when implementing new processes. This can involve time-consuming and costly approval processes, potentially slowing down the adoption of eco-friendly practices.
Supply chain considerations also play a crucial role. The availability and reliability of ethyl acetate supply, especially from sustainable sources, may be limited compared to well-established conventional solvents. This can lead to concerns about production continuity and potential disruptions in manufacturing schedules.
Furthermore, there is often a lack of comprehensive data and case studies on the long-term performance and environmental impact of ethyl acetate in various manufacturing applications. This knowledge gap can create uncertainty and hesitation among decision-makers when considering the switch to more sustainable practices.
Consumer perception and market demand present additional challenges. While there is a growing trend towards eco-friendly products, not all markets or consumer segments place equal value on sustainability. Manufacturers must carefully balance the adoption of green practices with market expectations and willingness to pay for environmentally conscious products.
Financial considerations pose another significant barrier. While ethyl acetate offers environmental benefits, its production and implementation may initially require substantial investments in new equipment, process modifications, and staff training. This upfront cost can be a deterrent for companies, especially small and medium-sized enterprises operating on tight budgets.
Technical challenges also abound in the integration of ethyl acetate into existing manufacturing systems. The solvent's properties, such as its lower boiling point compared to some traditional solvents, may necessitate adjustments in process parameters, safety protocols, and quality control measures. Ensuring consistent product quality and maintaining production efficiency during the transition can be a formidable task for manufacturers.
Regulatory compliance presents another hurdle. While ethyl acetate is generally considered environmentally friendly, manufacturers must navigate a complex landscape of environmental regulations, safety standards, and product certifications when implementing new processes. This can involve time-consuming and costly approval processes, potentially slowing down the adoption of eco-friendly practices.
Supply chain considerations also play a crucial role. The availability and reliability of ethyl acetate supply, especially from sustainable sources, may be limited compared to well-established conventional solvents. This can lead to concerns about production continuity and potential disruptions in manufacturing schedules.
Furthermore, there is often a lack of comprehensive data and case studies on the long-term performance and environmental impact of ethyl acetate in various manufacturing applications. This knowledge gap can create uncertainty and hesitation among decision-makers when considering the switch to more sustainable practices.
Consumer perception and market demand present additional challenges. While there is a growing trend towards eco-friendly products, not all markets or consumer segments place equal value on sustainability. Manufacturers must carefully balance the adoption of green practices with market expectations and willingness to pay for environmentally conscious products.
Current Green Applications
01 Production and purification of ethyl acetate
Various methods and processes for producing and purifying ethyl acetate are described. These include esterification reactions, distillation techniques, and the use of specific catalysts to improve yield and efficiency. The processes aim to produce high-purity ethyl acetate for industrial applications.- Production and purification of ethyl acetate: Various methods and processes for producing and purifying ethyl acetate are described. These include esterification reactions, distillation techniques, and the use of specific catalysts to improve yield and purity. The processes aim to optimize production efficiency and product quality.
- Applications of ethyl acetate in industrial processes: Ethyl acetate finds diverse applications in industrial processes, including as a solvent in various chemical reactions, as a component in coatings and adhesives, and in the production of other chemicals. Its properties make it suitable for use in multiple sectors such as pharmaceuticals, electronics, and manufacturing.
- Ethyl acetate in extraction and separation processes: Ethyl acetate is utilized in extraction and separation processes due to its solvent properties. It is employed in liquid-liquid extraction, azeotropic distillation, and as a component in separation media. These applications are particularly relevant in the purification of organic compounds and in the food and pharmaceutical industries.
- Environmental and safety considerations in ethyl acetate handling: The use and handling of ethyl acetate involve various environmental and safety considerations. This includes methods for reducing emissions, safe storage and transportation practices, and techniques for recovering and recycling ethyl acetate to minimize waste and environmental impact.
- Novel synthesis routes and derivatives of ethyl acetate: Research into novel synthesis routes for ethyl acetate and the development of its derivatives is ongoing. This includes exploring new catalysts, alternative feedstocks, and the creation of functionalized versions of ethyl acetate for specialized applications in various industries.
02 Applications of ethyl acetate in chemical processes
Ethyl acetate is utilized in various chemical processes and industries. It serves as a solvent, reactant, or intermediate in the production of other chemicals, pharmaceuticals, and materials. Its versatility makes it valuable in diverse applications such as coatings, adhesives, and extraction processes.Expand Specific Solutions03 Ethyl acetate in sustainable and green chemistry
Research focuses on developing environmentally friendly methods for producing and using ethyl acetate. This includes exploring bio-based feedstocks, improving energy efficiency in production processes, and finding sustainable alternatives to traditional petrochemical-based methods.Expand Specific Solutions04 Separation and recovery of ethyl acetate
Techniques for separating and recovering ethyl acetate from mixtures or waste streams are developed. These methods aim to improve the efficiency of ethyl acetate recycling in industrial processes, reducing waste and environmental impact while increasing economic viability.Expand Specific Solutions05 Ethyl acetate in specialized applications
Ethyl acetate finds use in specialized applications across various industries. These include its role in analytical chemistry, as a component in specific formulations, and in the development of novel materials or products with unique properties.Expand Specific Solutions
Key Industry Players
The ethyl acetate market is in a mature stage, with established players and steady demand across various industries. The global market size is estimated to be around $3 billion, growing at a moderate CAGR of 4-5%. Technologically, the production process is well-established, with major companies like Celanese, Eastman Chemical, and SABIC leading in production capacity and efficiency. However, there's increasing focus on eco-friendly manufacturing methods, with companies like LanzaTech and Viridis Chemical developing bio-based ethyl acetate production. Research institutions such as Tianjin University and University of Campinas are also contributing to advancements in sustainable production techniques, indicating a shift towards greener alternatives in this mature market.
Celanese International Corp.
Technical Solution: Celanese has developed a sustainable production process for ethyl acetate using bioethanol as a feedstock. Their innovative approach involves a two-step reaction: first, the dehydrogenation of ethanol to acetaldehyde, followed by the condensation of acetaldehyde with ethanol to form ethyl acetate[1]. This process significantly reduces carbon footprint compared to traditional petrochemical routes. Celanese has also implemented advanced catalytic systems that improve selectivity and yield, minimizing waste and energy consumption[2]. The company's commitment to eco-conscious manufacturing is further demonstrated by their use of renewable energy sources in production facilities and implementation of closed-loop recycling systems for solvents and byproducts[3].
Strengths: Utilizes renewable feedstock, reduces carbon emissions, and improves overall process efficiency. Weaknesses: May face challenges in scaling up production to meet global demand and potential higher costs compared to traditional methods.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed an innovative approach to ethyl acetate production that aligns with eco-conscious manufacturing goals. Their process utilizes a novel reactive distillation technology, which combines reaction and separation in a single unit operation, significantly reducing energy consumption and equipment footprint[7]. Sinopec has also implemented advanced process control systems and artificial intelligence algorithms to optimize reaction conditions in real-time, improving yield and reducing waste[8]. Furthermore, the company has invested in carbon capture and utilization technologies, converting CO2 emissions from the ethyl acetate production process into valuable chemicals, thereby closing the carbon loop[9].
Strengths: Energy-efficient process integration, advanced process control, and carbon utilization. Weaknesses: May face challenges in retrofitting existing plants and potential high initial investment costs.
Innovative EA Formulations
Process of low energy consumption for preparing a carboxylic acid ester
PatentWO2012123279A1
Innovation
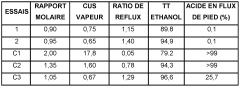
- A process involving the reaction of ethyl alcohol with acetic acid in the presence of a solid acid catalyst, using a reactive distillation system with a centrally placed reaction zone between upper and lower separation zones, optimizing the molar ratio of acetic acid to ethyl alcohol between 0.85 and 0.97, and controlling the reflux ratio between 1.0 and 1.5, significantly reduces energy costs and minimizes acetic acid at the column bottom.
Process for the production of ethyle acetate
PatentInactiveEP0339330A3
Innovation
- A process utilizing citric acid as a natural catalyst, combined with biologically produced ethanol and acetic acid, eliminates the need for mineral acids, employing a reactor system with specific heating and condensation steps to achieve high purity ethyl acetate production.
Regulatory Landscape
The regulatory landscape surrounding ethyl acetate usage in eco-conscious manufacturing is complex and evolving, reflecting growing global concerns about environmental sustainability and human health. At the international level, the United Nations' Sustainable Development Goals (SDGs) have set a framework for responsible production and consumption, indirectly influencing the use of chemicals like ethyl acetate in industrial processes.
In the European Union, the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) regulation plays a crucial role in governing the use of ethyl acetate. REACH requires manufacturers and importers to register chemicals and provide safety information, ensuring that potential risks are identified and managed. The EU has also implemented the Industrial Emissions Directive (IED), which sets strict limits on volatile organic compound (VOC) emissions, including those from ethyl acetate use in manufacturing.
The United States Environmental Protection Agency (EPA) regulates ethyl acetate under the Toxic Substances Control Act (TSCA) and the Clean Air Act. The EPA has established National Emission Standards for Hazardous Air Pollutants (NESHAP) that apply to industries using ethyl acetate, requiring the implementation of Maximum Achievable Control Technology (MACT) to minimize emissions.
In Asia, countries like China and Japan have been strengthening their chemical regulations. China's Measures for Environmental Management of New Chemical Substances and Japan's Chemical Substances Control Law both impact the use of ethyl acetate in manufacturing processes, emphasizing risk assessment and management.
Many countries have adopted occupational safety and health regulations that set exposure limits for workers handling ethyl acetate. These regulations often require proper ventilation, personal protective equipment, and safety training for employees working with the solvent.
The global trend towards circular economy principles is influencing regulations on waste management and recycling. This affects how manufacturers handle ethyl acetate waste and encourages the development of closed-loop systems for solvent recovery and reuse.
As sustainability becomes increasingly important, voluntary certification schemes and industry standards are emerging. These include green chemistry certifications and eco-labels that consider the environmental impact of chemicals used in manufacturing processes, potentially giving a competitive edge to companies using ethyl acetate in environmentally responsible ways.
In the European Union, the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) regulation plays a crucial role in governing the use of ethyl acetate. REACH requires manufacturers and importers to register chemicals and provide safety information, ensuring that potential risks are identified and managed. The EU has also implemented the Industrial Emissions Directive (IED), which sets strict limits on volatile organic compound (VOC) emissions, including those from ethyl acetate use in manufacturing.
The United States Environmental Protection Agency (EPA) regulates ethyl acetate under the Toxic Substances Control Act (TSCA) and the Clean Air Act. The EPA has established National Emission Standards for Hazardous Air Pollutants (NESHAP) that apply to industries using ethyl acetate, requiring the implementation of Maximum Achievable Control Technology (MACT) to minimize emissions.
In Asia, countries like China and Japan have been strengthening their chemical regulations. China's Measures for Environmental Management of New Chemical Substances and Japan's Chemical Substances Control Law both impact the use of ethyl acetate in manufacturing processes, emphasizing risk assessment and management.
Many countries have adopted occupational safety and health regulations that set exposure limits for workers handling ethyl acetate. These regulations often require proper ventilation, personal protective equipment, and safety training for employees working with the solvent.
The global trend towards circular economy principles is influencing regulations on waste management and recycling. This affects how manufacturers handle ethyl acetate waste and encourages the development of closed-loop systems for solvent recovery and reuse.
As sustainability becomes increasingly important, voluntary certification schemes and industry standards are emerging. These include green chemistry certifications and eco-labels that consider the environmental impact of chemicals used in manufacturing processes, potentially giving a competitive edge to companies using ethyl acetate in environmentally responsible ways.
Life Cycle Assessment
Life Cycle Assessment (LCA) plays a crucial role in evaluating the environmental impact of ethyl acetate throughout its entire lifecycle, from raw material extraction to disposal or recycling. This comprehensive analysis helps manufacturers make informed decisions to support eco-conscious manufacturing goals.
The LCA of ethyl acetate typically begins with the sourcing of raw materials, primarily ethanol and acetic acid. The production of these precursors involves agricultural processes for ethanol and petrochemical processes for acetic acid. Each step in the raw material production contributes to the overall environmental footprint, including energy consumption, water usage, and greenhouse gas emissions.
During the manufacturing phase, the esterification process to produce ethyl acetate requires energy input and may generate byproducts. Modern production methods have significantly improved efficiency, reducing energy consumption and minimizing waste. Some manufacturers have implemented closed-loop systems to recycle unreacted materials, further reducing environmental impact.
The distribution and use phase of ethyl acetate's lifecycle presents opportunities for eco-conscious practices. As a volatile organic compound (VOC), proper handling and storage are essential to prevent emissions. Many industries have adopted low-VOC or VOC-free formulations, incorporating ethyl acetate as a more environmentally friendly alternative to traditional solvents.
End-of-life considerations for ethyl acetate include its potential for recycling and biodegradability. When properly managed, ethyl acetate can be recovered and purified for reuse, reducing the demand for new production. In cases where recycling is not feasible, ethyl acetate's biodegradability ensures it does not persist in the environment, unlike many petroleum-based solvents.
Comparative LCAs have shown that ethyl acetate often has a lower environmental impact than alternative solvents, particularly in terms of global warming potential and ozone depletion. However, its production still contributes to acidification and eutrophication, areas where further improvements can be made.
To support eco-conscious manufacturing goals, companies are increasingly focusing on renewable sources for ethyl acetate production. Bio-based ethanol, derived from sustainable agricultural practices, can significantly reduce the carbon footprint of ethyl acetate. Additionally, innovative catalysts and process optimizations are being developed to enhance reaction efficiency and reduce energy requirements.
By conducting thorough LCAs, manufacturers can identify hotspots in the ethyl acetate lifecycle and implement targeted improvements. This data-driven approach enables companies to make strategic decisions that align with sustainability objectives while maintaining product quality and economic viability.
The LCA of ethyl acetate typically begins with the sourcing of raw materials, primarily ethanol and acetic acid. The production of these precursors involves agricultural processes for ethanol and petrochemical processes for acetic acid. Each step in the raw material production contributes to the overall environmental footprint, including energy consumption, water usage, and greenhouse gas emissions.
During the manufacturing phase, the esterification process to produce ethyl acetate requires energy input and may generate byproducts. Modern production methods have significantly improved efficiency, reducing energy consumption and minimizing waste. Some manufacturers have implemented closed-loop systems to recycle unreacted materials, further reducing environmental impact.
The distribution and use phase of ethyl acetate's lifecycle presents opportunities for eco-conscious practices. As a volatile organic compound (VOC), proper handling and storage are essential to prevent emissions. Many industries have adopted low-VOC or VOC-free formulations, incorporating ethyl acetate as a more environmentally friendly alternative to traditional solvents.
End-of-life considerations for ethyl acetate include its potential for recycling and biodegradability. When properly managed, ethyl acetate can be recovered and purified for reuse, reducing the demand for new production. In cases where recycling is not feasible, ethyl acetate's biodegradability ensures it does not persist in the environment, unlike many petroleum-based solvents.
Comparative LCAs have shown that ethyl acetate often has a lower environmental impact than alternative solvents, particularly in terms of global warming potential and ozone depletion. However, its production still contributes to acidification and eutrophication, areas where further improvements can be made.
To support eco-conscious manufacturing goals, companies are increasingly focusing on renewable sources for ethyl acetate production. Bio-based ethanol, derived from sustainable agricultural practices, can significantly reduce the carbon footprint of ethyl acetate. Additionally, innovative catalysts and process optimizations are being developed to enhance reaction efficiency and reduce energy requirements.
By conducting thorough LCAs, manufacturers can identify hotspots in the ethyl acetate lifecycle and implement targeted improvements. This data-driven approach enables companies to make strategic decisions that align with sustainability objectives while maintaining product quality and economic viability.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!