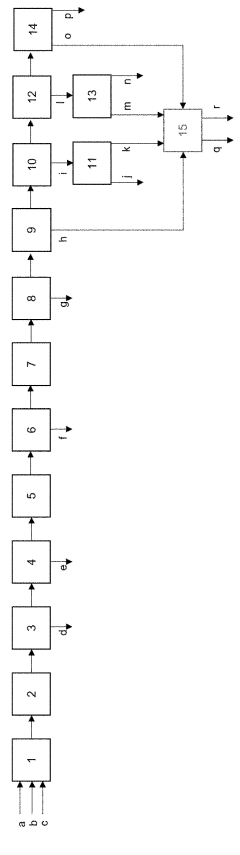
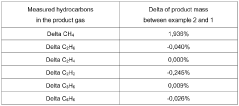
How Methane Pyrolysis Impacts Natural Gas Industries.
SEP 5, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Methane Pyrolysis Evolution and Objectives
Methane pyrolysis represents a significant evolution in natural gas processing technology, dating back to early laboratory experiments in the 1960s. Initially considered merely an academic curiosity, this process—which thermally decomposes methane into hydrogen and solid carbon without direct CO2 emissions—has gained substantial attention in recent decades as environmental concerns have intensified. The technology has evolved from basic thermal decomposition methods requiring temperatures exceeding 1000°C to more sophisticated approaches utilizing catalysts, plasma, and molten metal mediums that significantly reduce energy requirements.
The trajectory of methane pyrolysis development has been marked by several key technological breakthroughs. The 1990s saw the first viable catalytic methods using nickel and iron-based catalysts, while the 2000s brought innovations in plasma-assisted decomposition. The past decade has witnessed remarkable advancements in molten metal reactor designs, particularly those utilizing liquid metals like tin and bismuth as reaction mediums, dramatically improving conversion efficiency and carbon separation.
Current research objectives in methane pyrolysis center on addressing several critical challenges that have historically limited commercial viability. Primary among these is reducing the substantial energy input required for the endothermic reaction while simultaneously increasing methane conversion rates and hydrogen yield. Researchers aim to develop catalysts with enhanced stability that resist deactivation from carbon deposition—a persistent obstacle in continuous operation scenarios.
Another significant objective involves optimizing carbon handling and valorization pathways. The solid carbon byproduct, potentially valuable as a material resource, presents both an opportunity and a logistical challenge. Research efforts focus on producing carbon with controlled morphology and properties suitable for high-value applications in materials science and manufacturing.
Scale-up and process integration represent additional key objectives, with particular emphasis on heat management strategies and reactor designs capable of continuous operation at industrial scales. The technology aims to achieve economic viability at production scales relevant to existing natural gas infrastructure, with hydrogen production costs competitive with conventional steam methane reforming but without the associated carbon dioxide emissions.
The overarching goal driving methane pyrolysis development is establishing a transitional technology that leverages existing natural gas resources and infrastructure while significantly reducing greenhouse gas emissions. This positions methane pyrolysis as a potential bridge technology in the energy transition, allowing continued utilization of natural gas assets while moving toward decarbonization objectives and supporting the emerging hydrogen economy.
The trajectory of methane pyrolysis development has been marked by several key technological breakthroughs. The 1990s saw the first viable catalytic methods using nickel and iron-based catalysts, while the 2000s brought innovations in plasma-assisted decomposition. The past decade has witnessed remarkable advancements in molten metal reactor designs, particularly those utilizing liquid metals like tin and bismuth as reaction mediums, dramatically improving conversion efficiency and carbon separation.
Current research objectives in methane pyrolysis center on addressing several critical challenges that have historically limited commercial viability. Primary among these is reducing the substantial energy input required for the endothermic reaction while simultaneously increasing methane conversion rates and hydrogen yield. Researchers aim to develop catalysts with enhanced stability that resist deactivation from carbon deposition—a persistent obstacle in continuous operation scenarios.
Another significant objective involves optimizing carbon handling and valorization pathways. The solid carbon byproduct, potentially valuable as a material resource, presents both an opportunity and a logistical challenge. Research efforts focus on producing carbon with controlled morphology and properties suitable for high-value applications in materials science and manufacturing.
Scale-up and process integration represent additional key objectives, with particular emphasis on heat management strategies and reactor designs capable of continuous operation at industrial scales. The technology aims to achieve economic viability at production scales relevant to existing natural gas infrastructure, with hydrogen production costs competitive with conventional steam methane reforming but without the associated carbon dioxide emissions.
The overarching goal driving methane pyrolysis development is establishing a transitional technology that leverages existing natural gas resources and infrastructure while significantly reducing greenhouse gas emissions. This positions methane pyrolysis as a potential bridge technology in the energy transition, allowing continued utilization of natural gas assets while moving toward decarbonization objectives and supporting the emerging hydrogen economy.
Market Demand Analysis for Clean Hydrogen Production
The global hydrogen market is experiencing unprecedented growth, driven by the urgent need for clean energy alternatives to combat climate change. Current market analysis indicates that hydrogen demand could increase tenfold by 2050, reaching approximately 660 million tons annually, with clean hydrogen production methods becoming increasingly crucial. Traditional hydrogen production via steam methane reforming (SMR) accounts for over 95% of current hydrogen production but generates significant CO2 emissions—approximately 9-12 kg CO2 per kg H2 produced.
Methane pyrolysis represents a disruptive technology in this landscape, offering a pathway to produce "turquoise hydrogen" with substantially lower carbon emissions than conventional methods. Market research suggests that clean hydrogen production could capture 24% of final energy demand by 2050, with methane pyrolysis potentially addressing a significant portion of this market due to its environmental advantages.
The industrial sector currently represents the largest demand segment for hydrogen, particularly in refining, ammonia production, and methanol synthesis. However, emerging applications in transportation, power generation, and building heating are expected to drive substantial new demand. The transportation sector alone could require 140 million tons of hydrogen annually by 2050, creating a robust market for clean production methods like methane pyrolysis.
Economic analyses indicate that methane pyrolysis-derived hydrogen could achieve cost competitiveness with conventional SMR hydrogen when carbon pricing mechanisms are implemented. Current production costs for pyrolysis hydrogen range between $1.50-2.50/kg, compared to $1.00-1.80/kg for SMR hydrogen without carbon capture. This gap is expected to narrow as technology matures and economies of scale are realized.
Regional market assessment shows particularly strong potential in natural gas-rich regions seeking to decarbonize their energy systems while leveraging existing resources. North America, the Middle East, and Russia represent prime markets where methane pyrolysis could enable continued utilization of natural gas resources while meeting increasingly stringent carbon reduction targets.
Investment trends further validate market interest, with venture capital funding for methane pyrolysis technologies increasing by over 300% in the past five years. Major energy companies are also making strategic investments, recognizing the potential for methane pyrolysis to transform their natural gas assets into clean hydrogen production facilities while generating valuable solid carbon byproducts.
Consumer demand for low-carbon products is creating additional market pull, with industries seeking to reduce their carbon footprint through clean hydrogen adoption. This trend is particularly evident in hard-to-abate sectors like steel production, heavy transport, and chemical manufacturing, where electrification presents significant challenges.
Methane pyrolysis represents a disruptive technology in this landscape, offering a pathway to produce "turquoise hydrogen" with substantially lower carbon emissions than conventional methods. Market research suggests that clean hydrogen production could capture 24% of final energy demand by 2050, with methane pyrolysis potentially addressing a significant portion of this market due to its environmental advantages.
The industrial sector currently represents the largest demand segment for hydrogen, particularly in refining, ammonia production, and methanol synthesis. However, emerging applications in transportation, power generation, and building heating are expected to drive substantial new demand. The transportation sector alone could require 140 million tons of hydrogen annually by 2050, creating a robust market for clean production methods like methane pyrolysis.
Economic analyses indicate that methane pyrolysis-derived hydrogen could achieve cost competitiveness with conventional SMR hydrogen when carbon pricing mechanisms are implemented. Current production costs for pyrolysis hydrogen range between $1.50-2.50/kg, compared to $1.00-1.80/kg for SMR hydrogen without carbon capture. This gap is expected to narrow as technology matures and economies of scale are realized.
Regional market assessment shows particularly strong potential in natural gas-rich regions seeking to decarbonize their energy systems while leveraging existing resources. North America, the Middle East, and Russia represent prime markets where methane pyrolysis could enable continued utilization of natural gas resources while meeting increasingly stringent carbon reduction targets.
Investment trends further validate market interest, with venture capital funding for methane pyrolysis technologies increasing by over 300% in the past five years. Major energy companies are also making strategic investments, recognizing the potential for methane pyrolysis to transform their natural gas assets into clean hydrogen production facilities while generating valuable solid carbon byproducts.
Consumer demand for low-carbon products is creating additional market pull, with industries seeking to reduce their carbon footprint through clean hydrogen adoption. This trend is particularly evident in hard-to-abate sectors like steel production, heavy transport, and chemical manufacturing, where electrification presents significant challenges.
Current Methane Pyrolysis Technologies and Barriers
Methane pyrolysis represents a significant technological approach for natural gas utilization, offering a pathway to produce hydrogen while capturing solid carbon instead of generating CO2 emissions. Currently, several technological approaches dominate the methane pyrolysis landscape, each with distinct advantages and limitations.
Thermal pyrolysis methods operate at temperatures exceeding 1000°C, utilizing various heat sources to break down methane molecules. These include plasma-based systems that use electrical discharges to create high-temperature environments, and direct heating methods employing furnaces or concentrated solar energy. While these approaches achieve reasonable conversion rates, they face challenges related to energy efficiency and carbon fouling of reactor components.
Catalytic pyrolysis technologies operate at lower temperatures (700-900°C) by employing metal catalysts such as nickel, iron, or cobalt to reduce activation energy requirements. These systems demonstrate improved energy efficiency but struggle with catalyst deactivation due to carbon deposition. Recent innovations include fluidized bed reactors with continuous catalyst regeneration, though scaling these systems remains challenging.
Molten metal reactors represent another promising approach, using liquid metals like tin or bismuth as both heat transfer media and reaction surfaces. Companies like Monolith Materials and BASF have made significant progress with this technology, achieving stable operation at pilot scales. However, these systems face materials challenges related to high-temperature corrosion and metal loss during carbon separation processes.
Microwave-assisted pyrolysis has emerged as a novel technique, using electromagnetic radiation to selectively heat carbon-containing materials. This approach offers rapid heating and potentially lower energy requirements but faces uniformity issues in larger reactors and challenges in microwave delivery system design.
Despite technological advances, several barriers impede widespread commercial adoption of methane pyrolysis. Energy intensity remains a primary concern, with most processes requiring substantial thermal input that impacts overall efficiency and economics. Reactor design challenges persist, particularly regarding carbon handling and removal without disrupting continuous operation.
Scale-up represents another significant hurdle, as laboratory successes often encounter unforeseen complications when transitioning to industrial scales. Material limitations also constrain development, with few materials capable of withstanding the extreme conditions while maintaining structural integrity over commercially viable timeframes.
Economic barriers further complicate adoption, as the capital expenditure for pyrolysis facilities remains high compared to conventional steam methane reforming. Additionally, market uncertainties regarding solid carbon valorization create financial risk, as the commercial value of pyrolytic carbon varies significantly depending on its structural properties and purity.
Thermal pyrolysis methods operate at temperatures exceeding 1000°C, utilizing various heat sources to break down methane molecules. These include plasma-based systems that use electrical discharges to create high-temperature environments, and direct heating methods employing furnaces or concentrated solar energy. While these approaches achieve reasonable conversion rates, they face challenges related to energy efficiency and carbon fouling of reactor components.
Catalytic pyrolysis technologies operate at lower temperatures (700-900°C) by employing metal catalysts such as nickel, iron, or cobalt to reduce activation energy requirements. These systems demonstrate improved energy efficiency but struggle with catalyst deactivation due to carbon deposition. Recent innovations include fluidized bed reactors with continuous catalyst regeneration, though scaling these systems remains challenging.
Molten metal reactors represent another promising approach, using liquid metals like tin or bismuth as both heat transfer media and reaction surfaces. Companies like Monolith Materials and BASF have made significant progress with this technology, achieving stable operation at pilot scales. However, these systems face materials challenges related to high-temperature corrosion and metal loss during carbon separation processes.
Microwave-assisted pyrolysis has emerged as a novel technique, using electromagnetic radiation to selectively heat carbon-containing materials. This approach offers rapid heating and potentially lower energy requirements but faces uniformity issues in larger reactors and challenges in microwave delivery system design.
Despite technological advances, several barriers impede widespread commercial adoption of methane pyrolysis. Energy intensity remains a primary concern, with most processes requiring substantial thermal input that impacts overall efficiency and economics. Reactor design challenges persist, particularly regarding carbon handling and removal without disrupting continuous operation.
Scale-up represents another significant hurdle, as laboratory successes often encounter unforeseen complications when transitioning to industrial scales. Material limitations also constrain development, with few materials capable of withstanding the extreme conditions while maintaining structural integrity over commercially viable timeframes.
Economic barriers further complicate adoption, as the capital expenditure for pyrolysis facilities remains high compared to conventional steam methane reforming. Additionally, market uncertainties regarding solid carbon valorization create financial risk, as the commercial value of pyrolytic carbon varies significantly depending on its structural properties and purity.
Current Industrial Implementation Approaches
01 Catalytic methane pyrolysis processes
Catalytic processes for methane pyrolysis involve the use of specific catalysts to enhance the decomposition of methane into hydrogen and solid carbon. These catalysts typically include transition metals, metal oxides, or supported metal systems that lower the activation energy required for breaking the C-H bonds in methane. The catalytic approach allows for operation at lower temperatures compared to thermal pyrolysis, improving energy efficiency and reducing operational costs. Various reactor designs have been developed to optimize catalyst contact time and prevent catalyst deactivation from carbon deposition.- Catalytic methane pyrolysis processes: Catalytic processes for methane pyrolysis involve the use of specific catalysts to enhance the decomposition of methane into hydrogen and solid carbon. These catalysts typically include transition metals, metal oxides, or supported metal systems that lower the activation energy required for breaking carbon-hydrogen bonds. The catalytic approach allows for operation at lower temperatures compared to thermal pyrolysis, improving energy efficiency and controlling the morphology of the carbon products formed.
- Reactor designs for methane pyrolysis: Various reactor designs have been developed to optimize methane pyrolysis processes. These include fluidized bed reactors, molten metal reactors, plasma reactors, and fixed bed systems. Each design addresses specific challenges such as carbon separation, heat transfer efficiency, and continuous operation. Advanced reactor configurations incorporate features for improved temperature control, extended catalyst lifetime, and efficient removal of solid carbon byproducts to prevent reactor fouling.
- Carbon material production from methane pyrolysis: Methane pyrolysis produces valuable carbon materials as byproducts, including carbon black, carbon nanotubes, graphene, and other structured carbon forms. The morphology and properties of these carbon materials can be controlled through process parameters such as temperature, pressure, residence time, and catalyst selection. These carbon products have applications in various industries including rubber manufacturing, electronics, composite materials, and energy storage systems.
- Hydrogen production via methane pyrolysis: Methane pyrolysis offers a pathway for producing hydrogen with significantly lower carbon emissions compared to conventional steam methane reforming. The process directly converts methane into hydrogen and solid carbon, avoiding CO2 emissions. This approach is considered a promising technology for clean hydrogen production, particularly when integrated with renewable energy sources. The hydrogen produced can be used in fuel cells, as industrial feedstock, or for energy storage applications.
- Process integration and energy efficiency improvements: Innovations in methane pyrolysis focus on improving overall process efficiency through heat recovery systems, process integration, and coupling with other technologies. These approaches include utilizing waste heat, integrating with renewable energy sources, optimizing reaction conditions, and developing hybrid systems that combine pyrolysis with other conversion technologies. Advanced control systems and process intensification techniques are employed to reduce energy consumption and improve the economic viability of methane pyrolysis.
02 Thermal methane pyrolysis technologies
Thermal methane pyrolysis involves the direct decomposition of methane into hydrogen and carbon at high temperatures (typically 800-1200°C) without catalysts. This approach requires significant energy input but avoids catalyst-related issues such as deactivation and regeneration. Various reactor designs have been developed for thermal pyrolysis, including plasma reactors, molten metal reactors, and fluidized bed systems. These technologies focus on maximizing methane conversion while managing the heat transfer requirements and carbon separation challenges inherent to high-temperature operations.Expand Specific Solutions03 Carbon material recovery and utilization
Methods for recovering and utilizing the solid carbon byproduct from methane pyrolysis processes are essential for process economics and environmental sustainability. These approaches include techniques for separating carbon from reactor systems, purifying the carbon product, and processing it into valuable forms such as carbon black, graphite, carbon nanotubes, or activated carbon. The recovered carbon materials can be used in various applications including rubber reinforcement, electronics, construction materials, and environmental remediation, transforming what could be a waste product into a valuable resource.Expand Specific Solutions04 Reactor designs for methane pyrolysis
Specialized reactor designs for methane pyrolysis address challenges such as carbon handling, heat management, and continuous operation. These include molten metal or molten salt reactors where carbon floats to the surface for easy removal, fluidized bed reactors that maintain catalyst activity, plasma-assisted reactors that achieve high temperatures in localized zones, and membrane reactors that allow for continuous hydrogen separation. Advanced designs incorporate features for heat recovery, continuous carbon removal, and integration with downstream processes to improve overall system efficiency and economics.Expand Specific Solutions05 Hydrogen purification and system integration
Technologies for purifying the hydrogen product from methane pyrolysis and integrating pyrolysis systems into broader energy or chemical production facilities are critical for commercial viability. Purification methods include pressure swing adsorption, membrane separation, and cryogenic techniques to achieve the desired hydrogen purity for downstream applications. System integration approaches focus on heat recovery, process intensification, and coupling with renewable energy sources to improve energy efficiency. These integrated systems can connect methane pyrolysis with hydrogen utilization pathways such as fuel cells, ammonia production, or direct use as a clean fuel.Expand Specific Solutions
Leading Companies and Research Institutions in Pyrolysis
Methane pyrolysis is emerging as a transformative technology in the natural gas industry, currently in its early growth phase with expanding market potential. The competitive landscape is characterized by a mix of established energy giants (ConocoPhillips, Phillips 66, TotalEnergies, Sinopec) and specialized technology providers (UOP LLC, BASF, GTI Energy). Research institutions, particularly in China (Dalian Institute of Chemical Physics, Zhejiang University), are driving technological innovation alongside corporate R&D centers. While methane pyrolysis technology remains in development stages with varying degrees of commercial readiness, significant investments from major players indicate growing industry confidence in its potential to produce clean hydrogen while capturing solid carbon, positioning it as a strategic technology for natural gas industry decarbonization.
UOP LLC
Technical Solution: UOP LLC (a Honeywell company) has developed a proprietary methane pyrolysis technology called "TurboThermal Pyrolysis" that utilizes a novel rotating reactor design to enhance heat transfer and reaction kinetics. Their system operates at moderate temperatures (800-950°C) and employs specialized iron-based catalysts that promote carbon formation while maintaining catalyst activity through continuous mechanical agitation. The technology achieves methane conversion rates of 75-85% with hydrogen yields approaching theoretical limits. UOP's process incorporates a patented carbon separation system that continuously removes solid carbon from the reactor, preventing catalyst deactivation and system fouling. The technology has been demonstrated at pilot scale (processing 200-300 kg/day of natural gas) with plans for commercial deployment. A key innovation is their integrated heat management system that utilizes the exothermic carbon formation reactions to partially sustain the process temperature, reducing external energy requirements by up to 25% compared to conventional pyrolysis approaches.
Strengths: Continuous operation with minimal downtime; produces high-purity hydrogen suitable for fuel cells; scalable design applicable to both centralized and distributed production. Weaknesses: Mechanical complexity increases maintenance requirements; moderate carbon quality limits high-value applications; catalyst life remains a challenge under commercial operating conditions.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed an advanced methane pyrolysis technology that utilizes a molten metal catalyst system, primarily based on nickel-bismuth alloys. Their process operates at temperatures between 900-1100°C, enabling direct conversion of methane to hydrogen and solid carbon. The technology employs a fluidized bed reactor design that enhances heat transfer and catalyst contact efficiency, achieving conversion rates of up to 85% with hydrogen purity exceeding 95%. Sinopec has implemented this technology at pilot scale facilities processing 50-100 kg/hour of natural gas, demonstrating significant reduction in CO2 emissions compared to traditional steam methane reforming. Their system incorporates continuous carbon removal mechanisms that prevent catalyst deactivation and ensure sustained operation for extended periods without significant performance degradation.
Strengths: Produces zero-CO2 hydrogen with valuable carbon byproducts; eliminates need for carbon capture infrastructure; lower energy requirements than electrolysis. Weaknesses: High operating temperatures require significant energy input; molten metal handling presents safety and maintenance challenges; technology still at early commercial scale with limited long-term operational data.
Key Patents and Scientific Breakthroughs in Methane Pyrolysis
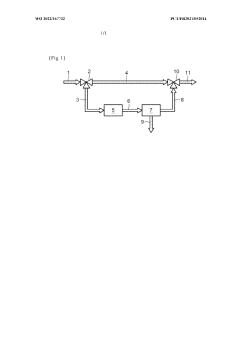
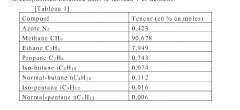
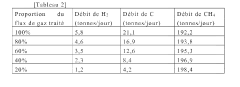
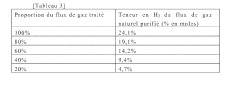
Method for purifying a flow of natural gas
PatentWO2022167732A1
Innovation
- A method involving pyrolysis of natural gas at 1000° C to 2000° C to decompose C2+ hydrocarbons into elemental carbon and dihydrogen, followed by carbon removal, and mixing the treated stream with untreated gas to increase methane content and produce dihydrogen, ensuring a high methane index and reduced emissions.
Method for operating a cracking process
PatentWO2024115488A1
Innovation
- A method utilizing a moving bed hydrocarbon pyrolysis process to convert cracker by-product streams into hydrogen, which is then used to provide thermal energy for the cracking process, reducing reliance on fossil fuels and minimizing CO2 emissions by using hydrogen combustion that produces only water as a by-product.
Carbon Management and Solid Carbon Valorization
Methane pyrolysis generates solid carbon as a primary byproduct, creating significant opportunities for carbon management and valorization strategies within natural gas industries. The produced solid carbon exists in various forms including carbon black, graphite, and carbon nanotubes, each offering distinct commercial applications and value propositions.
Carbon black derived from methane pyrolysis can be utilized in rubber manufacturing, particularly for tire production, where it enhances durability and performance characteristics. The electronics industry benefits from high-purity graphite applications in battery technologies, with potential integration into next-generation energy storage systems. Carbon nanotubes represent the highest-value carbon product, commanding premium prices in specialized markets for composite materials and advanced electronics.
The economic viability of methane pyrolysis significantly improves when considering the revenue potential from these solid carbon products. Market analyses indicate that carbon black currently trades at $800-1,500 per ton, while specialized carbon nanomaterials can reach values exceeding $100,000 per ton in certain applications. This value creation transforms what would otherwise be considered waste into valuable commodities, fundamentally altering the economics of hydrogen production from natural gas.
Effective carbon management strategies require developing robust supply chains and market connections between pyrolysis operators and carbon-consuming industries. Several natural gas companies have initiated partnerships with materials manufacturers to establish reliable offtake agreements for solid carbon products. These arrangements provide revenue certainty that supports investment in pyrolysis infrastructure while ensuring consistent material quality for end users.
Research and development efforts are increasingly focused on tailoring pyrolysis processes to produce specific carbon morphologies with enhanced properties for target applications. Temperature control, catalyst selection, and reactor design modifications allow operators to optimize carbon characteristics including particle size, surface area, and crystallinity. This customization capability represents a significant competitive advantage compared to conventional carbon black production methods.
Environmental certification systems for pyrolysis-derived carbon are emerging as important market differentiators. Carbon products generated through methane pyrolysis carry substantially lower environmental footprints than traditional carbon black manufacturing, which typically relies on incomplete combustion of fossil fuels. This environmental premium creates marketing opportunities in sustainability-focused sectors and potentially qualifies products for regulatory incentives in jurisdictions with carbon pricing mechanisms.
Carbon black derived from methane pyrolysis can be utilized in rubber manufacturing, particularly for tire production, where it enhances durability and performance characteristics. The electronics industry benefits from high-purity graphite applications in battery technologies, with potential integration into next-generation energy storage systems. Carbon nanotubes represent the highest-value carbon product, commanding premium prices in specialized markets for composite materials and advanced electronics.
The economic viability of methane pyrolysis significantly improves when considering the revenue potential from these solid carbon products. Market analyses indicate that carbon black currently trades at $800-1,500 per ton, while specialized carbon nanomaterials can reach values exceeding $100,000 per ton in certain applications. This value creation transforms what would otherwise be considered waste into valuable commodities, fundamentally altering the economics of hydrogen production from natural gas.
Effective carbon management strategies require developing robust supply chains and market connections between pyrolysis operators and carbon-consuming industries. Several natural gas companies have initiated partnerships with materials manufacturers to establish reliable offtake agreements for solid carbon products. These arrangements provide revenue certainty that supports investment in pyrolysis infrastructure while ensuring consistent material quality for end users.
Research and development efforts are increasingly focused on tailoring pyrolysis processes to produce specific carbon morphologies with enhanced properties for target applications. Temperature control, catalyst selection, and reactor design modifications allow operators to optimize carbon characteristics including particle size, surface area, and crystallinity. This customization capability represents a significant competitive advantage compared to conventional carbon black production methods.
Environmental certification systems for pyrolysis-derived carbon are emerging as important market differentiators. Carbon products generated through methane pyrolysis carry substantially lower environmental footprints than traditional carbon black manufacturing, which typically relies on incomplete combustion of fossil fuels. This environmental premium creates marketing opportunities in sustainability-focused sectors and potentially qualifies products for regulatory incentives in jurisdictions with carbon pricing mechanisms.
Economic Viability and Integration with Existing Infrastructure
The economic viability of methane pyrolysis represents a critical factor in determining its adoption within the natural gas industry. Current cost analyses indicate that methane pyrolysis processes require significant initial capital investment, ranging from $500-800 million for industrial-scale facilities. However, the operational costs can be offset by the production of valuable solid carbon, which commands market prices between $1,000-2,500 per ton depending on quality and purity levels.
Integration with existing natural gas infrastructure presents both opportunities and challenges. The advantage of methane pyrolysis lies in its compatibility with current natural gas transmission networks, requiring minimal modifications to pipeline systems for hydrogen blending up to certain percentages (typically 5-20% by volume without major infrastructure changes). This allows for gradual transition pathways rather than wholesale infrastructure replacement.
Financial modeling suggests that methane pyrolysis becomes economically competitive with steam methane reforming when carbon prices exceed $50-60 per ton or when hydrogen production scales reach approximately 50,000 tons annually. The break-even timeline for most facilities ranges between 7-12 years, depending on regional energy prices, carbon valuation, and regulatory frameworks.
Existing natural gas companies can leverage their established assets by repurposing processing facilities for methane pyrolysis operations. Studies indicate that approximately 60-70% of current natural gas processing equipment can be adapted or partially utilized in pyrolysis operations, significantly reducing transition costs compared to building entirely new facilities.
Grid integration considerations reveal that methane pyrolysis offers flexibility advantages over electrolytic hydrogen production, as it can operate continuously independent of renewable energy availability fluctuations. This provides stability benefits for energy systems while still producing low-carbon hydrogen with 80-90% fewer emissions than conventional methods when powered by renewable electricity sources.
Regional economic analyses demonstrate varying implementation potentials, with natural gas-rich regions like the United States, Russia, and Qatar showing the most favorable economics for large-scale adoption. The modularity of newer pyrolysis technologies also enables scalable implementation, allowing smaller gas producers to adopt the technology incrementally as economics improve and technology matures.
Integration with existing natural gas infrastructure presents both opportunities and challenges. The advantage of methane pyrolysis lies in its compatibility with current natural gas transmission networks, requiring minimal modifications to pipeline systems for hydrogen blending up to certain percentages (typically 5-20% by volume without major infrastructure changes). This allows for gradual transition pathways rather than wholesale infrastructure replacement.
Financial modeling suggests that methane pyrolysis becomes economically competitive with steam methane reforming when carbon prices exceed $50-60 per ton or when hydrogen production scales reach approximately 50,000 tons annually. The break-even timeline for most facilities ranges between 7-12 years, depending on regional energy prices, carbon valuation, and regulatory frameworks.
Existing natural gas companies can leverage their established assets by repurposing processing facilities for methane pyrolysis operations. Studies indicate that approximately 60-70% of current natural gas processing equipment can be adapted or partially utilized in pyrolysis operations, significantly reducing transition costs compared to building entirely new facilities.
Grid integration considerations reveal that methane pyrolysis offers flexibility advantages over electrolytic hydrogen production, as it can operate continuously independent of renewable energy availability fluctuations. This provides stability benefits for energy systems while still producing low-carbon hydrogen with 80-90% fewer emissions than conventional methods when powered by renewable electricity sources.
Regional economic analyses demonstrate varying implementation potentials, with natural gas-rich regions like the United States, Russia, and Qatar showing the most favorable economics for large-scale adoption. The modularity of newer pyrolysis technologies also enables scalable implementation, allowing smaller gas producers to adopt the technology incrementally as economics improve and technology matures.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!