Integration of Methane Pyrolysis in Petrochemical Industries.
SEP 5, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Methane Pyrolysis Evolution and Objectives
Methane pyrolysis represents a transformative approach to hydrogen production that has evolved significantly over the past decades. The process involves the thermal decomposition of methane (CH4) into hydrogen (H2) and solid carbon in the absence of oxygen, offering a potentially low-carbon alternative to traditional steam methane reforming. The evolution of this technology can be traced back to the early 20th century, but significant advancements have accelerated since the 1990s with the development of more efficient catalysts and reactor designs.
The historical trajectory of methane pyrolysis has been shaped by increasing environmental concerns and the growing demand for cleaner energy sources. Initially developed as a chemical process for carbon black production, its potential for hydrogen generation has gained prominence in recent years as global efforts to decarbonize intensify. The technology has evolved from basic thermal decomposition methods to more sophisticated approaches including catalytic, plasma-assisted, and molten metal pyrolysis techniques.
Current research objectives in methane pyrolysis integration within petrochemical industries focus on several key areas. Primary among these is enhancing process efficiency to reduce energy requirements, as traditional pyrolysis demands high temperatures (800-1200°C). Researchers aim to develop advanced catalysts that can lower activation energy and improve reaction kinetics while maintaining long-term stability under industrial conditions.
Another critical objective is scaling the technology for commercial viability. While laboratory demonstrations have shown promise, industrial implementation requires robust reactor designs capable of continuous operation with minimal maintenance. This includes addressing challenges related to carbon management, as the solid carbon byproduct can cause reactor fouling if not properly handled.
Integration with existing petrochemical infrastructure represents a significant technical goal. The ability to retrofit current facilities rather than building entirely new plants would substantially reduce implementation costs and accelerate adoption. This requires compatible process conditions and careful consideration of heat integration opportunities within existing plant configurations.
Economic viability remains a central objective, with research focused on reducing capital and operational expenditures to make methane pyrolysis competitive with conventional hydrogen production methods. This includes exploring value-added applications for the solid carbon byproduct, which could potentially offset production costs and enhance the overall economics of the process.
Environmental performance objectives include minimizing greenhouse gas emissions across the entire production chain and ensuring that the carbon byproduct can be permanently sequestered or utilized in environmentally beneficial applications. The ultimate goal is to develop a truly low-carbon or carbon-negative hydrogen production pathway that can contribute meaningfully to climate change mitigation efforts while meeting the growing hydrogen demand in petrochemical and other industries.
The historical trajectory of methane pyrolysis has been shaped by increasing environmental concerns and the growing demand for cleaner energy sources. Initially developed as a chemical process for carbon black production, its potential for hydrogen generation has gained prominence in recent years as global efforts to decarbonize intensify. The technology has evolved from basic thermal decomposition methods to more sophisticated approaches including catalytic, plasma-assisted, and molten metal pyrolysis techniques.
Current research objectives in methane pyrolysis integration within petrochemical industries focus on several key areas. Primary among these is enhancing process efficiency to reduce energy requirements, as traditional pyrolysis demands high temperatures (800-1200°C). Researchers aim to develop advanced catalysts that can lower activation energy and improve reaction kinetics while maintaining long-term stability under industrial conditions.
Another critical objective is scaling the technology for commercial viability. While laboratory demonstrations have shown promise, industrial implementation requires robust reactor designs capable of continuous operation with minimal maintenance. This includes addressing challenges related to carbon management, as the solid carbon byproduct can cause reactor fouling if not properly handled.
Integration with existing petrochemical infrastructure represents a significant technical goal. The ability to retrofit current facilities rather than building entirely new plants would substantially reduce implementation costs and accelerate adoption. This requires compatible process conditions and careful consideration of heat integration opportunities within existing plant configurations.
Economic viability remains a central objective, with research focused on reducing capital and operational expenditures to make methane pyrolysis competitive with conventional hydrogen production methods. This includes exploring value-added applications for the solid carbon byproduct, which could potentially offset production costs and enhance the overall economics of the process.
Environmental performance objectives include minimizing greenhouse gas emissions across the entire production chain and ensuring that the carbon byproduct can be permanently sequestered or utilized in environmentally beneficial applications. The ultimate goal is to develop a truly low-carbon or carbon-negative hydrogen production pathway that can contribute meaningfully to climate change mitigation efforts while meeting the growing hydrogen demand in petrochemical and other industries.
Market Demand for Clean Hydrogen Production
The global hydrogen market is experiencing a significant shift towards cleaner production methods, with demand for low-carbon hydrogen projected to reach 660 million tons by 2050, according to the Hydrogen Council. Traditional hydrogen production methods, primarily steam methane reforming (SMR), account for approximately 95% of current hydrogen production but generate substantial CO2 emissions—about 10 tons of CO2 per ton of hydrogen produced. This environmental impact has created an urgent market need for cleaner alternatives.
Methane pyrolysis represents a promising solution within this evolving market landscape. The process produces "turquoise hydrogen" with significantly lower carbon emissions than conventional methods, positioning it as a viable bridge between gray hydrogen (from SMR) and green hydrogen (from electrolysis). Market analysis indicates that petrochemical industries are particularly well-positioned to benefit from methane pyrolysis integration due to their existing natural gas infrastructure and carbon black utilization capabilities.
The demand drivers for clean hydrogen production through methane pyrolysis are multifaceted. Industrial decarbonization initiatives, particularly in hard-to-abate sectors like steel manufacturing, ammonia production, and refining operations, are creating substantial pull for low-carbon hydrogen solutions. These industries collectively represent over 90% of current hydrogen consumption and face increasing regulatory pressure to reduce emissions.
Policy frameworks worldwide are accelerating market development. The European Union's Hydrogen Strategy targets 40GW of clean hydrogen production capacity by 2030, while the US Inflation Reduction Act provides production tax credits of up to $3 per kilogram for clean hydrogen. These incentives are reshaping investment decisions and accelerating technology adoption timelines for methane pyrolysis systems.
Market segmentation analysis reveals that early adoption is concentrated in regions with robust natural gas infrastructure and stringent carbon regulations. Germany, the Netherlands, and parts of North America show particularly strong market potential, with several commercial-scale demonstration projects already underway. The solid carbon byproduct from methane pyrolysis—high-quality carbon black—represents an additional value stream, with growing markets in rubber manufacturing, plastics, and advanced materials.
Competitive analysis indicates that petrochemical companies integrating methane pyrolysis can achieve a 40-80% reduction in carbon emissions compared to conventional hydrogen production methods, while maintaining cost competitiveness, especially in regions with carbon pricing mechanisms. This dual advantage of environmental performance and economic viability is driving increased interest from both industry stakeholders and investors seeking sustainable technology solutions.
Methane pyrolysis represents a promising solution within this evolving market landscape. The process produces "turquoise hydrogen" with significantly lower carbon emissions than conventional methods, positioning it as a viable bridge between gray hydrogen (from SMR) and green hydrogen (from electrolysis). Market analysis indicates that petrochemical industries are particularly well-positioned to benefit from methane pyrolysis integration due to their existing natural gas infrastructure and carbon black utilization capabilities.
The demand drivers for clean hydrogen production through methane pyrolysis are multifaceted. Industrial decarbonization initiatives, particularly in hard-to-abate sectors like steel manufacturing, ammonia production, and refining operations, are creating substantial pull for low-carbon hydrogen solutions. These industries collectively represent over 90% of current hydrogen consumption and face increasing regulatory pressure to reduce emissions.
Policy frameworks worldwide are accelerating market development. The European Union's Hydrogen Strategy targets 40GW of clean hydrogen production capacity by 2030, while the US Inflation Reduction Act provides production tax credits of up to $3 per kilogram for clean hydrogen. These incentives are reshaping investment decisions and accelerating technology adoption timelines for methane pyrolysis systems.
Market segmentation analysis reveals that early adoption is concentrated in regions with robust natural gas infrastructure and stringent carbon regulations. Germany, the Netherlands, and parts of North America show particularly strong market potential, with several commercial-scale demonstration projects already underway. The solid carbon byproduct from methane pyrolysis—high-quality carbon black—represents an additional value stream, with growing markets in rubber manufacturing, plastics, and advanced materials.
Competitive analysis indicates that petrochemical companies integrating methane pyrolysis can achieve a 40-80% reduction in carbon emissions compared to conventional hydrogen production methods, while maintaining cost competitiveness, especially in regions with carbon pricing mechanisms. This dual advantage of environmental performance and economic viability is driving increased interest from both industry stakeholders and investors seeking sustainable technology solutions.
Technical Barriers and Global Development Status
Despite significant advancements in methane pyrolysis technology, several technical barriers continue to impede its widespread integration into petrochemical industries. The primary challenge remains the high energy requirement for breaking the strong C-H bonds in methane molecules, necessitating temperatures exceeding 700°C. This energy intensity significantly impacts the economic viability of industrial-scale implementation, particularly when competing with established steam methane reforming processes.
Catalyst deactivation presents another substantial hurdle. Carbon deposition on catalyst surfaces leads to rapid performance degradation, requiring frequent regeneration or replacement cycles that increase operational costs and downtime. While molten metal catalysts show promise in mitigating this issue, they introduce additional complexities in reactor design and material selection due to their corrosive nature and high operating temperatures.
Reactor engineering challenges persist across all methane pyrolysis approaches. Maintaining uniform temperature distribution, ensuring efficient heat transfer, and managing carbon separation in continuous operation remain problematic. Particularly in molten metal systems, the handling of solid carbon in liquid media presents unique engineering difficulties that have yet to be fully resolved at commercial scale.
Globally, methane pyrolysis development exhibits significant regional variations. Europe leads in research initiatives, with Germany's BASF and the Karlsruhe Institute of Technology pioneering molten metal catalyst technologies. Their 50 kg/h demonstration plant represents one of the most advanced implementations to date. North America follows closely, with companies like Monolith Materials operating a commercial-scale facility in Nebraska that produces carbon black and hydrogen through methane pyrolysis.
In Asia, Japan and South Korea are making notable progress in plasma-assisted pyrolysis methods, while China focuses on integrating pyrolysis with existing coal gasification infrastructure. Australia has emerged as a potential leader in hydrogen export strategies based on methane pyrolysis, with several pilot projects under development in collaboration with Japanese energy companies.
The technology readiness level (TRL) varies significantly across different pyrolysis approaches. Thermal pyrolysis methods have reached TRL 7-8 with demonstration plants in operation, while catalytic approaches generally remain at TRL 5-6 with pilot-scale testing. Plasma-assisted methods lag slightly at TRL 4-5, still requiring significant development before commercial viability.
Recent breakthroughs in novel structured catalysts and reactor designs suggest pathways to overcome current limitations, but substantial engineering challenges remain before methane pyrolysis can achieve widespread commercial adoption in petrochemical industries.
Catalyst deactivation presents another substantial hurdle. Carbon deposition on catalyst surfaces leads to rapid performance degradation, requiring frequent regeneration or replacement cycles that increase operational costs and downtime. While molten metal catalysts show promise in mitigating this issue, they introduce additional complexities in reactor design and material selection due to their corrosive nature and high operating temperatures.
Reactor engineering challenges persist across all methane pyrolysis approaches. Maintaining uniform temperature distribution, ensuring efficient heat transfer, and managing carbon separation in continuous operation remain problematic. Particularly in molten metal systems, the handling of solid carbon in liquid media presents unique engineering difficulties that have yet to be fully resolved at commercial scale.
Globally, methane pyrolysis development exhibits significant regional variations. Europe leads in research initiatives, with Germany's BASF and the Karlsruhe Institute of Technology pioneering molten metal catalyst technologies. Their 50 kg/h demonstration plant represents one of the most advanced implementations to date. North America follows closely, with companies like Monolith Materials operating a commercial-scale facility in Nebraska that produces carbon black and hydrogen through methane pyrolysis.
In Asia, Japan and South Korea are making notable progress in plasma-assisted pyrolysis methods, while China focuses on integrating pyrolysis with existing coal gasification infrastructure. Australia has emerged as a potential leader in hydrogen export strategies based on methane pyrolysis, with several pilot projects under development in collaboration with Japanese energy companies.
The technology readiness level (TRL) varies significantly across different pyrolysis approaches. Thermal pyrolysis methods have reached TRL 7-8 with demonstration plants in operation, while catalytic approaches generally remain at TRL 5-6 with pilot-scale testing. Plasma-assisted methods lag slightly at TRL 4-5, still requiring significant development before commercial viability.
Recent breakthroughs in novel structured catalysts and reactor designs suggest pathways to overcome current limitations, but substantial engineering challenges remain before methane pyrolysis can achieve widespread commercial adoption in petrochemical industries.
Current Methane Pyrolysis Integration Solutions
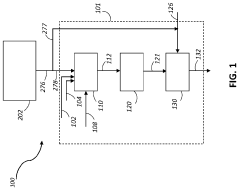
01 Catalytic methane pyrolysis processes
Catalytic processes for methane pyrolysis involve the use of specific catalysts to enhance the decomposition of methane into hydrogen and solid carbon. These catalysts typically include transition metals, metal oxides, or supported metal systems that lower the activation energy required for breaking carbon-hydrogen bonds. The catalytic approach allows for operation at lower temperatures compared to thermal pyrolysis, improving energy efficiency and controlling the morphology of the carbon products formed during the reaction.- Catalytic methane pyrolysis processes: Catalytic processes for methane pyrolysis involve the use of specific catalysts to enhance the decomposition of methane into hydrogen and solid carbon. These catalysts typically include transition metals, metal oxides, or supported metal systems that lower the activation energy required for breaking the C-H bonds in methane. The catalytic approach allows for operation at lower temperatures compared to thermal pyrolysis, typically in the range of 500-900°C, which improves energy efficiency and reduces operational costs. The selection of appropriate catalysts can significantly influence the reaction kinetics, product selectivity, and carbon morphology.
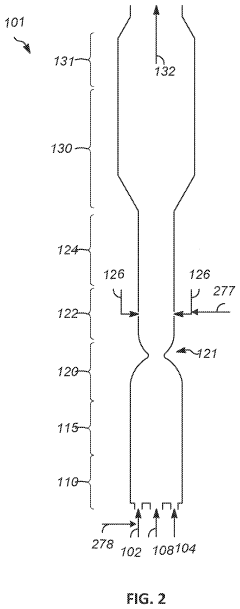
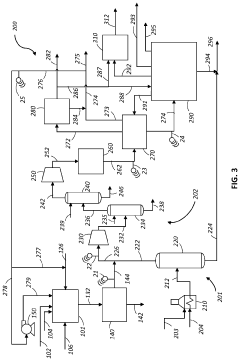
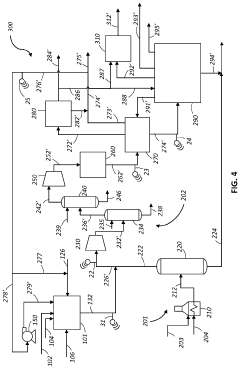
- Reactor designs for methane pyrolysis: Various reactor designs have been developed for methane pyrolysis, each with specific advantages for different applications. These include fluidized bed reactors, which provide excellent heat transfer and continuous carbon removal; molten metal reactors, where liquid metals serve as both heat transfer medium and catalyst; fixed bed reactors for catalyst-based systems; and plasma reactors for high-temperature, catalyst-free decomposition. Advanced reactor designs incorporate features for continuous carbon removal to prevent reactor fouling, efficient heat management systems to optimize energy use, and integrated product separation units to enhance overall process efficiency.
- Hydrogen production through methane pyrolysis: Methane pyrolysis offers a promising route for hydrogen production with significantly lower carbon emissions compared to conventional steam methane reforming. The process directly converts methane into hydrogen and solid carbon, avoiding CO2 emissions associated with traditional hydrogen production methods. The hydrogen produced is of high purity and can be used in various applications including fuel cells, chemical synthesis, and as a clean energy carrier. The process efficiency can be enhanced through heat recovery systems, optimized reactor designs, and integration with renewable energy sources to provide the necessary heat for the endothermic reaction.
- Carbon material recovery and utilization: The solid carbon produced during methane pyrolysis can be recovered and utilized in various applications, adding economic value to the process. Depending on the process conditions and catalysts used, the carbon can be produced in different forms including carbon black, carbon nanotubes, graphene, or amorphous carbon. These carbon materials have applications in rubber reinforcement, pigments, electronics, construction materials, and advanced composites. The quality and properties of the carbon can be controlled by adjusting process parameters such as temperature, pressure, residence time, and catalyst selection, allowing for targeted production of specific carbon materials.
- Process integration and sustainability aspects: Methane pyrolysis can be integrated with other processes to enhance overall efficiency and sustainability. Integration opportunities include coupling with renewable energy sources for heat supply, combining with biogas upgrading systems to utilize biomethane, and incorporating carbon sequestration or utilization pathways. The process offers environmental benefits through reduced greenhouse gas emissions compared to conventional hydrogen production methods. Life cycle assessments indicate that methane pyrolysis can achieve significant carbon emission reductions, especially when renewable energy is used for process heating and when the produced carbon is permanently sequestered or used in long-lasting products.
02 Reactor designs for methane pyrolysis
Various reactor designs have been developed for methane pyrolysis, including fluidized bed reactors, molten metal reactors, and plasma reactors. These designs address challenges such as carbon separation, heat transfer efficiency, and continuous operation. Advanced reactor configurations incorporate features for improved residence time distribution, enhanced mixing, and effective carbon product removal, which collectively contribute to higher conversion rates and process stability during the pyrolysis of methane to produce hydrogen and carbon.Expand Specific Solutions03 Carbon product recovery and utilization
Methane pyrolysis produces valuable solid carbon products that can be recovered and utilized in various applications. The carbon products range from carbon black to graphitic materials and can be tailored by controlling pyrolysis conditions. Recovery methods include filtration, cyclone separation, and electrostatic precipitation. The recovered carbon can be used in applications such as rubber reinforcement, pigments, construction materials, and advanced materials for energy storage, providing additional economic value to the methane pyrolysis process beyond hydrogen production.Expand Specific Solutions04 Thermal and plasma-assisted pyrolysis techniques
Thermal pyrolysis of methane involves high-temperature decomposition without catalysts, typically requiring temperatures above 1000°C. Plasma-assisted pyrolysis uses electrical discharges to create highly reactive plasma environments that can break down methane at lower bulk temperatures. These techniques offer advantages in terms of carbon product purity and process simplicity, though they may have higher energy requirements. Recent innovations focus on improving energy efficiency through heat recovery systems and renewable energy integration to make these processes more economically viable and environmentally sustainable.Expand Specific Solutions05 Integration with hydrogen production systems
Methane pyrolysis is increasingly being integrated into comprehensive hydrogen production systems as a low-carbon hydrogen generation method. These integrated systems may combine pyrolysis with purification technologies, hydrogen storage solutions, and distribution infrastructure. The integration allows for efficient handling of the produced hydrogen while managing carbon byproducts. Some advanced systems also incorporate renewable energy sources to power the pyrolysis process, creating pathways for carbon-neutral or carbon-negative hydrogen production that can support decarbonization efforts across various industrial sectors.Expand Specific Solutions
Leading Companies and Competitive Landscape
The methane pyrolysis market in petrochemical industries is in an early growth phase, characterized by increasing R&D investments and pilot projects. The global market is expanding as companies seek carbon-neutral hydrogen production methods, with projections indicating significant growth potential. Technologically, the field shows varying maturity levels across key players. Industry leaders like SABIC, Saudi Aramco, and Sinopec are advancing commercial-scale implementations, while research institutions such as Dalian Institute of Chemical Physics and Taiyuan University of Technology are developing next-generation catalysts. Companies including Shell, ExxonMobil, and Haldor Topsøe are focusing on process optimization and scalability, with newer entrants like Hazer Group and Molten Industries introducing innovative approaches to methane decomposition technologies.
China Petroleum & Chemical Corp. (Sinopec)
Technical Solution: Sinopec has pioneered a methane pyrolysis process utilizing molten metal technology, specifically employing liquid nickel-bismuth alloys as both catalyst and heat transfer medium. Their system operates at temperatures of 800-1000°C and achieves methane conversion rates of up to 85% with hydrogen selectivity exceeding 95%. The molten metal bath facilitates efficient heat transfer while simultaneously separating the solid carbon that floats to the surface for continuous harvesting. Sinopec has implemented this technology at demonstration scale (processing 500 Nm³/h of natural gas) at their Qingdao petrochemical complex, where the hydrogen produced is directed to hydrodesulfurization units and other hydrogen-consuming processes. The company has also developed specialized carbon handling systems that allow the recovered carbon to be utilized in polymer reinforcement applications, creating additional value streams from what would otherwise be a waste product.
Strengths: High methane conversion efficiency; continuous carbon removal capability; integration with existing hydrogen-consuming units at refineries. Weaknesses: Energy-intensive process requiring significant heat input; complex molten metal handling systems; potential metal contamination in carbon product.
UOP LLC
Technical Solution: UOP has developed a proprietary methane pyrolysis technology called "ThermoCarb" that employs a moving bed reactor system with specialized metal-supported catalysts. Their process operates at moderate temperatures (650-850°C) and utilizes pressure swing to enhance methane conversion while minimizing energy requirements. The ThermoCarb system features a unique catalyst regeneration cycle that addresses carbon deposition issues, allowing for extended catalyst lifetime exceeding 2,000 hours. UOP's integrated approach connects methane pyrolysis with downstream petrochemical processes, enabling direct hydrogen utilization for hydroprocessing applications while capturing high-purity carbon nanostructures. The company has successfully demonstrated this technology at pilot scale, processing up to 200 kg/day of methane with hydrogen yields approaching theoretical limits. UOP's system design includes heat recovery mechanisms that reduce overall energy consumption by approximately 30% compared to conventional pyrolysis approaches.
Strengths: Lower operating temperature than competing technologies; extended catalyst lifetime through regeneration cycle; efficient heat integration with existing refinery operations. Weaknesses: More complex reactor design increases capital costs; requires specialized catalyst materials; carbon product quality can vary depending on operating conditions.
Key Patents and Technical Innovations
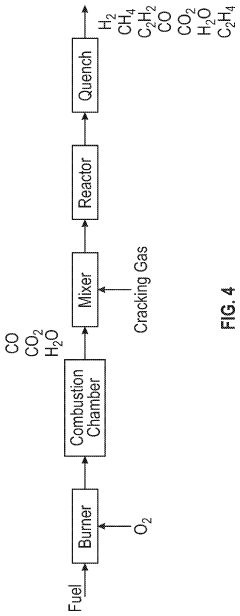
Integrated process for producing acetylene
PatentInactiveUS20220064083A1
Innovation
- An integrated process that recovers a fuel gas stream from a product recovery unit, combusts a fuel and oxidizer in a pyrolytic reactor to create a supersonic combustion gas stream, and injects a light hydrocarbon stream to produce a reaction mixture comprising acetylene, methane, and carbon oxides, which is then separated to maximize acetylene recovery and reduce waste, integrating with existing refinery processes to enhance hydrocarbon recovery and reduce costs.
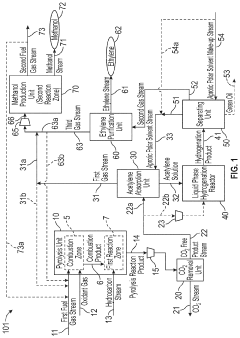
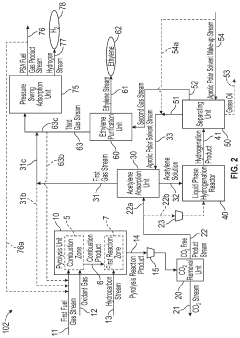
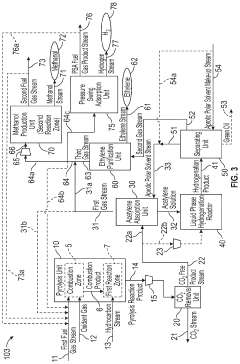
Novel Process Integration of Methane or Higher Hydrocarbon Pyrolysis Step to Produce Ethylene and Methanol and/or Hydrogen
PatentInactiveUS20200055731A1
Innovation
- A method integrating hydrocarbon pyrolysis with subsequent steps involving combustion, pyrolysis reactions, carbon dioxide removal, acetylene absorption, liquid phase hydrogenation, and methanol production, utilizing aprotic polar solvents and pressure swing adsorption to efficiently produce ethylene and methanol from natural gas and higher hydrocarbons.
Carbon Management and Utilization Strategies
Carbon management and utilization strategies represent a critical component in the integration of methane pyrolysis within petrochemical industries. The primary advantage of methane pyrolysis lies in its ability to produce hydrogen while generating solid carbon instead of CO2, creating significant opportunities for carbon management. This process yields high-value carbon materials that can be utilized across various industrial applications, transforming what would traditionally be considered emissions into valuable products.
The solid carbon byproduct from methane pyrolysis exists primarily as carbon black or graphitic carbon, depending on the specific process conditions. These materials have established markets in rubber manufacturing, pigments, plastics, and emerging applications in advanced materials. Petrochemical facilities implementing methane pyrolysis can develop integrated carbon utilization pathways that create additional revenue streams while simultaneously reducing the carbon footprint of operations.
Strategic carbon management approaches include the development of on-site carbon processing facilities that can convert raw carbon outputs into market-ready products. This vertical integration minimizes transportation costs and ensures quality control throughout the value chain. Some facilities have implemented carbon upgrading processes to produce higher-value forms such as graphene or carbon nanotubes, significantly increasing the economic returns from the pyrolysis process.
Carbon sequestration represents another viable management strategy, particularly for carbon outputs that cannot be immediately commercialized. Recent advancements in carbon stabilization techniques have made long-term storage increasingly feasible, with some petrochemical companies exploring underground storage in depleted natural gas reservoirs or dedicated geological formations.
Market development initiatives form a crucial component of successful carbon management strategies. Industry leaders have established partnerships with carbon-consuming industries to create stable demand channels for pyrolytic carbon. These collaborative approaches often include joint research programs to develop new applications for pyrolytic carbon, expanding potential markets beyond traditional uses.
Regulatory frameworks increasingly recognize the carbon benefits of methane pyrolysis, with several jurisdictions developing carbon credit mechanisms that reward the permanent sequestration or utilization of carbon. Forward-thinking petrochemical companies are positioning themselves to capitalize on these incentives by implementing comprehensive carbon tracking systems that document the fate of all carbon produced through pyrolysis operations.
The economic viability of carbon management strategies varies significantly based on regional factors, including local market demand for carbon products, transportation infrastructure, and regulatory environments. Successful implementation requires tailored approaches that consider these variables while maintaining flexibility to adapt to evolving market conditions and technological developments.
The solid carbon byproduct from methane pyrolysis exists primarily as carbon black or graphitic carbon, depending on the specific process conditions. These materials have established markets in rubber manufacturing, pigments, plastics, and emerging applications in advanced materials. Petrochemical facilities implementing methane pyrolysis can develop integrated carbon utilization pathways that create additional revenue streams while simultaneously reducing the carbon footprint of operations.
Strategic carbon management approaches include the development of on-site carbon processing facilities that can convert raw carbon outputs into market-ready products. This vertical integration minimizes transportation costs and ensures quality control throughout the value chain. Some facilities have implemented carbon upgrading processes to produce higher-value forms such as graphene or carbon nanotubes, significantly increasing the economic returns from the pyrolysis process.
Carbon sequestration represents another viable management strategy, particularly for carbon outputs that cannot be immediately commercialized. Recent advancements in carbon stabilization techniques have made long-term storage increasingly feasible, with some petrochemical companies exploring underground storage in depleted natural gas reservoirs or dedicated geological formations.
Market development initiatives form a crucial component of successful carbon management strategies. Industry leaders have established partnerships with carbon-consuming industries to create stable demand channels for pyrolytic carbon. These collaborative approaches often include joint research programs to develop new applications for pyrolytic carbon, expanding potential markets beyond traditional uses.
Regulatory frameworks increasingly recognize the carbon benefits of methane pyrolysis, with several jurisdictions developing carbon credit mechanisms that reward the permanent sequestration or utilization of carbon. Forward-thinking petrochemical companies are positioning themselves to capitalize on these incentives by implementing comprehensive carbon tracking systems that document the fate of all carbon produced through pyrolysis operations.
The economic viability of carbon management strategies varies significantly based on regional factors, including local market demand for carbon products, transportation infrastructure, and regulatory environments. Successful implementation requires tailored approaches that consider these variables while maintaining flexibility to adapt to evolving market conditions and technological developments.
Economic Feasibility and ROI Analysis
The economic feasibility of integrating methane pyrolysis into petrochemical industries hinges on several critical factors. Initial capital expenditure for methane pyrolysis facilities ranges from $50-150 million depending on scale, with operational costs primarily driven by energy consumption at approximately $300-500 per ton of hydrogen produced. This represents a significant investment that must be evaluated against potential returns.
Return on investment calculations indicate promising prospects, with payback periods typically ranging from 5-8 years under current market conditions. The economic model benefits substantially from the production of solid carbon as a valuable by-product, which can be sold at $1,000-2,500 per ton depending on purity and form. This dual-revenue stream significantly enhances the financial viability compared to traditional hydrogen production methods.
Sensitivity analysis reveals that methane pyrolysis economics are particularly responsive to natural gas prices, electricity costs, and carbon market valuations. A 10% reduction in energy costs can improve ROI by approximately 15-20%, highlighting the importance of energy efficiency in implementation strategies. The technology becomes increasingly competitive as carbon pricing mechanisms mature globally, with each $10/ton increase in carbon pricing improving annual returns by an estimated 8-12%.
Comparative cost analysis against conventional steam methane reforming (SMR) shows that while pyrolysis has higher upfront costs, it offers superior long-term economics when accounting for carbon pricing and emissions regulations. The levelized cost of hydrogen production via pyrolysis is estimated at $1.8-2.5/kg compared to $1.5-2.0/kg for SMR without carbon capture, but this gap narrows or reverses when environmental costs are internalized.
Regional economic variations are significant, with methane pyrolysis showing enhanced feasibility in regions with low natural gas prices and stringent carbon regulations. North American implementations benefit from abundant natural gas resources, while European installations gain advantage from progressive carbon pricing frameworks. Middle Eastern petrochemical hubs present unique opportunities due to extremely low feedstock costs, potentially reducing payback periods to 3-5 years.
Financing models also impact feasibility, with public-private partnerships emerging as particularly effective structures for managing the technology's risk profile. Green bonds and sustainability-linked loans offer favorable terms that can improve project economics by 10-15% through reduced capital costs.
Return on investment calculations indicate promising prospects, with payback periods typically ranging from 5-8 years under current market conditions. The economic model benefits substantially from the production of solid carbon as a valuable by-product, which can be sold at $1,000-2,500 per ton depending on purity and form. This dual-revenue stream significantly enhances the financial viability compared to traditional hydrogen production methods.
Sensitivity analysis reveals that methane pyrolysis economics are particularly responsive to natural gas prices, electricity costs, and carbon market valuations. A 10% reduction in energy costs can improve ROI by approximately 15-20%, highlighting the importance of energy efficiency in implementation strategies. The technology becomes increasingly competitive as carbon pricing mechanisms mature globally, with each $10/ton increase in carbon pricing improving annual returns by an estimated 8-12%.
Comparative cost analysis against conventional steam methane reforming (SMR) shows that while pyrolysis has higher upfront costs, it offers superior long-term economics when accounting for carbon pricing and emissions regulations. The levelized cost of hydrogen production via pyrolysis is estimated at $1.8-2.5/kg compared to $1.5-2.0/kg for SMR without carbon capture, but this gap narrows or reverses when environmental costs are internalized.
Regional economic variations are significant, with methane pyrolysis showing enhanced feasibility in regions with low natural gas prices and stringent carbon regulations. North American implementations benefit from abundant natural gas resources, while European installations gain advantage from progressive carbon pricing frameworks. Middle Eastern petrochemical hubs present unique opportunities due to extremely low feedstock costs, potentially reducing payback periods to 3-5 years.
Financing models also impact feasibility, with public-private partnerships emerging as particularly effective structures for managing the technology's risk profile. Green bonds and sustainability-linked loans offer favorable terms that can improve project economics by 10-15% through reduced capital costs.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!