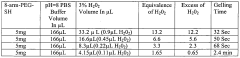
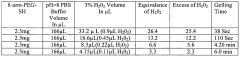
Investigating PTFE hydrogel blends for wound dressing
JUN 27, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
PTFE Hydrogel Background
Polytetrafluoroethylene (PTFE) hydrogel blends have emerged as a promising material for wound dressing applications, combining the unique properties of PTFE with the beneficial characteristics of hydrogels. PTFE, known for its exceptional chemical resistance and low friction coefficient, has been widely used in various medical applications. When blended with hydrogels, it creates a composite material that offers enhanced performance in wound healing processes.
The development of PTFE hydrogel blends for wound dressing can be traced back to the early 2000s when researchers began exploring ways to improve the properties of traditional wound dressings. The primary goal was to create a material that could provide a moist wound environment, promote healing, and prevent bacterial infection while maintaining the durability and non-adherent nature of PTFE.
Hydrogels, which are three-dimensional networks of hydrophilic polymers, have been extensively studied for their ability to absorb and retain large amounts of water. This property makes them ideal for maintaining a moist wound environment, which is crucial for optimal healing. By incorporating PTFE into hydrogel matrices, researchers aimed to combine the moisture-retaining capabilities of hydrogels with the non-stick and protective properties of PTFE.
The evolution of PTFE hydrogel blends has been driven by advancements in polymer science and biomaterials engineering. Early attempts focused on simple physical blending of PTFE particles with hydrogel precursors. However, this approach often resulted in poor mechanical properties and inhomogeneous distribution of PTFE within the hydrogel matrix.
Subsequent research efforts have explored more sophisticated techniques, such as in-situ polymerization of hydrogel components in the presence of PTFE particles, surface modification of PTFE to enhance its compatibility with hydrogels, and the development of novel crosslinking methods to improve the overall stability of the blends.
The potential of PTFE hydrogel blends in wound dressing applications has attracted significant attention from both academia and industry. These materials offer several advantages over traditional wound dressings, including improved moisture management, reduced bacterial adhesion, and enhanced mechanical strength. Additionally, the non-adherent nature of PTFE helps prevent tissue damage during dressing changes, making the process less painful for patients.
As research in this field continues to progress, the focus has shifted towards developing PTFE hydrogel blends with advanced functionalities. This includes incorporating antimicrobial agents, growth factors, and other bioactive molecules to promote faster wound healing and prevent infections. The potential for creating smart wound dressings that can respond to changes in the wound environment is also being explored, opening up new possibilities for personalized wound care.
The development of PTFE hydrogel blends for wound dressing can be traced back to the early 2000s when researchers began exploring ways to improve the properties of traditional wound dressings. The primary goal was to create a material that could provide a moist wound environment, promote healing, and prevent bacterial infection while maintaining the durability and non-adherent nature of PTFE.
Hydrogels, which are three-dimensional networks of hydrophilic polymers, have been extensively studied for their ability to absorb and retain large amounts of water. This property makes them ideal for maintaining a moist wound environment, which is crucial for optimal healing. By incorporating PTFE into hydrogel matrices, researchers aimed to combine the moisture-retaining capabilities of hydrogels with the non-stick and protective properties of PTFE.
The evolution of PTFE hydrogel blends has been driven by advancements in polymer science and biomaterials engineering. Early attempts focused on simple physical blending of PTFE particles with hydrogel precursors. However, this approach often resulted in poor mechanical properties and inhomogeneous distribution of PTFE within the hydrogel matrix.
Subsequent research efforts have explored more sophisticated techniques, such as in-situ polymerization of hydrogel components in the presence of PTFE particles, surface modification of PTFE to enhance its compatibility with hydrogels, and the development of novel crosslinking methods to improve the overall stability of the blends.
The potential of PTFE hydrogel blends in wound dressing applications has attracted significant attention from both academia and industry. These materials offer several advantages over traditional wound dressings, including improved moisture management, reduced bacterial adhesion, and enhanced mechanical strength. Additionally, the non-adherent nature of PTFE helps prevent tissue damage during dressing changes, making the process less painful for patients.
As research in this field continues to progress, the focus has shifted towards developing PTFE hydrogel blends with advanced functionalities. This includes incorporating antimicrobial agents, growth factors, and other bioactive molecules to promote faster wound healing and prevent infections. The potential for creating smart wound dressings that can respond to changes in the wound environment is also being explored, opening up new possibilities for personalized wound care.
Wound Dressing Market
The global wound dressing market has been experiencing significant growth in recent years, driven by factors such as the increasing prevalence of chronic wounds, rising geriatric population, and advancements in wound care technologies. The market is characterized by a diverse range of products, including traditional wound dressings, advanced wound dressings, and bioactive dressings.
Traditional wound dressings, such as gauze and bandages, continue to hold a substantial market share due to their cost-effectiveness and widespread availability. However, advanced wound dressings, including hydrogels, foams, and films, are gaining traction due to their superior healing properties and ability to maintain a moist wound environment.
The market for hydrogel-based wound dressings has been particularly dynamic, with increasing interest in novel materials and formulations. PTFE (polytetrafluoroethylene) hydrogel blends represent an emerging area of research and development within this segment, offering potential advantages in terms of biocompatibility, moisture retention, and antimicrobial properties.
Geographically, North America and Europe dominate the wound dressing market, owing to their well-established healthcare infrastructure and higher healthcare expenditure. However, the Asia-Pacific region is expected to witness the fastest growth in the coming years, driven by improving healthcare access and rising awareness about advanced wound care products.
Key market players in the wound dressing industry include 3M Company, Smith & Nephew, Mölnlycke Health Care, and ConvaTec Group. These companies are actively investing in research and development to introduce innovative products and expand their market presence.
The wound dressing market is also influenced by evolving healthcare policies and reimbursement scenarios. Increasing focus on reducing hospital-acquired infections and promoting faster wound healing has led to greater adoption of advanced wound dressings in healthcare settings.
Looking ahead, the market is poised for further growth, with emerging trends such as the integration of smart technologies in wound dressings and the development of personalized wound care solutions. The ongoing research into PTFE hydrogel blends for wound dressing applications aligns well with these market trends, potentially offering new opportunities for product innovation and market expansion.
Traditional wound dressings, such as gauze and bandages, continue to hold a substantial market share due to their cost-effectiveness and widespread availability. However, advanced wound dressings, including hydrogels, foams, and films, are gaining traction due to their superior healing properties and ability to maintain a moist wound environment.
The market for hydrogel-based wound dressings has been particularly dynamic, with increasing interest in novel materials and formulations. PTFE (polytetrafluoroethylene) hydrogel blends represent an emerging area of research and development within this segment, offering potential advantages in terms of biocompatibility, moisture retention, and antimicrobial properties.
Geographically, North America and Europe dominate the wound dressing market, owing to their well-established healthcare infrastructure and higher healthcare expenditure. However, the Asia-Pacific region is expected to witness the fastest growth in the coming years, driven by improving healthcare access and rising awareness about advanced wound care products.
Key market players in the wound dressing industry include 3M Company, Smith & Nephew, Mölnlycke Health Care, and ConvaTec Group. These companies are actively investing in research and development to introduce innovative products and expand their market presence.
The wound dressing market is also influenced by evolving healthcare policies and reimbursement scenarios. Increasing focus on reducing hospital-acquired infections and promoting faster wound healing has led to greater adoption of advanced wound dressings in healthcare settings.
Looking ahead, the market is poised for further growth, with emerging trends such as the integration of smart technologies in wound dressings and the development of personalized wound care solutions. The ongoing research into PTFE hydrogel blends for wound dressing applications aligns well with these market trends, potentially offering new opportunities for product innovation and market expansion.
PTFE Hydrogel Challenges
The development of PTFE hydrogel blends for wound dressing applications faces several significant challenges. One of the primary obstacles is achieving optimal mechanical properties that can withstand the dynamic environment of a wound site. PTFE, known for its hydrophobicity and chemical inertness, often exhibits poor adhesion to hydrophilic components in hydrogels, leading to potential phase separation and compromised structural integrity.
Another critical challenge lies in balancing the water absorption capacity of the hydrogel with the hydrophobic nature of PTFE. While hydrogels are valued for their ability to maintain a moist wound environment, excessive water uptake can lead to maceration of the surrounding healthy tissue. Conversely, insufficient moisture retention may impede the healing process. Achieving this delicate equilibrium requires precise control over the blend composition and crosslinking density.
Biocompatibility and biodegradability present additional hurdles in PTFE hydrogel blend development. PTFE's non-biodegradable nature may limit its use in certain wound healing applications where complete absorption of the dressing is desired. Furthermore, ensuring that the blend does not elicit adverse immune responses or impede cellular processes crucial for wound healing is paramount.
The manufacturing process of PTFE hydrogel blends poses its own set of challenges. Achieving uniform dispersion of PTFE particles within the hydrogel matrix without compromising the network structure is technically demanding. Traditional blending methods may not be sufficient, necessitating the development of novel processing techniques that can maintain the integrity of both components while ensuring homogeneous distribution.
Sterilization of PTFE hydrogel blends presents another significant challenge. Common sterilization methods such as autoclaving or gamma irradiation may alter the physical and chemical properties of the blend, potentially compromising its performance as a wound dressing. Developing sterilization protocols that preserve the blend's structural and functional characteristics is crucial for clinical application.
Lastly, the long-term stability of PTFE hydrogel blends under various storage conditions and throughout their shelf life is a concern that requires extensive investigation. Factors such as temperature fluctuations, humidity, and exposure to light can potentially affect the blend's properties over time, necessitating the development of appropriate packaging and storage solutions to maintain efficacy until use.
Addressing these challenges requires a multidisciplinary approach, combining expertise in materials science, polymer chemistry, and biomedical engineering. Innovative strategies such as surface modification of PTFE particles, development of novel crosslinking agents, and exploration of advanced manufacturing techniques like 3D printing may hold the key to overcoming these obstacles and realizing the full potential of PTFE hydrogel blends in wound dressing applications.
Another critical challenge lies in balancing the water absorption capacity of the hydrogel with the hydrophobic nature of PTFE. While hydrogels are valued for their ability to maintain a moist wound environment, excessive water uptake can lead to maceration of the surrounding healthy tissue. Conversely, insufficient moisture retention may impede the healing process. Achieving this delicate equilibrium requires precise control over the blend composition and crosslinking density.
Biocompatibility and biodegradability present additional hurdles in PTFE hydrogel blend development. PTFE's non-biodegradable nature may limit its use in certain wound healing applications where complete absorption of the dressing is desired. Furthermore, ensuring that the blend does not elicit adverse immune responses or impede cellular processes crucial for wound healing is paramount.
The manufacturing process of PTFE hydrogel blends poses its own set of challenges. Achieving uniform dispersion of PTFE particles within the hydrogel matrix without compromising the network structure is technically demanding. Traditional blending methods may not be sufficient, necessitating the development of novel processing techniques that can maintain the integrity of both components while ensuring homogeneous distribution.
Sterilization of PTFE hydrogel blends presents another significant challenge. Common sterilization methods such as autoclaving or gamma irradiation may alter the physical and chemical properties of the blend, potentially compromising its performance as a wound dressing. Developing sterilization protocols that preserve the blend's structural and functional characteristics is crucial for clinical application.
Lastly, the long-term stability of PTFE hydrogel blends under various storage conditions and throughout their shelf life is a concern that requires extensive investigation. Factors such as temperature fluctuations, humidity, and exposure to light can potentially affect the blend's properties over time, necessitating the development of appropriate packaging and storage solutions to maintain efficacy until use.
Addressing these challenges requires a multidisciplinary approach, combining expertise in materials science, polymer chemistry, and biomedical engineering. Innovative strategies such as surface modification of PTFE particles, development of novel crosslinking agents, and exploration of advanced manufacturing techniques like 3D printing may hold the key to overcoming these obstacles and realizing the full potential of PTFE hydrogel blends in wound dressing applications.
Current PTFE Solutions
01 PTFE-hydrogel composite materials
Blends of polytetrafluoroethylene (PTFE) and hydrogels are developed to create composite materials with unique properties. These composites combine the hydrophobicity and chemical resistance of PTFE with the water-absorbing capabilities of hydrogels, resulting in materials with potential applications in biomedical and industrial fields.- PTFE-hydrogel composite materials: Blends of polytetrafluoroethylene (PTFE) and hydrogels are developed to create composite materials with unique properties. These composites combine the hydrophobicity and chemical resistance of PTFE with the water-absorbing capabilities of hydrogels, resulting in materials with enhanced performance for various applications.
- Biomedical applications of PTFE-hydrogel blends: PTFE-hydrogel blends are utilized in biomedical applications due to their biocompatibility and unique properties. These materials can be used for tissue engineering, drug delivery systems, and medical implants, offering improved performance and integration with biological tissues.
- Manufacturing processes for PTFE-hydrogel blends: Various manufacturing processes are developed to create PTFE-hydrogel blends, including in-situ polymerization, solution blending, and melt processing techniques. These methods aim to achieve uniform dispersion of PTFE within the hydrogel matrix and optimize the properties of the resulting composite materials.
- Surface modification of PTFE-hydrogel blends: Surface modification techniques are applied to PTFE-hydrogel blends to enhance their properties and functionality. These methods include plasma treatment, chemical grafting, and the incorporation of functional groups, which can improve adhesion, wettability, and biocompatibility of the composite materials.
- Industrial applications of PTFE-hydrogel blends: PTFE-hydrogel blends find applications in various industrial sectors, including filtration, coatings, and lubrication. These materials offer advantages such as improved wear resistance, self-lubricating properties, and enhanced chemical resistance, making them suitable for use in demanding environments.
02 Fabrication methods for PTFE-hydrogel blends
Various techniques are employed to create PTFE-hydrogel blends, including in-situ polymerization, solution blending, and melt processing. These methods aim to achieve uniform dispersion of PTFE particles within the hydrogel matrix, optimizing the properties of the resulting composite materials.Expand Specific Solutions03 Applications in biomedical engineering
PTFE-hydrogel blends find applications in biomedical engineering, such as tissue engineering scaffolds, drug delivery systems, and wound dressings. The combination of PTFE's biocompatibility and hydrogel's ability to mimic biological tissues makes these blends suitable for various medical applications.Expand Specific Solutions04 Enhanced mechanical properties
The incorporation of PTFE into hydrogel matrices can lead to improved mechanical properties, including increased tensile strength, elasticity, and durability. These enhanced properties make PTFE-hydrogel blends suitable for applications requiring both flexibility and strength.Expand Specific Solutions05 Controlled release and permeability
PTFE-hydrogel blends can be designed to exhibit controlled release properties and selective permeability. By adjusting the composition and structure of the blend, researchers can tailor the material's ability to release drugs or allow specific molecules to pass through, making them useful in drug delivery systems and membrane technologies.Expand Specific Solutions
Key Industry Players
The development of PTFE hydrogel blends for wound dressing is in an emerging phase, with significant potential for market growth due to increasing demand for advanced wound care solutions. The global wound dressing market is expanding, driven by rising chronic diseases and an aging population. Technologically, PTFE hydrogel blends are still evolving, with ongoing research to optimize their properties for wound healing. Companies like Paul Hartmann AG, T&R Biofab, and Convatec Ltd. are at the forefront of this field, leveraging their expertise in medical devices and wound care. Academic institutions such as the University of Maryland and Ghent University are contributing to the advancement of this technology through collaborative research efforts.
PAUL HARTMANN AG
Technical Solution: PAUL HARTMANN AG has developed advanced PTFE hydrogel blends for wound dressing applications. Their technology incorporates a unique combination of PTFE and hydrogel materials to create a highly effective wound healing environment. The company's approach involves creating a microporous PTFE membrane infused with a hydrogel matrix, allowing for optimal moisture management and gas exchange at the wound site[1]. This innovative blend provides a barrier against external contaminants while maintaining a moist wound environment conducive to healing. The hydrogel component is engineered to absorb excess exudate and release it gradually, preventing maceration of surrounding healthy tissue[3]. Additionally, HARTMANN's PTFE hydrogel blends are designed to be non-adherent, reducing trauma during dressing changes and promoting patient comfort[5].
Strengths: Excellent moisture management, reduced dressing change frequency, and enhanced patient comfort. Weaknesses: Potentially higher production costs compared to traditional dressings and limited suitability for heavily exuding wounds.
Ghent University
Technical Solution: Ghent University has pioneered research into PTFE hydrogel blends for wound dressing applications. Their approach focuses on developing a novel composite material that combines the non-stick properties of PTFE with the moisture-retaining capabilities of hydrogels. The university's research team has successfully created a PTFE-hydrogel hybrid that demonstrates superior wound healing properties[2]. This blend utilizes a nanoscale PTFE network interpenetrated with a biocompatible hydrogel, resulting in a material that provides excellent oxygen permeability while maintaining an optimal moist wound environment[4]. The hydrogel component is engineered to incorporate antimicrobial agents, enhancing the dressing's ability to prevent infection. Furthermore, Ghent University's PTFE hydrogel blend has shown remarkable mechanical strength and flexibility, allowing for easy application and conformability to various wound shapes[6].
Strengths: High oxygen permeability, antimicrobial properties, and excellent conformability to wound surfaces. Weaknesses: Complex manufacturing process may lead to higher costs, and long-term clinical studies are still ongoing.
PTFE Hydrogel Patents
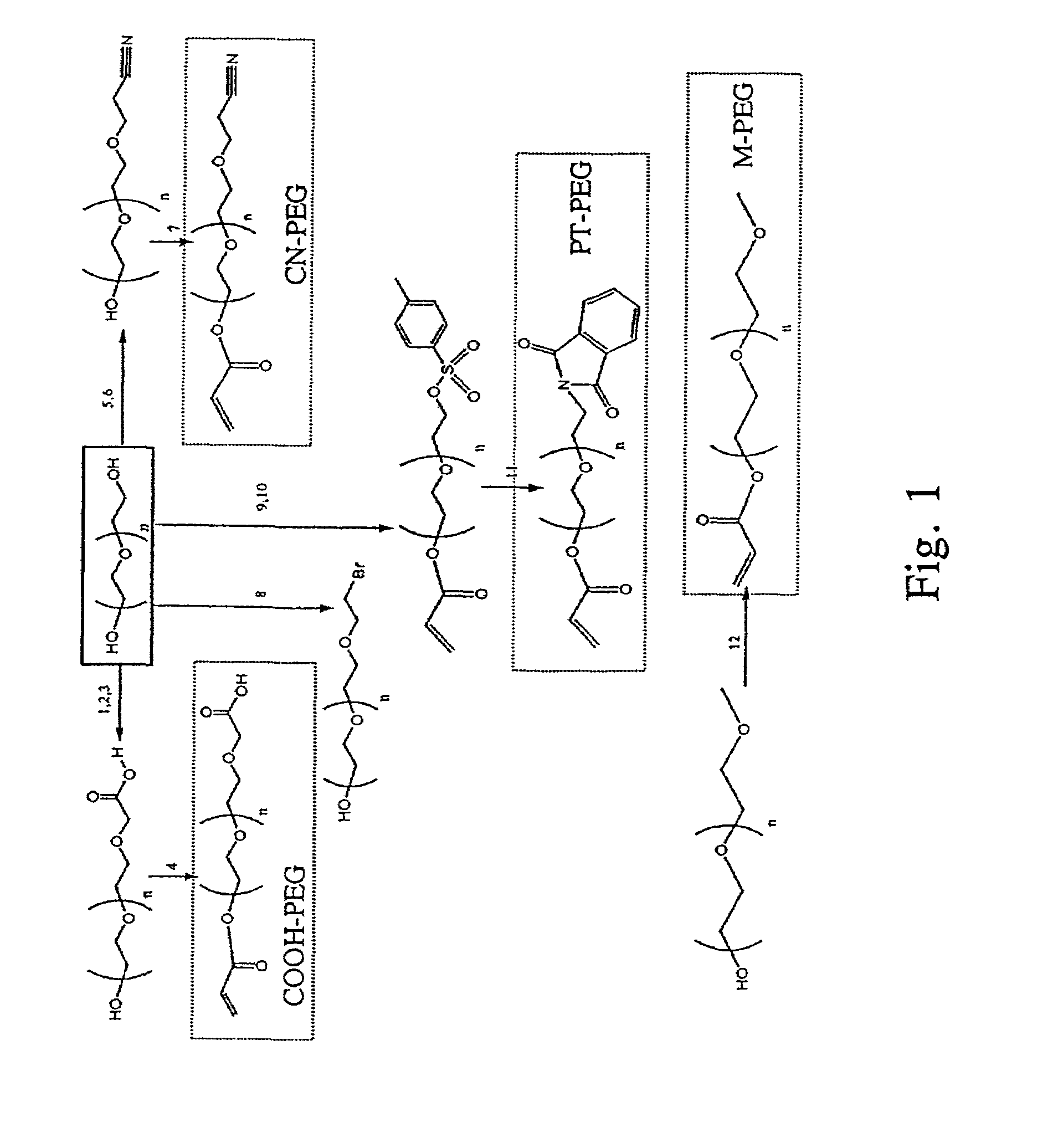
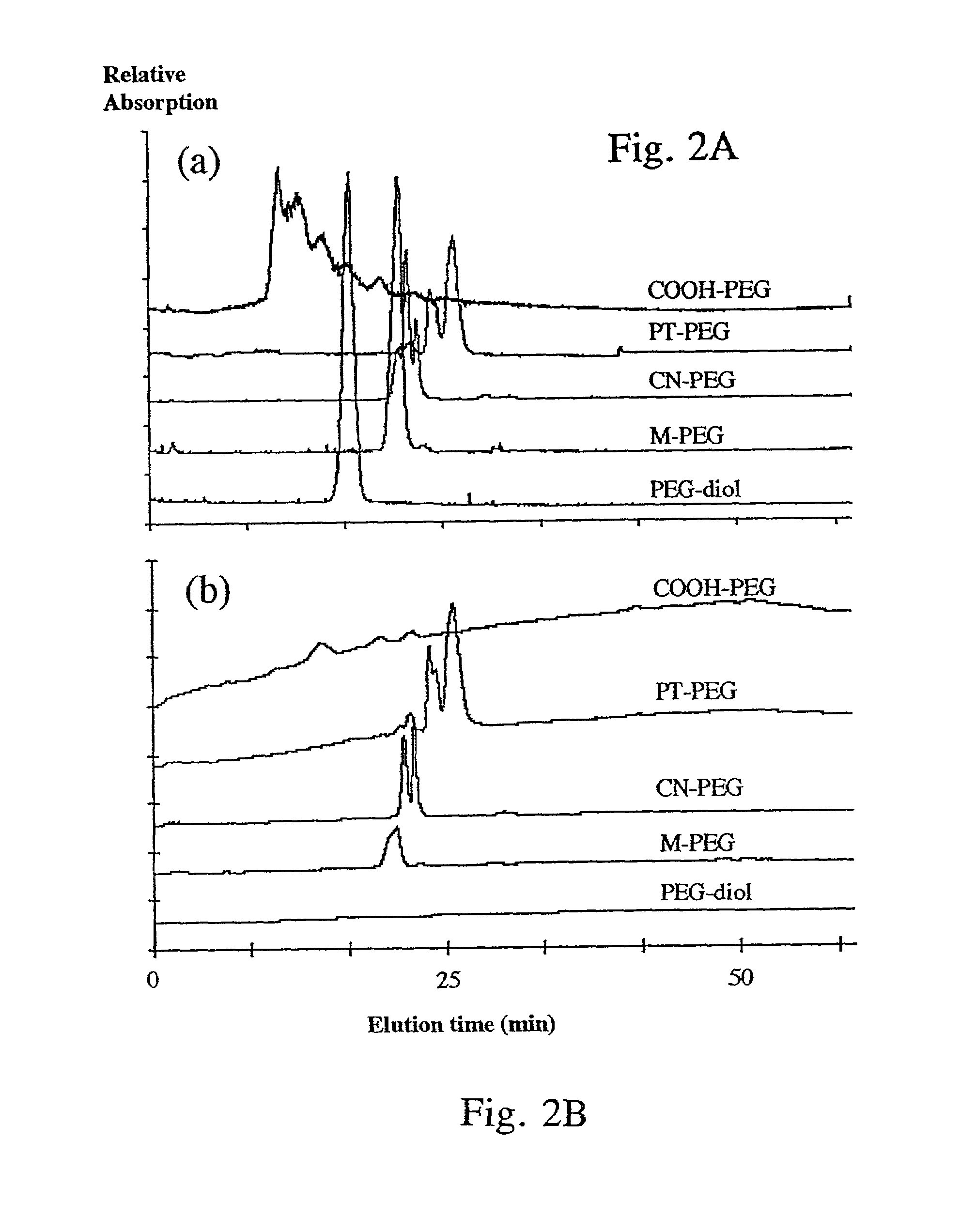
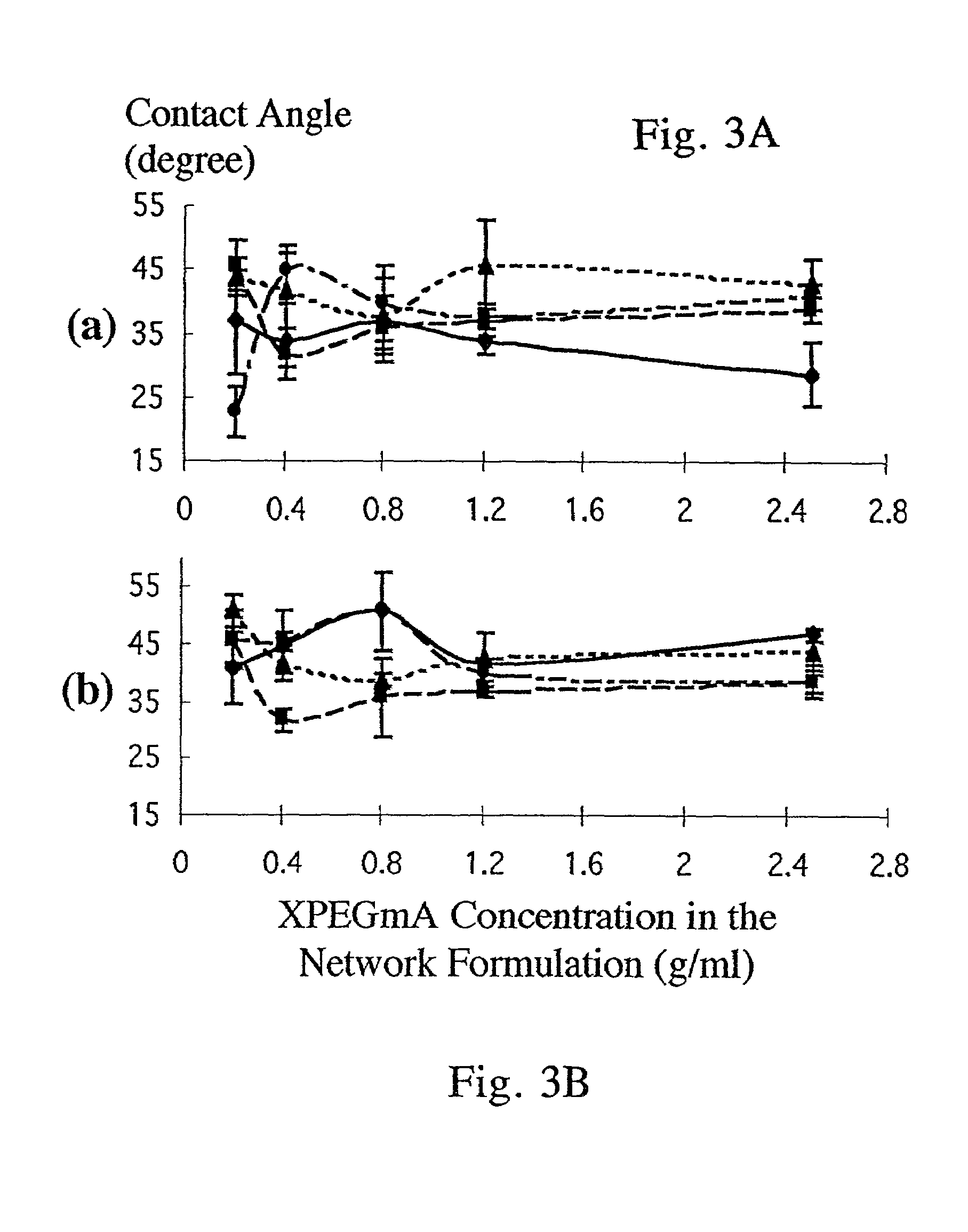
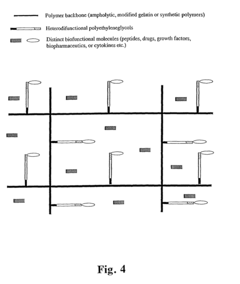
Bifunctional-modified hydrogels
PatentInactiveUS7615593B2
Innovation
- Development of hydrogels with a polymer matrix modified using bifunctional poly(alkylene glycols) that covalently bond pharmacologically-active agents, providing a homogenous, mechanically stable, and porous structure for controlled drug delivery and enhanced wound healing.
Dressing compositions and methods
PatentWO2008133918A1
Innovation
- Development of a reversibly cross-linked in situ-forming hydrogel composition using a hydrophilic polymer with sulfhydryl or mercaptan moieties and a cross-linker, allowing for easy dissolution and removal, enhanced conformability, and improved drug retention through RGD peptide linkage.
Biocompatibility Tests
Biocompatibility testing is a critical aspect of developing PTFE hydrogel blends for wound dressing applications. These tests are designed to evaluate the safety and compatibility of the material with biological systems, ensuring that the wound dressing will not cause adverse reactions when in contact with human tissue.
The first step in biocompatibility testing typically involves in vitro cytotoxicity assays. These tests assess the potential of the PTFE hydrogel blend to cause cell death or inhibit cell growth. Common methods include the MTT assay and the neutral red uptake assay, which measure cell viability and metabolic activity. For wound dressing applications, it is crucial to test the material's effects on various cell types, including fibroblasts, keratinocytes, and endothelial cells, which are integral to the wound healing process.
Following cytotoxicity testing, sensitization and irritation tests are conducted to evaluate the potential for allergic reactions or local tissue irritation. These tests often involve animal models, such as the guinea pig maximization test or the local lymph node assay. For PTFE hydrogel blends, it is particularly important to assess any potential leachables or degradation products that may cause sensitization over time.
Hemocompatibility testing is another crucial aspect, especially if the wound dressing may come into contact with blood. This involves evaluating the material's potential to cause hemolysis, platelet activation, or coagulation. Standard tests include the hemolysis assay and the platelet adhesion test.
In vivo biocompatibility studies are typically conducted in animal models to assess the overall tissue response to the PTFE hydrogel blend. These studies evaluate parameters such as inflammation, foreign body response, and the material's integration with surrounding tissue. For wound dressing applications, it is essential to assess the material's impact on wound healing rates and the quality of healed tissue.
Long-term implantation studies may also be necessary to evaluate the chronic effects of the PTFE hydrogel blend. These studies assess the material's degradation profile, potential for calcification, and any long-term tissue reactions. For wound dressings, the focus would be on repeated application scenarios and potential cumulative effects.
Genotoxicity and carcinogenicity tests are conducted to ensure that the PTFE hydrogel blend does not cause DNA damage or promote tumor formation. These tests typically include in vitro assays such as the Ames test and in vivo studies in rodent models.
Finally, specific tests related to wound healing efficacy should be included in the biocompatibility assessment. These may involve evaluating the material's ability to maintain a moist wound environment, promote cell migration and proliferation, and support the formation of granulation tissue.
The first step in biocompatibility testing typically involves in vitro cytotoxicity assays. These tests assess the potential of the PTFE hydrogel blend to cause cell death or inhibit cell growth. Common methods include the MTT assay and the neutral red uptake assay, which measure cell viability and metabolic activity. For wound dressing applications, it is crucial to test the material's effects on various cell types, including fibroblasts, keratinocytes, and endothelial cells, which are integral to the wound healing process.
Following cytotoxicity testing, sensitization and irritation tests are conducted to evaluate the potential for allergic reactions or local tissue irritation. These tests often involve animal models, such as the guinea pig maximization test or the local lymph node assay. For PTFE hydrogel blends, it is particularly important to assess any potential leachables or degradation products that may cause sensitization over time.
Hemocompatibility testing is another crucial aspect, especially if the wound dressing may come into contact with blood. This involves evaluating the material's potential to cause hemolysis, platelet activation, or coagulation. Standard tests include the hemolysis assay and the platelet adhesion test.
In vivo biocompatibility studies are typically conducted in animal models to assess the overall tissue response to the PTFE hydrogel blend. These studies evaluate parameters such as inflammation, foreign body response, and the material's integration with surrounding tissue. For wound dressing applications, it is essential to assess the material's impact on wound healing rates and the quality of healed tissue.
Long-term implantation studies may also be necessary to evaluate the chronic effects of the PTFE hydrogel blend. These studies assess the material's degradation profile, potential for calcification, and any long-term tissue reactions. For wound dressings, the focus would be on repeated application scenarios and potential cumulative effects.
Genotoxicity and carcinogenicity tests are conducted to ensure that the PTFE hydrogel blend does not cause DNA damage or promote tumor formation. These tests typically include in vitro assays such as the Ames test and in vivo studies in rodent models.
Finally, specific tests related to wound healing efficacy should be included in the biocompatibility assessment. These may involve evaluating the material's ability to maintain a moist wound environment, promote cell migration and proliferation, and support the formation of granulation tissue.
Regulatory Compliance
Regulatory compliance is a critical aspect of developing and commercializing PTFE hydrogel blends for wound dressing applications. The regulatory landscape for medical devices, including wound dressings, is complex and varies across different regions and countries. In the United States, the Food and Drug Administration (FDA) regulates wound dressings as medical devices under the Federal Food, Drug, and Cosmetic Act.
For PTFE hydrogel blends, manufacturers must navigate the appropriate regulatory pathway, which typically involves either the 510(k) premarket notification process or the more rigorous Premarket Approval (PMA) process, depending on the device's classification and intended use. The 510(k) pathway is more common for wound dressings, requiring manufacturers to demonstrate substantial equivalence to a legally marketed predicate device.
Compliance with Good Manufacturing Practices (GMP) is essential throughout the development and production of PTFE hydrogel blends. This ensures consistent quality, safety, and efficacy of the final product. Manufacturers must implement and maintain a quality management system that adheres to ISO 13485 standards, specifically designed for medical devices.
Biocompatibility testing is a crucial regulatory requirement for wound dressings. PTFE hydrogel blends must undergo a series of tests as outlined in ISO 10993, including cytotoxicity, sensitization, and irritation studies. Additionally, depending on the specific claims and intended use of the wound dressing, further testing may be required to demonstrate antimicrobial efficacy, fluid absorption capacity, or other performance characteristics.
In the European Union, PTFE hydrogel blends for wound dressing would fall under the Medical Device Regulation (MDR). Manufacturers must obtain CE marking by demonstrating compliance with the Essential Requirements outlined in the MDR. This process involves conducting a conformity assessment, preparing technical documentation, and obtaining certification from a Notified Body.
Post-market surveillance is an ongoing regulatory requirement for wound dressings. Manufacturers must monitor the performance and safety of their products in real-world settings, report adverse events, and implement corrective actions when necessary. This continuous vigilance helps ensure the long-term safety and efficacy of PTFE hydrogel blends in wound care applications.
As the regulatory landscape evolves, manufacturers must stay informed about changes in requirements and guidelines. This includes monitoring updates from regulatory bodies, participating in industry forums, and engaging with regulatory experts to ensure ongoing compliance and market access for their PTFE hydrogel blend wound dressings.
For PTFE hydrogel blends, manufacturers must navigate the appropriate regulatory pathway, which typically involves either the 510(k) premarket notification process or the more rigorous Premarket Approval (PMA) process, depending on the device's classification and intended use. The 510(k) pathway is more common for wound dressings, requiring manufacturers to demonstrate substantial equivalence to a legally marketed predicate device.
Compliance with Good Manufacturing Practices (GMP) is essential throughout the development and production of PTFE hydrogel blends. This ensures consistent quality, safety, and efficacy of the final product. Manufacturers must implement and maintain a quality management system that adheres to ISO 13485 standards, specifically designed for medical devices.
Biocompatibility testing is a crucial regulatory requirement for wound dressings. PTFE hydrogel blends must undergo a series of tests as outlined in ISO 10993, including cytotoxicity, sensitization, and irritation studies. Additionally, depending on the specific claims and intended use of the wound dressing, further testing may be required to demonstrate antimicrobial efficacy, fluid absorption capacity, or other performance characteristics.
In the European Union, PTFE hydrogel blends for wound dressing would fall under the Medical Device Regulation (MDR). Manufacturers must obtain CE marking by demonstrating compliance with the Essential Requirements outlined in the MDR. This process involves conducting a conformity assessment, preparing technical documentation, and obtaining certification from a Notified Body.
Post-market surveillance is an ongoing regulatory requirement for wound dressings. Manufacturers must monitor the performance and safety of their products in real-world settings, report adverse events, and implement corrective actions when necessary. This continuous vigilance helps ensure the long-term safety and efficacy of PTFE hydrogel blends in wound care applications.
As the regulatory landscape evolves, manufacturers must stay informed about changes in requirements and guidelines. This includes monitoring updates from regulatory bodies, participating in industry forums, and engaging with regulatory experts to ensure ongoing compliance and market access for their PTFE hydrogel blend wound dressings.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!