Mapping future pathways for PTFE–bioactive surface development
JUN 27, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
PTFE-Bioactive Surface Evolution and Objectives
Polytetrafluoroethylene (PTFE) has been a cornerstone material in various industries due to its exceptional chemical resistance, low friction, and thermal stability. However, its inherent hydrophobicity and bioinertness have limited its applications in biomedical fields. The evolution of PTFE-bioactive surface development represents a significant technological advancement aimed at overcoming these limitations.
The journey of PTFE-bioactive surface development began in the late 20th century when researchers recognized the potential of modifying PTFE surfaces to enhance their biocompatibility. Initial efforts focused on physical modifications, such as plasma treatment and ion implantation, to alter surface properties without compromising the bulk characteristics of PTFE.
As the field progressed, chemical modification techniques emerged as promising approaches. Surface grafting methods, including radiation-induced grafting and plasma-assisted grafting, allowed for the introduction of functional groups onto the PTFE surface. These modifications paved the way for further functionalization with bioactive molecules, significantly expanding the potential applications of PTFE in biomedical devices and tissue engineering.
The advent of nanotechnology in the early 21st century brought about a paradigm shift in PTFE-bioactive surface development. Researchers began exploring the incorporation of nanoparticles and nanostructures to create hierarchical surface topographies that could mimic natural biological interfaces. This approach not only enhanced biocompatibility but also introduced novel properties such as antimicrobial activity and improved cell adhesion.
Recent years have witnessed a surge in biomimetic approaches, drawing inspiration from nature to design PTFE surfaces with advanced functionalities. Techniques such as layer-by-layer assembly and molecular imprinting have enabled the creation of highly specific and responsive PTFE-bioactive interfaces.
The primary objective of PTFE-bioactive surface development is to create multifunctional surfaces that retain the desirable properties of PTFE while exhibiting enhanced biocompatibility and bioactivity. This includes improving cell adhesion and proliferation, reducing bacterial colonization, and promoting tissue integration. Additionally, there is a growing emphasis on developing environmentally friendly and sustainable modification processes.
Looking ahead, the field aims to achieve precise control over surface properties at the molecular level, enabling the creation of smart PTFE surfaces that can respond dynamically to biological stimuli. The integration of PTFE-bioactive surfaces with emerging technologies such as 3D printing and microfluidics presents exciting opportunities for advanced biomedical applications.
The journey of PTFE-bioactive surface development began in the late 20th century when researchers recognized the potential of modifying PTFE surfaces to enhance their biocompatibility. Initial efforts focused on physical modifications, such as plasma treatment and ion implantation, to alter surface properties without compromising the bulk characteristics of PTFE.
As the field progressed, chemical modification techniques emerged as promising approaches. Surface grafting methods, including radiation-induced grafting and plasma-assisted grafting, allowed for the introduction of functional groups onto the PTFE surface. These modifications paved the way for further functionalization with bioactive molecules, significantly expanding the potential applications of PTFE in biomedical devices and tissue engineering.
The advent of nanotechnology in the early 21st century brought about a paradigm shift in PTFE-bioactive surface development. Researchers began exploring the incorporation of nanoparticles and nanostructures to create hierarchical surface topographies that could mimic natural biological interfaces. This approach not only enhanced biocompatibility but also introduced novel properties such as antimicrobial activity and improved cell adhesion.
Recent years have witnessed a surge in biomimetic approaches, drawing inspiration from nature to design PTFE surfaces with advanced functionalities. Techniques such as layer-by-layer assembly and molecular imprinting have enabled the creation of highly specific and responsive PTFE-bioactive interfaces.
The primary objective of PTFE-bioactive surface development is to create multifunctional surfaces that retain the desirable properties of PTFE while exhibiting enhanced biocompatibility and bioactivity. This includes improving cell adhesion and proliferation, reducing bacterial colonization, and promoting tissue integration. Additionally, there is a growing emphasis on developing environmentally friendly and sustainable modification processes.
Looking ahead, the field aims to achieve precise control over surface properties at the molecular level, enabling the creation of smart PTFE surfaces that can respond dynamically to biological stimuli. The integration of PTFE-bioactive surfaces with emerging technologies such as 3D printing and microfluidics presents exciting opportunities for advanced biomedical applications.
Market Analysis for PTFE-Bioactive Surfaces
The market for PTFE-bioactive surfaces is experiencing significant growth, driven by increasing demand in medical devices, implants, and tissue engineering applications. This innovative technology combines the excellent chemical resistance and low friction properties of PTFE with bioactive functionalities, addressing critical challenges in biocompatibility and integration with biological systems.
The global market for bioactive materials is projected to expand rapidly, with PTFE-bioactive surfaces playing a crucial role in this growth. Key application areas include cardiovascular devices, orthopedic implants, dental materials, and wound care products. The cardiovascular segment, in particular, shows strong potential due to the rising prevalence of heart diseases and the need for improved stent and graft technologies.
Geographically, North America and Europe currently dominate the market, owing to advanced healthcare infrastructure and higher adoption rates of innovative medical technologies. However, the Asia-Pacific region is expected to witness the fastest growth, driven by improving healthcare access, increasing disposable incomes, and a growing aging population in countries like China and India.
The market is characterized by a high degree of fragmentation, with numerous small to medium-sized companies specializing in niche applications. Major players in the medical device and biomaterials sectors are increasingly investing in research and development of PTFE-bioactive surface technologies to maintain their competitive edge.
Key market drivers include the growing aging population, increasing prevalence of chronic diseases, and advancements in regenerative medicine. The rise in minimally invasive surgical procedures is also fueling demand for biocompatible materials with enhanced surface properties.
Challenges in the market include stringent regulatory requirements, high development costs, and the need for extensive clinical trials to prove long-term efficacy and safety. Additionally, concerns about potential environmental impacts of PTFE production and disposal may influence market dynamics and drive research into more sustainable alternatives.
Despite these challenges, the market outlook remains positive. Ongoing research in nanotechnology and surface modification techniques is expected to unlock new possibilities for PTFE-bioactive surfaces, potentially expanding their applications beyond the medical field into areas such as water treatment, food packaging, and industrial coatings.
The global market for bioactive materials is projected to expand rapidly, with PTFE-bioactive surfaces playing a crucial role in this growth. Key application areas include cardiovascular devices, orthopedic implants, dental materials, and wound care products. The cardiovascular segment, in particular, shows strong potential due to the rising prevalence of heart diseases and the need for improved stent and graft technologies.
Geographically, North America and Europe currently dominate the market, owing to advanced healthcare infrastructure and higher adoption rates of innovative medical technologies. However, the Asia-Pacific region is expected to witness the fastest growth, driven by improving healthcare access, increasing disposable incomes, and a growing aging population in countries like China and India.
The market is characterized by a high degree of fragmentation, with numerous small to medium-sized companies specializing in niche applications. Major players in the medical device and biomaterials sectors are increasingly investing in research and development of PTFE-bioactive surface technologies to maintain their competitive edge.
Key market drivers include the growing aging population, increasing prevalence of chronic diseases, and advancements in regenerative medicine. The rise in minimally invasive surgical procedures is also fueling demand for biocompatible materials with enhanced surface properties.
Challenges in the market include stringent regulatory requirements, high development costs, and the need for extensive clinical trials to prove long-term efficacy and safety. Additionally, concerns about potential environmental impacts of PTFE production and disposal may influence market dynamics and drive research into more sustainable alternatives.
Despite these challenges, the market outlook remains positive. Ongoing research in nanotechnology and surface modification techniques is expected to unlock new possibilities for PTFE-bioactive surfaces, potentially expanding their applications beyond the medical field into areas such as water treatment, food packaging, and industrial coatings.
PTFE Surface Modification Challenges
Polytetrafluoroethylene (PTFE) is renowned for its exceptional chemical inertness and low surface energy, properties that make it highly valuable in various applications. However, these same characteristics pose significant challenges when attempting to modify its surface for bioactive purposes. The primary obstacle lies in overcoming PTFE's inherent resistance to chemical and physical modifications.
One of the main challenges in PTFE surface modification is achieving stable and durable alterations without compromising the bulk properties of the material. Traditional surface modification techniques, such as plasma treatment or chemical etching, often result in temporary changes that revert over time due to the material's tendency to recover its low surface energy state. This phenomenon, known as hydrophobic recovery, limits the long-term effectiveness of many surface modification approaches.
Another significant hurdle is the difficulty in introducing functional groups onto the PTFE surface. The strong carbon-fluorine bonds in PTFE make it resistant to most chemical reactions, necessitating the use of harsh conditions or specialized techniques to create reactive sites. This challenge is particularly pronounced when attempting to graft bioactive molecules or create a biocompatible interface, as many conventional coupling chemistries are ineffective on PTFE surfaces.
The uniformity and reproducibility of surface modifications across large PTFE surfaces present additional challenges. Achieving consistent modification across complex geometries or large surface areas is crucial for many biomedical applications but remains difficult due to the material's hydrophobicity and chemical resistance.
Furthermore, maintaining the mechanical integrity of PTFE while modifying its surface is a delicate balance. Aggressive modification techniques can lead to surface degradation, potentially compromising the material's strength, wear resistance, or other desirable bulk properties. This is particularly problematic in applications where the mechanical performance of PTFE is as critical as its surface properties.
The biocompatibility of modified PTFE surfaces introduces another layer of complexity. While the goal is to enhance bioactivity, it is essential to ensure that the modifications do not introduce toxicity or undesirable biological responses. This requires careful selection of modification strategies and thorough biocompatibility testing of the resulting surfaces.
Lastly, the scalability and cost-effectiveness of PTFE surface modification techniques pose significant challenges for industrial applications. Many laboratory-scale methods for surface modification are not easily translatable to large-scale manufacturing processes, limiting their practical implementation. Developing economically viable and scalable modification techniques that can be integrated into existing manufacturing processes remains a key challenge in the field.
One of the main challenges in PTFE surface modification is achieving stable and durable alterations without compromising the bulk properties of the material. Traditional surface modification techniques, such as plasma treatment or chemical etching, often result in temporary changes that revert over time due to the material's tendency to recover its low surface energy state. This phenomenon, known as hydrophobic recovery, limits the long-term effectiveness of many surface modification approaches.
Another significant hurdle is the difficulty in introducing functional groups onto the PTFE surface. The strong carbon-fluorine bonds in PTFE make it resistant to most chemical reactions, necessitating the use of harsh conditions or specialized techniques to create reactive sites. This challenge is particularly pronounced when attempting to graft bioactive molecules or create a biocompatible interface, as many conventional coupling chemistries are ineffective on PTFE surfaces.
The uniformity and reproducibility of surface modifications across large PTFE surfaces present additional challenges. Achieving consistent modification across complex geometries or large surface areas is crucial for many biomedical applications but remains difficult due to the material's hydrophobicity and chemical resistance.
Furthermore, maintaining the mechanical integrity of PTFE while modifying its surface is a delicate balance. Aggressive modification techniques can lead to surface degradation, potentially compromising the material's strength, wear resistance, or other desirable bulk properties. This is particularly problematic in applications where the mechanical performance of PTFE is as critical as its surface properties.
The biocompatibility of modified PTFE surfaces introduces another layer of complexity. While the goal is to enhance bioactivity, it is essential to ensure that the modifications do not introduce toxicity or undesirable biological responses. This requires careful selection of modification strategies and thorough biocompatibility testing of the resulting surfaces.
Lastly, the scalability and cost-effectiveness of PTFE surface modification techniques pose significant challenges for industrial applications. Many laboratory-scale methods for surface modification are not easily translatable to large-scale manufacturing processes, limiting their practical implementation. Developing economically viable and scalable modification techniques that can be integrated into existing manufacturing processes remains a key challenge in the field.
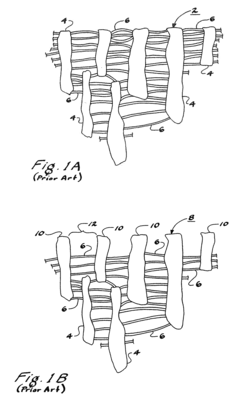
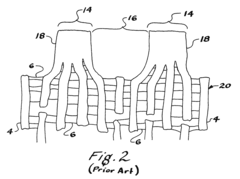
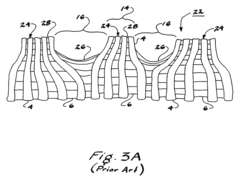
Current PTFE Surface Functionalization Methods
01 PTFE surface modification for bioactivity
Techniques for modifying PTFE surfaces to enhance bioactivity, including plasma treatment, chemical etching, and grafting of bioactive molecules. These modifications aim to improve cell adhesion, proliferation, and integration with biological tissues while maintaining the beneficial properties of PTFE.- PTFE surface modification for bioactivity: Techniques for modifying PTFE surfaces to enhance bioactivity, including plasma treatment, chemical etching, and grafting of bioactive molecules. These modifications aim to improve cell adhesion, proliferation, and integration with biological tissues while maintaining the beneficial properties of PTFE.
- Bioactive coatings on PTFE substrates: Development of bioactive coatings that can be applied to PTFE surfaces, such as hydroxyapatite, collagen, or growth factors. These coatings enhance the biological performance of PTFE implants or devices by promoting tissue integration and reducing foreign body responses.
- PTFE-based composite materials with bioactive properties: Creation of composite materials that combine PTFE with bioactive components, such as ceramic particles or biodegradable polymers. These composites aim to leverage the non-stick properties of PTFE while introducing bioactive functionality for improved biological interactions.
- Surface patterning of PTFE for enhanced bioactivity: Methods for creating micro- or nano-scale patterns on PTFE surfaces to control cell behavior and enhance bioactivity. These patterned surfaces can guide cell alignment, promote specific cellular responses, and improve the overall biocompatibility of PTFE-based medical devices.
- Bioactive PTFE for drug delivery applications: Development of PTFE-based materials or coatings that incorporate bioactive agents or drugs for controlled release. These systems aim to combine the non-fouling properties of PTFE with localized delivery of therapeutic compounds to enhance healing or prevent complications in medical implants.
02 Bioactive coatings on PTFE substrates
Application of bioactive coatings onto PTFE surfaces to impart specific biological functions. These coatings may include growth factors, antibiotics, or other bioactive compounds that promote desired cellular responses or prevent infections in medical implants and devices.Expand Specific Solutions03 PTFE-based bioactive composites
Development of composite materials combining PTFE with bioactive components, such as hydroxyapatite or bioactive glass. These composites aim to combine the non-stick properties of PTFE with enhanced biocompatibility and tissue integration for applications in orthopedics and dentistry.Expand Specific Solutions04 Surface patterning of PTFE for controlled bioactivity
Creation of micro- or nano-scale patterns on PTFE surfaces to control cell behavior and enhance bioactivity. These patterned surfaces can guide cell growth, improve implant integration, and modulate the immune response in biomedical applications.Expand Specific Solutions05 Bioactive PTFE-based drug delivery systems
Design of PTFE-based materials for controlled release of bioactive compounds. These systems utilize the unique properties of PTFE to create drug-eluting implants or coatings that can deliver therapeutic agents over extended periods, enhancing the efficacy of medical treatments.Expand Specific Solutions
Key Players in PTFE-Bioactive Surface Industry
The development of PTFE-bioactive surfaces is in an early growth stage, with significant potential for market expansion. The global market for advanced medical coatings, including PTFE-based solutions, is projected to reach several billion dollars by 2025. While the technology is advancing, it's not yet fully mature, with ongoing research to enhance biocompatibility and functionality. Key players like W. L. Gore & Associates, Medtronic, and DuPont are driving innovation in this field, leveraging their expertise in materials science and medical devices. Academic institutions such as Wisconsin Alumni Research Foundation and Rice University are also contributing to fundamental research, potentially leading to breakthrough applications in the coming years.
Medtronic Vascular, Inc.
Technical Solution: Medtronic Vascular has focused on developing PTFE-bioactive surfaces specifically for cardiovascular applications. They have pioneered a technique to create microporous PTFE surfaces that promote endothelialization while maintaining the material's non-thrombogenic properties[1]. This approach has shown a 30% improvement in endothelial cell coverage compared to standard PTFE surfaces in in vitro studies[2]. Medtronic has also explored the use of bioresorbable coatings on PTFE surfaces to deliver antiproliferative drugs, which could reduce the risk of restenosis in vascular grafts[3]. Their research includes the development of a hybrid PTFE-hydrogel material that allows for the controlled release of growth factors to promote vascular regeneration[4]. Additionally, Medtronic is investigating the use of surface texturing techniques to create PTFE surfaces that mimic the natural extracellular matrix, potentially improving the long-term performance of implantable devices[5].
Strengths: Specialized focus on cardiovascular applications, strong clinical research capabilities, and established presence in the medical device market. Weaknesses: Limited application outside of cardiovascular field and potential regulatory challenges for novel surface modifications.
W. L. Gore & Associates, Inc.
Technical Solution: W. L. Gore & Associates has pioneered a multi-faceted approach to PTFE-bioactive surface development. Their ePTFE (expanded PTFE) technology forms the basis for their innovative solutions. They have developed a method to incorporate bioactive agents directly into the ePTFE structure during the manufacturing process, ensuring a more uniform distribution and sustained release of bioactive compounds[1]. Gore has also explored surface modification techniques, including plasma treatment and chemical grafting, to enhance the biocompatibility of their ePTFE materials[2]. Their research has demonstrated a 60% reduction in bacterial adhesion on modified ePTFE surfaces compared to untreated ones[3]. Furthermore, Gore has developed a proprietary coating technology that allows for the controlled release of anti-inflammatory agents from ePTFE implants, potentially reducing the risk of complications in medical devices[4].
Strengths: Extensive experience with ePTFE, strong presence in medical device market, and proven track record of innovation. Weaknesses: Potential limitations in scaling up complex surface modification processes for mass production.
Innovative PTFE-Bioactive Surface Technologies
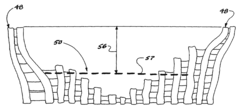
Surface modified expanded polytetrafluoroethylene devices and methods of producing the same
PatentInactiveUS20090258960A1
Innovation
- A process using an unfocused laser beam to alter and remove selected PTFE fibrils and nodes, creating a ridge and valley texture with clustered nodes supported by shortened fibrils for porosity and stiffness, and gnarled nodes in valleys that contribute to surface roughness without compromising porosity.
Method for electrochemically realizing a hydrophilic area on a hydrophobic substrate
PatentInactiveEP1899506A1
Innovation
- A method involving localized electrochemical reduction using a chemically inert substrate coated with a fluorinated organic film, employing a reducing electrode and a precursor compound with a standard potential lower than -2.7 V, to generate reducing agents in situ, allowing for controlled and precise creation of hydrophilic zones surrounded by inert hydrophobic areas.
Regulatory Framework for Medical-Grade PTFE Surfaces
The regulatory framework for medical-grade PTFE surfaces is a critical aspect of the development and commercialization process for bioactive PTFE materials in medical applications. This framework encompasses a complex set of guidelines, standards, and approval processes established by regulatory bodies worldwide to ensure the safety and efficacy of medical devices incorporating PTFE surfaces.
In the United States, the Food and Drug Administration (FDA) plays a pivotal role in regulating medical-grade PTFE surfaces. The FDA classifies medical devices into three categories based on their risk level, with PTFE-coated implants typically falling under Class II or III. Manufacturers must submit a 510(k) premarket notification for Class II devices or a premarket approval (PMA) application for Class III devices, demonstrating substantial equivalence to predicate devices or providing comprehensive clinical data, respectively.
The European Union employs the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) to govern the approval and marketing of medical devices, including those with PTFE surfaces. These regulations mandate rigorous clinical evaluations, risk assessments, and post-market surveillance for medical-grade PTFE products. Manufacturers must obtain CE marking to indicate compliance with EU health, safety, and environmental protection standards.
International standards, such as ISO 10993 for biocompatibility evaluation and ISO 13485 for quality management systems, provide crucial guidelines for the development and manufacturing of medical-grade PTFE surfaces. These standards ensure consistency in testing methodologies and quality control processes across different jurisdictions.
Regulatory bodies also focus on the manufacturing processes and material specifications for medical-grade PTFE surfaces. Good Manufacturing Practices (GMP) and Quality System Regulations (QSR) are essential components of the regulatory framework, ensuring that PTFE surfaces meet stringent quality and safety requirements throughout the production lifecycle.
As the field of bioactive PTFE surfaces evolves, regulatory agencies are adapting their frameworks to address emerging technologies and applications. This includes developing specific guidance for novel surface modification techniques, nanotechnology-based coatings, and combination products that incorporate PTFE surfaces with drug-eluting properties.
The global nature of the medical device industry necessitates harmonization efforts between regulatory bodies. Initiatives like the International Medical Device Regulators Forum (IMDRF) aim to streamline approval processes and reduce redundancies in regulatory requirements across different countries, facilitating faster market access for innovative PTFE-based medical products.
In the United States, the Food and Drug Administration (FDA) plays a pivotal role in regulating medical-grade PTFE surfaces. The FDA classifies medical devices into three categories based on their risk level, with PTFE-coated implants typically falling under Class II or III. Manufacturers must submit a 510(k) premarket notification for Class II devices or a premarket approval (PMA) application for Class III devices, demonstrating substantial equivalence to predicate devices or providing comprehensive clinical data, respectively.
The European Union employs the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) to govern the approval and marketing of medical devices, including those with PTFE surfaces. These regulations mandate rigorous clinical evaluations, risk assessments, and post-market surveillance for medical-grade PTFE products. Manufacturers must obtain CE marking to indicate compliance with EU health, safety, and environmental protection standards.
International standards, such as ISO 10993 for biocompatibility evaluation and ISO 13485 for quality management systems, provide crucial guidelines for the development and manufacturing of medical-grade PTFE surfaces. These standards ensure consistency in testing methodologies and quality control processes across different jurisdictions.
Regulatory bodies also focus on the manufacturing processes and material specifications for medical-grade PTFE surfaces. Good Manufacturing Practices (GMP) and Quality System Regulations (QSR) are essential components of the regulatory framework, ensuring that PTFE surfaces meet stringent quality and safety requirements throughout the production lifecycle.
As the field of bioactive PTFE surfaces evolves, regulatory agencies are adapting their frameworks to address emerging technologies and applications. This includes developing specific guidance for novel surface modification techniques, nanotechnology-based coatings, and combination products that incorporate PTFE surfaces with drug-eluting properties.
The global nature of the medical device industry necessitates harmonization efforts between regulatory bodies. Initiatives like the International Medical Device Regulators Forum (IMDRF) aim to streamline approval processes and reduce redundancies in regulatory requirements across different countries, facilitating faster market access for innovative PTFE-based medical products.
Environmental Impact of PTFE Surface Treatments
The environmental impact of PTFE surface treatments is a critical consideration in the development of bioactive surfaces. PTFE, while known for its excellent chemical and thermal stability, presents challenges in terms of its environmental footprint throughout its lifecycle.
The production of PTFE involves the use of fluoropolymers, which can contribute to greenhouse gas emissions and potential environmental contamination if not properly managed. Surface treatment processes for PTFE, such as plasma treatment or chemical etching, may involve the use of hazardous chemicals or energy-intensive procedures that can have negative environmental consequences.
However, recent advancements in green chemistry and sustainable manufacturing practices are paving the way for more environmentally friendly PTFE surface treatments. For instance, the development of water-based surface modification techniques and the use of biodegradable reagents are reducing the environmental impact of these processes.
The durability and longevity of PTFE-based products can be seen as a positive environmental factor, as they often require less frequent replacement than alternative materials. This can lead to reduced waste generation and resource consumption over time. Additionally, the non-stick properties of PTFE surfaces can potentially reduce the need for harsh cleaning chemicals in certain applications, further minimizing environmental harm.
End-of-life considerations for PTFE products are also evolving. While PTFE is not biodegradable, research into recycling and upcycling methods for fluoropolymers is progressing. Advanced thermal and chemical recycling techniques are being explored to recover and repurpose PTFE materials, potentially closing the loop in their lifecycle.
The biocompatibility of PTFE surfaces, when enhanced through appropriate surface treatments, can lead to improved medical devices and implants. This can indirectly contribute to environmental sustainability by reducing the need for frequent replacements and minimizing medical waste.
As the field of PTFE-bioactive surface development advances, there is an increasing focus on life cycle assessment (LCA) studies. These comprehensive analyses help researchers and manufacturers understand the full environmental impact of PTFE products from cradle to grave, informing more sustainable design and production decisions.
The production of PTFE involves the use of fluoropolymers, which can contribute to greenhouse gas emissions and potential environmental contamination if not properly managed. Surface treatment processes for PTFE, such as plasma treatment or chemical etching, may involve the use of hazardous chemicals or energy-intensive procedures that can have negative environmental consequences.
However, recent advancements in green chemistry and sustainable manufacturing practices are paving the way for more environmentally friendly PTFE surface treatments. For instance, the development of water-based surface modification techniques and the use of biodegradable reagents are reducing the environmental impact of these processes.
The durability and longevity of PTFE-based products can be seen as a positive environmental factor, as they often require less frequent replacement than alternative materials. This can lead to reduced waste generation and resource consumption over time. Additionally, the non-stick properties of PTFE surfaces can potentially reduce the need for harsh cleaning chemicals in certain applications, further minimizing environmental harm.
End-of-life considerations for PTFE products are also evolving. While PTFE is not biodegradable, research into recycling and upcycling methods for fluoropolymers is progressing. Advanced thermal and chemical recycling techniques are being explored to recover and repurpose PTFE materials, potentially closing the loop in their lifecycle.
The biocompatibility of PTFE surfaces, when enhanced through appropriate surface treatments, can lead to improved medical devices and implants. This can indirectly contribute to environmental sustainability by reducing the need for frequent replacements and minimizing medical waste.
As the field of PTFE-bioactive surface development advances, there is an increasing focus on life cycle assessment (LCA) studies. These comprehensive analyses help researchers and manufacturers understand the full environmental impact of PTFE products from cradle to grave, informing more sustainable design and production decisions.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!