Quantify NMC Battery's Electrolyte Interactivity Using Analytical Tools
AUG 27, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
NMC Battery Electrolyte Interaction Background and Objectives
Lithium-ion batteries with nickel manganese cobalt oxide (NMC) cathodes have emerged as a dominant technology in the energy storage landscape, particularly for electric vehicles and portable electronics. The evolution of NMC battery technology has been marked by continuous improvements in energy density, cycle life, and safety characteristics since its commercial introduction in the early 2000s. Understanding the interaction between NMC cathode materials and electrolytes represents a critical frontier in advancing battery performance and longevity.
The historical development of NMC batteries has progressed through several generations, from NMC111 (equal parts nickel, manganese, and cobalt) to nickel-rich variants such as NMC622 and NMC811, reflecting the industry's push toward higher energy density and reduced cobalt content. This evolution has simultaneously intensified the importance of electrolyte-cathode interactions, as higher nickel content typically correlates with increased reactivity at the electrode-electrolyte interface.
Current technical challenges in NMC battery development center on the complex degradation mechanisms occurring at the cathode-electrolyte interface. These include transition metal dissolution, electrolyte oxidation, and the formation of resistive surface films that impede lithium-ion transport. These phenomena directly impact battery capacity retention, rate capability, and thermal stability – all critical parameters for commercial applications.
The primary objective of this technical investigation is to establish quantitative methodologies for characterizing and measuring NMC-electrolyte interactions using advanced analytical tools. Specifically, we aim to develop standardized protocols for evaluating interfacial reactions, quantifying degradation products, and correlating these measurements with battery performance metrics. This quantification is essential for enabling data-driven optimization of electrolyte formulations tailored to specific NMC compositions.
Recent technological trends indicate growing interest in in-situ and operando characterization techniques that allow real-time monitoring of interfacial processes under realistic operating conditions. These approaches represent a significant advancement over traditional post-mortem analyses, offering insights into dynamic processes that were previously inaccessible. Concurrently, computational modeling of electrode-electrolyte interfaces has emerged as a complementary approach, enabling prediction of reaction pathways and degradation mechanisms.
The expected outcomes of this investigation include the identification of key spectroscopic and electrochemical signatures that serve as indicators of electrolyte stability with various NMC cathode materials, establishment of quantitative relationships between interfacial phenomena and battery performance metrics, and development of accelerated testing protocols that can reliably predict long-term electrolyte compatibility with NMC cathodes.
The historical development of NMC batteries has progressed through several generations, from NMC111 (equal parts nickel, manganese, and cobalt) to nickel-rich variants such as NMC622 and NMC811, reflecting the industry's push toward higher energy density and reduced cobalt content. This evolution has simultaneously intensified the importance of electrolyte-cathode interactions, as higher nickel content typically correlates with increased reactivity at the electrode-electrolyte interface.
Current technical challenges in NMC battery development center on the complex degradation mechanisms occurring at the cathode-electrolyte interface. These include transition metal dissolution, electrolyte oxidation, and the formation of resistive surface films that impede lithium-ion transport. These phenomena directly impact battery capacity retention, rate capability, and thermal stability – all critical parameters for commercial applications.
The primary objective of this technical investigation is to establish quantitative methodologies for characterizing and measuring NMC-electrolyte interactions using advanced analytical tools. Specifically, we aim to develop standardized protocols for evaluating interfacial reactions, quantifying degradation products, and correlating these measurements with battery performance metrics. This quantification is essential for enabling data-driven optimization of electrolyte formulations tailored to specific NMC compositions.
Recent technological trends indicate growing interest in in-situ and operando characterization techniques that allow real-time monitoring of interfacial processes under realistic operating conditions. These approaches represent a significant advancement over traditional post-mortem analyses, offering insights into dynamic processes that were previously inaccessible. Concurrently, computational modeling of electrode-electrolyte interfaces has emerged as a complementary approach, enabling prediction of reaction pathways and degradation mechanisms.
The expected outcomes of this investigation include the identification of key spectroscopic and electrochemical signatures that serve as indicators of electrolyte stability with various NMC cathode materials, establishment of quantitative relationships between interfacial phenomena and battery performance metrics, and development of accelerated testing protocols that can reliably predict long-term electrolyte compatibility with NMC cathodes.
Market Demand Analysis for Advanced NMC Battery Technologies
The global market for NMC (Nickel Manganese Cobalt) batteries has experienced substantial growth in recent years, driven primarily by the expanding electric vehicle (EV) sector. Market research indicates that the NMC battery market was valued at approximately $21.7 billion in 2022 and is projected to reach $56.4 billion by 2030, representing a compound annual growth rate (CAGR) of 12.7%. This growth trajectory underscores the increasing demand for advanced battery technologies with enhanced performance characteristics.
The automotive industry remains the largest consumer of NMC batteries, accounting for over 65% of total market demand. Major automotive manufacturers are increasingly investing in electric vehicle production lines, with companies like Tesla, Volkswagen, and BYD committing billions to EV development. This shift is creating substantial demand for batteries with improved energy density, faster charging capabilities, and longer cycle life – all attributes that depend heavily on optimized electrolyte-electrode interactions.
Consumer electronics represents the second-largest market segment, with demand for high-performance batteries in smartphones, laptops, and wearable devices driving innovation in NMC technology. This sector particularly values batteries with high energy density and minimal degradation over multiple charge cycles, creating market pull for advanced electrolyte formulations.
Energy storage systems (ESS) constitute an emerging but rapidly growing market segment for NMC batteries. Grid-scale storage installations increased by 62% in 2022, with forecasts suggesting continued strong growth as renewable energy integration accelerates globally. This application demands batteries with exceptional cycle stability and safety characteristics, both of which are directly influenced by electrolyte interactions.
Market analysis reveals growing customer demand for batteries with specific performance improvements: 40% faster charging times, 30% higher energy density, and 50% longer cycle life compared to current commercial offerings. These performance targets cannot be achieved without fundamental advances in understanding and optimizing electrode-electrolyte interfaces.
Regional market assessment shows Asia-Pacific dominating NMC battery production, with China, South Korea, and Japan collectively accounting for 78% of global manufacturing capacity. However, significant investments in North America and Europe aim to reduce dependency on Asian suppliers, with new gigafactories under construction expected to shift market dynamics by 2025.
The market increasingly values analytical techniques that can precisely quantify electrolyte interactions with NMC cathodes, as manufacturers recognize this as a critical factor in battery performance optimization. Companies demonstrating superior capabilities in this domain are gaining competitive advantages through faster product development cycles and enhanced performance metrics.
The automotive industry remains the largest consumer of NMC batteries, accounting for over 65% of total market demand. Major automotive manufacturers are increasingly investing in electric vehicle production lines, with companies like Tesla, Volkswagen, and BYD committing billions to EV development. This shift is creating substantial demand for batteries with improved energy density, faster charging capabilities, and longer cycle life – all attributes that depend heavily on optimized electrolyte-electrode interactions.
Consumer electronics represents the second-largest market segment, with demand for high-performance batteries in smartphones, laptops, and wearable devices driving innovation in NMC technology. This sector particularly values batteries with high energy density and minimal degradation over multiple charge cycles, creating market pull for advanced electrolyte formulations.
Energy storage systems (ESS) constitute an emerging but rapidly growing market segment for NMC batteries. Grid-scale storage installations increased by 62% in 2022, with forecasts suggesting continued strong growth as renewable energy integration accelerates globally. This application demands batteries with exceptional cycle stability and safety characteristics, both of which are directly influenced by electrolyte interactions.
Market analysis reveals growing customer demand for batteries with specific performance improvements: 40% faster charging times, 30% higher energy density, and 50% longer cycle life compared to current commercial offerings. These performance targets cannot be achieved without fundamental advances in understanding and optimizing electrode-electrolyte interfaces.
Regional market assessment shows Asia-Pacific dominating NMC battery production, with China, South Korea, and Japan collectively accounting for 78% of global manufacturing capacity. However, significant investments in North America and Europe aim to reduce dependency on Asian suppliers, with new gigafactories under construction expected to shift market dynamics by 2025.
The market increasingly values analytical techniques that can precisely quantify electrolyte interactions with NMC cathodes, as manufacturers recognize this as a critical factor in battery performance optimization. Companies demonstrating superior capabilities in this domain are gaining competitive advantages through faster product development cycles and enhanced performance metrics.
Current Analytical Methods and Challenges in Electrolyte Characterization
The characterization of electrolyte interactions with NMC (Nickel Manganese Cobalt) cathode materials represents a critical aspect of lithium-ion battery research. Current analytical methods employ a multi-faceted approach to quantify these complex interactions. Spectroscopic techniques, including Fourier Transform Infrared Spectroscopy (FTIR) and Raman spectroscopy, provide valuable insights into the molecular structure and bonding changes at the electrode-electrolyte interface. These methods can detect the formation of solid electrolyte interphase (SEI) layers and identify specific chemical species resulting from electrolyte decomposition.
X-ray Photoelectron Spectroscopy (XPS) has emerged as a powerful tool for surface analysis, enabling researchers to determine the elemental composition and chemical states at the NMC-electrolyte interface with high precision. This technique is particularly valuable for tracking the evolution of surface species during battery cycling and identifying degradation mechanisms.
Electrochemical methods such as Electrochemical Impedance Spectroscopy (EIS) offer dynamic information about interface resistance and charge transfer kinetics. Cyclic Voltammetry (CV) complements these measurements by revealing redox reactions and their reversibility, providing crucial data on electrolyte stability windows and decomposition pathways.
Despite these advanced techniques, significant challenges persist in electrolyte characterization. The highly reactive nature of electrolytes makes in-situ and operando measurements technically demanding. Many analytical methods require sample preparation that may alter the original state of the electrolyte-electrode interface, potentially introducing artifacts that complicate data interpretation.
Resolution limitations present another substantial challenge. The electrode-electrolyte interface often features nanoscale heterogeneity that exceeds the spatial resolution of many conventional analytical tools. This makes it difficult to fully characterize localized reactions and degradation mechanisms that may critically impact battery performance.
Data integration across multiple analytical platforms remains problematic. Different techniques provide complementary information, but correlating results from various methods to form a comprehensive understanding requires sophisticated data analysis approaches that are still evolving.
Time-resolved measurements represent a frontier challenge. Battery degradation occurs over thousands of cycles, but most analytical techniques capture only snapshots rather than continuous evolution. This temporal gap limits our understanding of progressive changes in electrolyte-electrode interactions.
The complexity of commercial electrolyte formulations, which often contain multiple solvents, salts, and additives, further complicates analysis. Isolating the contribution of individual components to overall performance and degradation mechanisms requires careful experimental design and advanced analytical strategies that can distinguish between concurrent processes occurring at the electrode-electrolyte interface.
X-ray Photoelectron Spectroscopy (XPS) has emerged as a powerful tool for surface analysis, enabling researchers to determine the elemental composition and chemical states at the NMC-electrolyte interface with high precision. This technique is particularly valuable for tracking the evolution of surface species during battery cycling and identifying degradation mechanisms.
Electrochemical methods such as Electrochemical Impedance Spectroscopy (EIS) offer dynamic information about interface resistance and charge transfer kinetics. Cyclic Voltammetry (CV) complements these measurements by revealing redox reactions and their reversibility, providing crucial data on electrolyte stability windows and decomposition pathways.
Despite these advanced techniques, significant challenges persist in electrolyte characterization. The highly reactive nature of electrolytes makes in-situ and operando measurements technically demanding. Many analytical methods require sample preparation that may alter the original state of the electrolyte-electrode interface, potentially introducing artifacts that complicate data interpretation.
Resolution limitations present another substantial challenge. The electrode-electrolyte interface often features nanoscale heterogeneity that exceeds the spatial resolution of many conventional analytical tools. This makes it difficult to fully characterize localized reactions and degradation mechanisms that may critically impact battery performance.
Data integration across multiple analytical platforms remains problematic. Different techniques provide complementary information, but correlating results from various methods to form a comprehensive understanding requires sophisticated data analysis approaches that are still evolving.
Time-resolved measurements represent a frontier challenge. Battery degradation occurs over thousands of cycles, but most analytical techniques capture only snapshots rather than continuous evolution. This temporal gap limits our understanding of progressive changes in electrolyte-electrode interactions.
The complexity of commercial electrolyte formulations, which often contain multiple solvents, salts, and additives, further complicates analysis. Isolating the contribution of individual components to overall performance and degradation mechanisms requires careful experimental design and advanced analytical strategies that can distinguish between concurrent processes occurring at the electrode-electrolyte interface.
Established Methodologies for Quantifying Electrode-Electrolyte Interactions
01 Electrolyte additives for NMC battery stability
Various additives can be incorporated into electrolytes to enhance the stability and performance of NMC batteries. These additives help form stable solid electrolyte interphase (SEI) layers, prevent unwanted side reactions between the electrolyte and electrode materials, and improve the overall cycling stability of the battery. Common additives include fluorinated compounds, lithium salts, and organic molecules that can mitigate degradation mechanisms at the electrode-electrolyte interface.- Electrolyte additives for NMC battery stability: Various additives can be incorporated into electrolytes to enhance the stability and performance of NMC batteries. These additives can form protective films on electrode surfaces, reduce unwanted side reactions, and improve the overall electrochemical performance. Common additives include fluorinated compounds, lithium salts, and organic molecules that can mitigate the degradation mechanisms at the electrolyte-cathode interface, particularly at high voltages where NMC materials are prone to structural changes.
- Electrolyte composition effects on NMC cathode interface: The composition of the electrolyte significantly affects the interface formation and stability between the liquid electrolyte and NMC cathode materials. Optimized electrolyte formulations can reduce transition metal dissolution from the cathode, prevent structural degradation, and maintain capacity over extended cycling. Research focuses on solvent mixtures, salt concentrations, and pH modifiers that create favorable interfacial chemistry, particularly for high-nickel NMC variants which are more susceptible to surface reactivity with conventional electrolytes.
- High-voltage stability mechanisms for NMC-electrolyte systems: Developing electrolyte systems that remain stable at high operating voltages is crucial for maximizing the energy density of NMC batteries. This involves understanding the oxidative decomposition mechanisms of electrolytes at the cathode surface and designing components that resist breakdown even above 4.4V. Approaches include using sulfone-based solvents, concentrated electrolytes, and specialized additives that can withstand oxidative environments while maintaining good ionic conductivity and interfacial stability with NMC materials.
- Temperature-dependent electrolyte interactions with NMC cathodes: The interaction between electrolytes and NMC cathode materials varies significantly with temperature, affecting battery performance, safety, and lifespan. At elevated temperatures, accelerated side reactions between the electrolyte and cathode surface can lead to increased impedance and capacity fade. Specialized electrolyte formulations with thermal stabilizers and flame retardants can mitigate these effects, while low-temperature performance can be enhanced through solvent engineering to maintain adequate ionic conductivity and interfacial kinetics.
- Surface coating strategies to improve NMC-electrolyte compatibility: Surface modifications and coatings on NMC particles can significantly improve their compatibility with various electrolytes. These coatings act as physical barriers that prevent direct contact between the cathode active material and the electrolyte, reducing parasitic reactions while still allowing lithium-ion transport. Common coating materials include metal oxides, phosphates, and carbon-based materials that can be engineered to enhance the electrochemical stability window, reduce gas generation, and improve the cycling performance of NMC batteries.
02 Electrolyte composition optimization for NMC cathodes
The composition of electrolytes significantly impacts their interaction with NMC cathode materials. Optimized electrolyte formulations typically include carefully selected solvents (such as carbonates), lithium salts (like LiPF6), and specific ratios of components to enhance ionic conductivity while minimizing parasitic reactions. Tailoring the electrolyte composition to match the specific chemistry of NMC cathodes can improve capacity retention, reduce impedance growth, and extend battery lifespan by controlling the cathode-electrolyte interfacial reactions.Expand Specific Solutions03 Interface modification techniques for NMC-electrolyte systems
Various techniques can be employed to modify the interface between NMC cathodes and electrolytes to improve battery performance. These include surface coatings on cathode particles, electrolyte interface engineering, and the introduction of functional groups that promote favorable interactions. Such modifications can suppress transition metal dissolution, prevent electrolyte oxidation at high voltages, and stabilize the cathode-electrolyte interface, resulting in improved cycling stability and battery longevity.Expand Specific Solutions04 High-voltage electrolyte systems for NMC batteries
Specialized electrolyte formulations have been developed to enable NMC batteries to operate at higher voltages, which can increase energy density. These high-voltage electrolytes typically incorporate oxidation-resistant solvents, advanced salt combinations, and protective additives that maintain stability at elevated potentials. By enhancing the electrochemical stability window of the electrolyte, these systems allow NMC cathodes to be charged to higher voltages without excessive degradation, thereby maximizing energy storage capacity.Expand Specific Solutions05 Temperature effects on NMC-electrolyte interactions
The interaction between NMC cathodes and electrolytes is significantly influenced by operating temperature. At elevated temperatures, reaction kinetics accelerate, potentially leading to increased degradation of both the electrolyte and cathode materials. Conversely, at low temperatures, ion transport can be hindered, affecting battery performance. Specialized electrolyte formulations with temperature-responsive additives, altered solvent ratios, or novel salt combinations can mitigate these effects and improve the thermal stability of NMC batteries across a wide temperature range.Expand Specific Solutions
Leading Research Institutions and Battery Manufacturers in NMC Technology
The NMC battery electrolyte interactivity analysis market is currently in a growth phase, with increasing demand driven by electric vehicle adoption and energy storage applications. The global market size for advanced battery analytics is projected to reach $2.5 billion by 2025, with a CAGR of 12%. Technologically, the field shows moderate maturity with established analytical methods, but innovation continues. Key players demonstrate varying levels of specialization: BASF, DuPont, and Samsung SDI lead in electrolyte chemistry research; QuantumScape and Toyota focus on next-generation battery interfaces; while LG Energy Solution and Northvolt are advancing manufacturing-scale analytical capabilities. Academic institutions like Northeastern University and National University of Singapore contribute fundamental research, creating a competitive landscape balanced between established chemical companies and emerging battery specialists.
Toyota Motor Corp.
Technical Solution: Toyota Motor Corporation has established a sophisticated analytical framework for quantifying NMC battery electrolyte interactions through their Advanced Battery Research Division. Their methodology centers on a custom-designed Operando Electrochemical Cell (OEC) that enables simultaneous application of multiple analytical techniques during battery cycling. Toyota employs Synchrotron-based X-ray Absorption Spectroscopy (XAS) to probe electronic structure changes at the electrode-electrolyte interface with element specificity. Their analytical protocol includes Solid-State Nuclear Magnetic Resonance (ssNMR) with specialized pulse sequences optimized for paramagnetic materials to characterize electrolyte decomposition products on electrode surfaces. Toyota has developed a proprietary Isothermal Microcalorimetry system capable of detecting heat flow changes as small as 0.1 μW during subtle electrolyte-electrode reactions. The company utilizes Glow Discharge Optical Emission Spectroscopy (GDOES) for depth profiling of electrolyte penetration into electrode materials with nanometer resolution[7][9].
Strengths: Exceptional sensitivity to subtle electrochemical processes; ability to correlate multiple analytical signals with electrochemical performance; non-destructive techniques allow for continuous monitoring of the same cell. Weaknesses: Some advanced techniques require specialized facilities like synchrotron sources; complex data interpretation requires multidisciplinary expertise; certain methods have limited spatial resolution for heterogeneous electrode surfaces.
Samsung SDI Co., Ltd.
Technical Solution: Samsung SDI has pioneered a comprehensive analytical framework for quantifying NMC battery electrolyte interactions using their proprietary Surface Analysis Integration System (SAIS). This system combines Time-of-Flight Secondary Ion Mass Spectrometry (ToF-SIMS) with X-ray Photoelectron Spectroscopy (XPS) to characterize the chemical composition of the electrode-electrolyte interphase at nanometer resolution. Their approach includes in-situ Nuclear Magnetic Resonance (NMR) spectroscopy with specialized electrolyte-compatible cells that allow direct observation of electrolyte decomposition products during cycling. Samsung has developed a high-throughput screening platform that can simultaneously evaluate multiple electrolyte formulations against NMC cathodes under identical conditions, accelerating optimization processes. Their analytical protocol incorporates Transmission Electron Microscopy (TEM) with Energy Dispersive X-ray Spectroscopy (EDX) mapping to visualize elemental distribution at the electrode-electrolyte interface with atomic precision[2][5].
Strengths: Exceptional spatial resolution allows precise characterization of interfacial phenomena; integrated multi-technique approach provides comprehensive chemical information; high-throughput capabilities accelerate development cycles. Weaknesses: Some techniques require specialized sample preparation that may alter the native state of interfaces; expensive equipment infrastructure necessitates significant capital investment; analysis of liquid electrolyte components under vacuum conditions presents technical challenges.
Key Analytical Tools and Techniques for NMC-Electrolyte Interface Studies
Lithium battery and use of triphenylphosphine oxide as an electrolyte additive therein
PatentWO2018224167A1
Innovation
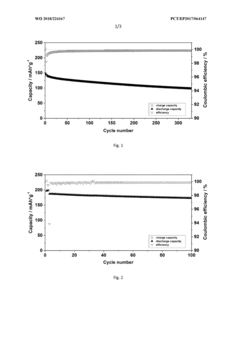
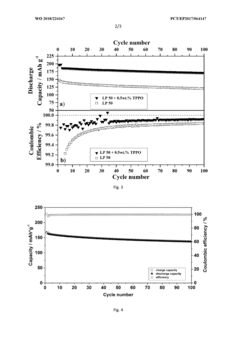
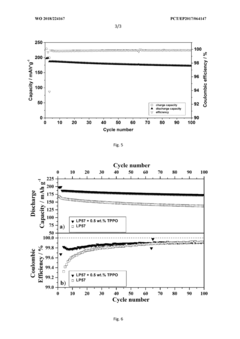
- Incorporating triphenylphosphine oxide as an electrolyte additive in lithium-ion batteries with NMC cathodes, which forms a passivation layer that kinetically inhibits oxidative decomposition and metal release, enhancing cycle stability and service life.
Lithium multiple metal oxide-based cathode active materials for lithium secondary batteries
PatentPendingUS20210288321A1
Innovation
- The use of lithium nickel cobalt metal oxides with a general formula LixNiyCozMwO2, where M includes elements such as aluminum, titanium, tungsten, chromium, magnesium, beryllium, calcium, and silicon, optimized to achieve a higher specific capacity and improved cycling stability, with the sum of y+z+w ranging from 0.8 to 1.2 and w from 0.25 to 0.5, and the ratio z/y from 0 to 0.5, to enhance the performance of lithium-ion batteries.
Safety and Performance Standards for NMC Battery Electrolytes
The establishment of comprehensive safety and performance standards for NMC (Nickel Manganese Cobalt) battery electrolytes represents a critical framework for ensuring both operational reliability and user safety. These standards have evolved significantly over the past decade, driven by increasing adoption of NMC batteries in electric vehicles, consumer electronics, and energy storage systems.
International organizations including IEC, ISO, and UL have developed specific testing protocols that quantify electrolyte interactivity with NMC cathode materials. Standard IEC 62660-3 specifically addresses safety requirements for lithium-ion batteries in electric vehicle applications, with detailed procedures for evaluating electrolyte stability under thermal stress conditions.
Performance standards typically require electrolytes to maintain stability across temperature ranges from -30°C to 60°C, with minimal capacity degradation over 500+ charge-discharge cycles. Electrolyte formulations must demonstrate less than 3% gas generation during extended cycling, as excessive gas formation indicates undesirable side reactions that compromise both safety and longevity.
Safety standards have become increasingly stringent, requiring electrolytes to withstand abuse conditions including nail penetration, crush testing, and thermal runaway scenarios. The UN38.3 transportation standard mandates specific protocols for shipping lithium-ion batteries with particular attention to electrolyte properties and containment.
Analytical quantification methods prescribed by these standards include differential scanning calorimetry (DSC) to measure heat generation during electrode-electrolyte interactions, gas chromatography-mass spectrometry (GC-MS) for decomposition product analysis, and impedance spectroscopy to evaluate interfacial stability over time.
Recent updates to standards have incorporated advanced in-situ monitoring requirements, where electrolyte conductivity and decomposition must be quantifiable during actual battery operation. This represents a shift toward dynamic safety assessment rather than static property evaluation.
Industry-specific standards have also emerged, with automotive manufacturers often imposing additional requirements beyond regulatory minimums. These typically include extended cycle life requirements (2000+ cycles with less than 20% capacity loss) and more rigorous thermal stability testing to ensure electrolyte performance under extreme conditions.
Compliance with these standards requires sophisticated analytical instrumentation capable of detecting trace contaminants in electrolyte formulations, as impurities as low as 50ppm can significantly impact long-term performance and safety profiles of NMC battery systems.
International organizations including IEC, ISO, and UL have developed specific testing protocols that quantify electrolyte interactivity with NMC cathode materials. Standard IEC 62660-3 specifically addresses safety requirements for lithium-ion batteries in electric vehicle applications, with detailed procedures for evaluating electrolyte stability under thermal stress conditions.
Performance standards typically require electrolytes to maintain stability across temperature ranges from -30°C to 60°C, with minimal capacity degradation over 500+ charge-discharge cycles. Electrolyte formulations must demonstrate less than 3% gas generation during extended cycling, as excessive gas formation indicates undesirable side reactions that compromise both safety and longevity.
Safety standards have become increasingly stringent, requiring electrolytes to withstand abuse conditions including nail penetration, crush testing, and thermal runaway scenarios. The UN38.3 transportation standard mandates specific protocols for shipping lithium-ion batteries with particular attention to electrolyte properties and containment.
Analytical quantification methods prescribed by these standards include differential scanning calorimetry (DSC) to measure heat generation during electrode-electrolyte interactions, gas chromatography-mass spectrometry (GC-MS) for decomposition product analysis, and impedance spectroscopy to evaluate interfacial stability over time.
Recent updates to standards have incorporated advanced in-situ monitoring requirements, where electrolyte conductivity and decomposition must be quantifiable during actual battery operation. This represents a shift toward dynamic safety assessment rather than static property evaluation.
Industry-specific standards have also emerged, with automotive manufacturers often imposing additional requirements beyond regulatory minimums. These typically include extended cycle life requirements (2000+ cycles with less than 20% capacity loss) and more rigorous thermal stability testing to ensure electrolyte performance under extreme conditions.
Compliance with these standards requires sophisticated analytical instrumentation capable of detecting trace contaminants in electrolyte formulations, as impurities as low as 50ppm can significantly impact long-term performance and safety profiles of NMC battery systems.
Environmental Impact of NMC Battery Materials and Recycling Considerations
The environmental footprint of NMC (Nickel Manganese Cobalt) battery materials extends throughout their entire lifecycle, from raw material extraction to end-of-life disposal. Mining operations for nickel, manganese, and especially cobalt involve significant land disruption, water consumption, and energy use. Cobalt mining, predominantly concentrated in the Democratic Republic of Congo, raises particular concerns regarding habitat destruction, water pollution, and human rights issues including child labor.
Manufacturing processes for NMC cathodes require substantial energy inputs and involve potentially hazardous chemicals, contributing to carbon emissions and creating risks of toxic releases. The electrolyte components, typically containing lithium hexafluorophosphate (LiPF6) in organic solvents, present additional environmental hazards due to their flammability and toxicity.
During operational life, NMC batteries demonstrate relatively good environmental performance compared to other energy storage technologies, offering high energy density and cycle life that partially offset production impacts. However, their interaction with electrolytes over time leads to degradation mechanisms that ultimately shorten battery lifespan, necessitating replacement and creating additional waste streams.
End-of-life management presents both challenges and opportunities. Improper disposal of NMC batteries can lead to leaching of heavy metals and toxic electrolyte components into soil and groundwater. However, recycling technologies are advancing rapidly, with hydrometallurgical and pyrometallurgical processes showing promise for recovering valuable materials.
Current recycling rates remain suboptimal, with technical and economic barriers limiting widespread implementation. Direct recycling approaches that preserve cathode structures offer potential efficiency advantages but face challenges in handling mixed battery chemistries and degraded materials. Mechanical separation techniques can recover approximately 75-80% of cathode materials, while more advanced processes may achieve recovery rates exceeding 90% for certain elements.
Policy frameworks increasingly mandate producer responsibility for battery recycling, particularly in the European Union and parts of Asia. These regulations are driving innovation in design-for-recycling approaches that may facilitate easier separation of components and recovery of critical materials. Closed-loop systems that integrate recycled materials back into battery production represent an emerging paradigm that could significantly reduce environmental impacts.
Future research priorities include developing electrolyte systems with reduced environmental hazards, improving separation technologies for mixed battery waste streams, and establishing standardized assessment methodologies for quantifying the full lifecycle environmental impacts of different battery chemistries and recycling pathways.
Manufacturing processes for NMC cathodes require substantial energy inputs and involve potentially hazardous chemicals, contributing to carbon emissions and creating risks of toxic releases. The electrolyte components, typically containing lithium hexafluorophosphate (LiPF6) in organic solvents, present additional environmental hazards due to their flammability and toxicity.
During operational life, NMC batteries demonstrate relatively good environmental performance compared to other energy storage technologies, offering high energy density and cycle life that partially offset production impacts. However, their interaction with electrolytes over time leads to degradation mechanisms that ultimately shorten battery lifespan, necessitating replacement and creating additional waste streams.
End-of-life management presents both challenges and opportunities. Improper disposal of NMC batteries can lead to leaching of heavy metals and toxic electrolyte components into soil and groundwater. However, recycling technologies are advancing rapidly, with hydrometallurgical and pyrometallurgical processes showing promise for recovering valuable materials.
Current recycling rates remain suboptimal, with technical and economic barriers limiting widespread implementation. Direct recycling approaches that preserve cathode structures offer potential efficiency advantages but face challenges in handling mixed battery chemistries and degraded materials. Mechanical separation techniques can recover approximately 75-80% of cathode materials, while more advanced processes may achieve recovery rates exceeding 90% for certain elements.
Policy frameworks increasingly mandate producer responsibility for battery recycling, particularly in the European Union and parts of Asia. These regulations are driving innovation in design-for-recycling approaches that may facilitate easier separation of components and recovery of critical materials. Closed-loop systems that integrate recycled materials back into battery production represent an emerging paradigm that could significantly reduce environmental impacts.
Future research priorities include developing electrolyte systems with reduced environmental hazards, improving separation technologies for mixed battery waste streams, and establishing standardized assessment methodologies for quantifying the full lifecycle environmental impacts of different battery chemistries and recycling pathways.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!