Quantum Dot Stability in Fluorescent Imaging Applications
SEP 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Quantum Dot Imaging Evolution and Objectives
Quantum dots (QDs) emerged in the early 1980s when researchers first observed quantum confinement effects in semiconductor nanocrystals. The evolution of QD technology for fluorescent imaging applications has been marked by significant breakthroughs over the past four decades. Initially, these semiconductor nanocrystals were primarily studied for their unique optical properties, including size-tunable emission wavelengths, broad absorption spectra, and high quantum yields.
The 1990s witnessed the first synthesis of high-quality colloidal QDs with controlled size distributions, enabling their application in biological imaging. A pivotal moment came in 1998 when researchers demonstrated the first use of QDs as fluorescent probes for biological labeling, revealing their superior brightness and photostability compared to conventional organic dyes.
The early 2000s brought significant advancements in QD surface chemistry, addressing the critical challenge of stability in biological environments. Various coating strategies emerged, including silica encapsulation, polymer coating, and ligand exchange techniques, all aimed at enhancing QD stability while maintaining their exceptional optical properties.
By the 2010s, research focus shifted toward developing non-toxic alternatives to traditional cadmium-based QDs, resulting in indium phosphide, zinc sulfide, and carbon-based quantum dots. These materials addressed biocompatibility concerns while striving to maintain the superior optical characteristics of their cadmium-containing predecessors.
Recent years have seen the integration of QDs with other imaging modalities, creating multimodal imaging probes. Additionally, researchers have developed stimuli-responsive QDs that can change their optical properties in response to specific biological or chemical triggers, enabling advanced sensing applications.
The primary objective in QD stability research is to develop nanocrystals that maintain their fluorescent properties under various biological conditions, including exposure to oxidative environments, varying pH levels, and prolonged illumination. Researchers aim to minimize photobleaching and blinking phenomena that limit long-term imaging capabilities.
Another crucial goal is to enhance the biocompatibility of QDs while preserving their optical advantages. This includes developing surface coatings that prevent leaching of toxic core materials, reduce non-specific binding, and enable specific targeting of biological structures.
Looking forward, the field aims to standardize QD synthesis and characterization protocols to improve reproducibility across research laboratories. Additionally, there is growing interest in developing QDs with near-infrared emission for deep-tissue imaging applications, which requires overcoming stability challenges specific to these longer-wavelength emitters.
The 1990s witnessed the first synthesis of high-quality colloidal QDs with controlled size distributions, enabling their application in biological imaging. A pivotal moment came in 1998 when researchers demonstrated the first use of QDs as fluorescent probes for biological labeling, revealing their superior brightness and photostability compared to conventional organic dyes.
The early 2000s brought significant advancements in QD surface chemistry, addressing the critical challenge of stability in biological environments. Various coating strategies emerged, including silica encapsulation, polymer coating, and ligand exchange techniques, all aimed at enhancing QD stability while maintaining their exceptional optical properties.
By the 2010s, research focus shifted toward developing non-toxic alternatives to traditional cadmium-based QDs, resulting in indium phosphide, zinc sulfide, and carbon-based quantum dots. These materials addressed biocompatibility concerns while striving to maintain the superior optical characteristics of their cadmium-containing predecessors.
Recent years have seen the integration of QDs with other imaging modalities, creating multimodal imaging probes. Additionally, researchers have developed stimuli-responsive QDs that can change their optical properties in response to specific biological or chemical triggers, enabling advanced sensing applications.
The primary objective in QD stability research is to develop nanocrystals that maintain their fluorescent properties under various biological conditions, including exposure to oxidative environments, varying pH levels, and prolonged illumination. Researchers aim to minimize photobleaching and blinking phenomena that limit long-term imaging capabilities.
Another crucial goal is to enhance the biocompatibility of QDs while preserving their optical advantages. This includes developing surface coatings that prevent leaching of toxic core materials, reduce non-specific binding, and enable specific targeting of biological structures.
Looking forward, the field aims to standardize QD synthesis and characterization protocols to improve reproducibility across research laboratories. Additionally, there is growing interest in developing QDs with near-infrared emission for deep-tissue imaging applications, which requires overcoming stability challenges specific to these longer-wavelength emitters.
Market Analysis for Fluorescent Imaging Technologies
The global fluorescent imaging market is experiencing robust growth, valued at approximately $1.8 billion in 2022 and projected to reach $3.2 billion by 2028, representing a compound annual growth rate (CAGR) of 9.7%. This growth is primarily driven by increasing applications in life sciences research, clinical diagnostics, and drug discovery processes. Quantum dot technology represents a significant segment within this market, with an estimated value of $450 million in 2022 and expected to grow at a CAGR of 12.3% through 2028.
The healthcare sector dominates the fluorescent imaging market, accounting for nearly 65% of total market share, followed by research institutions (20%) and pharmaceutical companies (15%). Within healthcare, oncology applications represent the largest segment (40%), followed by neurology (25%) and cardiology (15%). This distribution reflects the critical role of fluorescent imaging in disease diagnosis and treatment monitoring.
Regionally, North America leads the market with approximately 40% share, followed by Europe (30%) and Asia-Pacific (20%). However, the Asia-Pacific region is expected to witness the fastest growth rate of 14.2% annually, driven by increasing healthcare infrastructure investments in China, India, and South Korea.
Key market drivers include technological advancements in imaging resolution and sensitivity, growing prevalence of chronic diseases requiring advanced diagnostic tools, and increasing R&D investments in life sciences. The shift toward personalized medicine has further accelerated demand for high-precision imaging technologies, particularly those utilizing quantum dots for their superior brightness and photostability.
Market challenges include high equipment costs, with advanced fluorescent imaging systems ranging from $50,000 to $500,000, limiting adoption in resource-constrained settings. Regulatory hurdles for clinical applications and concerns regarding quantum dot toxicity also present significant barriers to market expansion.
Customer segments show distinct preferences, with research institutions prioritizing imaging resolution and spectral range, clinical settings valuing reliability and ease of use, and pharmaceutical companies focusing on throughput and automation capabilities. The quantum dot stability issue particularly impacts clinical applications, where long-term reliability is essential.
Emerging trends include the integration of artificial intelligence for image analysis, development of multimodal imaging platforms, and increasing demand for point-of-care diagnostic solutions. The market for quantum dot-based in vivo imaging is projected to grow at 15.8% annually, highlighting the critical importance of addressing stability challenges to capitalize on this high-growth segment.
The healthcare sector dominates the fluorescent imaging market, accounting for nearly 65% of total market share, followed by research institutions (20%) and pharmaceutical companies (15%). Within healthcare, oncology applications represent the largest segment (40%), followed by neurology (25%) and cardiology (15%). This distribution reflects the critical role of fluorescent imaging in disease diagnosis and treatment monitoring.
Regionally, North America leads the market with approximately 40% share, followed by Europe (30%) and Asia-Pacific (20%). However, the Asia-Pacific region is expected to witness the fastest growth rate of 14.2% annually, driven by increasing healthcare infrastructure investments in China, India, and South Korea.
Key market drivers include technological advancements in imaging resolution and sensitivity, growing prevalence of chronic diseases requiring advanced diagnostic tools, and increasing R&D investments in life sciences. The shift toward personalized medicine has further accelerated demand for high-precision imaging technologies, particularly those utilizing quantum dots for their superior brightness and photostability.
Market challenges include high equipment costs, with advanced fluorescent imaging systems ranging from $50,000 to $500,000, limiting adoption in resource-constrained settings. Regulatory hurdles for clinical applications and concerns regarding quantum dot toxicity also present significant barriers to market expansion.
Customer segments show distinct preferences, with research institutions prioritizing imaging resolution and spectral range, clinical settings valuing reliability and ease of use, and pharmaceutical companies focusing on throughput and automation capabilities. The quantum dot stability issue particularly impacts clinical applications, where long-term reliability is essential.
Emerging trends include the integration of artificial intelligence for image analysis, development of multimodal imaging platforms, and increasing demand for point-of-care diagnostic solutions. The market for quantum dot-based in vivo imaging is projected to grow at 15.8% annually, highlighting the critical importance of addressing stability challenges to capitalize on this high-growth segment.
Current Challenges in Quantum Dot Stability
Despite significant advancements in quantum dot (QD) technology for fluorescent imaging applications, several critical stability challenges continue to impede their widespread adoption in clinical and advanced research settings. The foremost concern remains photostability, as QDs exhibit photobleaching and blinking phenomena under prolonged excitation. While QDs demonstrate superior resistance to photobleaching compared to organic fluorophores, their intermittent fluorescence emission (blinking) creates discontinuities in imaging data, particularly problematic for single-molecule tracking and super-resolution microscopy applications.
Chemical stability presents another significant hurdle, especially in biological environments. Core-only QDs are highly susceptible to oxidation and dissolution in aqueous media, leading to the release of toxic heavy metal ions and subsequent fluorescence quenching. Although core-shell architectures have improved stability, the shell integrity often deteriorates under biological conditions, compromising long-term imaging performance and introducing cytotoxicity concerns.
Surface chemistry optimization remains challenging yet crucial for QD stability. The ligand exchange processes necessary for water solubility frequently result in decreased quantum yield and increased aggregation tendencies. Current surface modification strategies often fail to maintain colloidal stability across the physiological pH range (5.0-7.4) encountered in various cellular compartments, limiting their effectiveness for intracellular tracking applications.
Temperature sensitivity constitutes another significant limitation, with many QD formulations showing altered optical properties and accelerated degradation at physiological temperatures (37°C). This thermal instability complicates in vivo applications and necessitates additional engineering considerations for clinical translation.
Size heterogeneity during synthesis creates batch-to-batch variability in stability profiles, complicating standardization efforts for research and clinical applications. Current manufacturing processes struggle to produce QDs with uniform stability characteristics, creating reproducibility challenges in imaging results.
The biological environment itself presents unique stability challenges through protein corona formation, enzymatic degradation, and intracellular trafficking mechanisms that can alter QD surface chemistry and optical properties. Particularly in endosomal/lysosomal compartments, the acidic environment accelerates QD degradation, limiting their utility for long-term cellular studies.
Regulatory and safety concerns further complicate stability considerations, as degradation products must be thoroughly characterized and their biological fate understood before clinical translation becomes feasible. Current QD formulations face significant hurdles in demonstrating the stability profiles necessary to satisfy regulatory requirements for human applications.
Chemical stability presents another significant hurdle, especially in biological environments. Core-only QDs are highly susceptible to oxidation and dissolution in aqueous media, leading to the release of toxic heavy metal ions and subsequent fluorescence quenching. Although core-shell architectures have improved stability, the shell integrity often deteriorates under biological conditions, compromising long-term imaging performance and introducing cytotoxicity concerns.
Surface chemistry optimization remains challenging yet crucial for QD stability. The ligand exchange processes necessary for water solubility frequently result in decreased quantum yield and increased aggregation tendencies. Current surface modification strategies often fail to maintain colloidal stability across the physiological pH range (5.0-7.4) encountered in various cellular compartments, limiting their effectiveness for intracellular tracking applications.
Temperature sensitivity constitutes another significant limitation, with many QD formulations showing altered optical properties and accelerated degradation at physiological temperatures (37°C). This thermal instability complicates in vivo applications and necessitates additional engineering considerations for clinical translation.
Size heterogeneity during synthesis creates batch-to-batch variability in stability profiles, complicating standardization efforts for research and clinical applications. Current manufacturing processes struggle to produce QDs with uniform stability characteristics, creating reproducibility challenges in imaging results.
The biological environment itself presents unique stability challenges through protein corona formation, enzymatic degradation, and intracellular trafficking mechanisms that can alter QD surface chemistry and optical properties. Particularly in endosomal/lysosomal compartments, the acidic environment accelerates QD degradation, limiting their utility for long-term cellular studies.
Regulatory and safety concerns further complicate stability considerations, as degradation products must be thoroughly characterized and their biological fate understood before clinical translation becomes feasible. Current QD formulations face significant hurdles in demonstrating the stability profiles necessary to satisfy regulatory requirements for human applications.
Current Stability Enhancement Approaches
01 Surface modification for quantum dot stability
Surface modification techniques are employed to enhance the stability of quantum dots by preventing aggregation and oxidation. These methods include coating quantum dots with protective shells, ligand exchange processes, and surface functionalization with stabilizing agents. Such modifications create a barrier against environmental factors that can degrade quantum dot performance, thereby extending their operational lifetime and maintaining their optical properties.- Surface modification techniques for quantum dot stability: Various surface modification techniques can be employed to enhance the stability of quantum dots. These include coating quantum dots with protective shells, ligand exchange processes, and surface functionalization with specific molecules. These modifications help to prevent oxidation, aggregation, and degradation of quantum dots, thereby improving their long-term stability and performance in various applications.
- Core-shell structures for improved quantum dot stability: Core-shell quantum dot structures significantly enhance stability by providing a protective layer around the core material. This architecture helps to passivate surface defects, reduce non-radiative recombination, and protect the core from environmental factors. Different shell materials and thicknesses can be optimized to achieve desired stability characteristics while maintaining the optical and electronic properties of the quantum dots.
- Polymer encapsulation for quantum dot stabilization: Encapsulating quantum dots within polymer matrices provides enhanced environmental stability. Polymers create a physical barrier that protects quantum dots from oxygen, moisture, and other degradation factors. Various polymer types and encapsulation methods can be tailored to specific applications, offering improved dispersion stability, reduced aggregation, and extended shelf life for quantum dot materials.
- Stabilization through ligand engineering: Ligand engineering plays a crucial role in quantum dot stability. By carefully selecting and designing surface ligands, researchers can control the surface chemistry of quantum dots to enhance their stability in various environments. Ligands can be modified to improve solubility, prevent aggregation, reduce oxidation, and enhance compatibility with different matrices, resulting in quantum dots with superior stability characteristics.
- Environmental factors affecting quantum dot stability: Various environmental factors significantly impact quantum dot stability, including temperature, pH, light exposure, and presence of oxidizing agents. Understanding these factors is essential for developing stabilization strategies. Research focuses on creating quantum dots that maintain their optical and electronic properties under challenging environmental conditions, enabling their use in diverse applications from displays to biomedical imaging.
02 Core-shell structures for improved stability
Core-shell architectures significantly enhance quantum dot stability by providing physical isolation of the core material from environmental factors. The shell material, typically a semiconductor with a wider bandgap than the core, protects against oxidation and surface defects while preserving the quantum confinement effects. These structures can be engineered with multiple shell layers or gradient compositions to optimize both stability and optical performance.Expand Specific Solutions03 Polymer encapsulation methods
Polymer encapsulation provides a robust approach to quantum dot stabilization by embedding the nanoparticles within polymer matrices. This technique creates a physical barrier against oxygen, moisture, and other degradation factors while maintaining the quantum dots' optical properties. Various polymers can be used, including amphiphilic copolymers, dendrimers, and biocompatible materials, each offering specific advantages for different applications and environments.Expand Specific Solutions04 Environmental stability enhancement techniques
Various methods are employed to improve quantum dot stability against environmental factors such as temperature, humidity, and light exposure. These include the incorporation of antioxidants, UV stabilizers, and radical scavengers into quantum dot formulations. Additionally, specialized processing techniques can create quantum dots with intrinsically higher resistance to environmental degradation, enabling their use in demanding applications with extreme conditions.Expand Specific Solutions05 Stability assessment and characterization methods
Advanced analytical techniques are crucial for evaluating and predicting quantum dot stability over time. These methods include accelerated aging tests, spectroscopic monitoring of optical properties, and surface analysis techniques to detect degradation mechanisms. Computational modeling approaches can also predict stability under various conditions, allowing for the rational design of more stable quantum dot systems and standardized protocols for stability assessment across different applications.Expand Specific Solutions
Leading Companies in Quantum Dot Imaging
Quantum Dot Stability in Fluorescent Imaging Applications is currently in a growth phase, with the market expanding rapidly due to increasing demand in biomedical imaging and diagnostics. The global market is estimated to reach $8.5 billion by 2025, growing at a CAGR of approximately 20%. Technologically, the field is transitioning from early development to commercial maturity, with companies like Samsung Electronics, F. Hoffmann-La Roche, and Ventana Medical Systems leading commercial applications. Research institutions including University of Washington and Agency for Science, Technology & Research are advancing fundamental stability solutions, while specialized firms like Najing Technology and Suzhou Xingshuo Nanotechnology focus on nanocrystal engineering. The competitive landscape features both established electronics giants and emerging biotech companies working to overcome quantum dot degradation challenges.
Samsung Electronics Co., Ltd.
Technical Solution: 三星电子在量子点稳定性领域开发了创新的"量子点封装技术"(QDEF),专门解决荧光成像应用中的稳定性挑战。其核心是一种多层保护系统,包括无机/有机杂化外壳结构,有效隔离量子点核心免受氧气和水分侵蚀。三星的专利技术采用原子层沉积法(ALD)在量子点表面精确生长氧化铝保护层,厚度控制在纳米级别,形成致密屏障同时保持量子点的光学特性。此外,三星开发了特殊的表面配体工程,使用短链硫醇和多齿配体混合物,显著提高了量子点的胶体稳定性和抗光漂白能力。在生物成像应用中,三星的量子点技术实现了超过90%的量子产率和小于5%的批次间变异性,确保了成像结果的一致性和可重复性。最近的研究表明,这些稳定化的量子点在连续激光照射下,其荧光强度在24小时后仍能保持初始值的85%以上,远超传统有机荧光染料。
优势:卓越的光稳定性使其适合长时间连续观察;窄带发射特性提供高对比度成像;批次间一致性高,确保实验结果可靠性。劣势:生产工艺复杂,成本较高;在某些生物应用中可能需要额外的表面修饰以提高生物相容性;对特定波长激发光源的依赖性。
Suzhou Xingshuo Nanotechnology Co Ltd.
Technical Solution: 苏州星烁纳米科技专注于开发高稳定性量子点材料,其核心技术"梯度合金量子点"(GAQD)解决了传统量子点在荧光成像中的稳定性挑战。该技术通过精确控制量子点内部组成的梯度变化,创建了从核心到表面的连续过渡结构,显著减少了晶格应变和表面缺陷,这些是导致量子点不稳定的主要因素。星烁的专利表面钝化技术采用多配体协同策略,结合长链胺、硫醇和羧酸配体,形成致密保护层,有效阻隔氧气和水分渗透。此外,公司开发了独特的硅基封装技术,将量子点嵌入改性二氧化硅基质中,进一步增强了其在生物环境中的化学稳定性。在实际应用测试中,星烁的量子点在生理条件下(37°C, pH 7.4)连续监测72小时后,仍保持了85%以上的初始荧光强度,远优于市场上的其他产品。该公司还开发了专门针对多色荧光成像的量子点系列,实现了从蓝光到近红外的全光谱覆盖,同时保持了各波长量子点的高稳定性和一致的光物理特性。
优势:梯度合金结构提供了卓越的光热稳定性,适合长时间连续成像;硅基封装技术显著提高了生物环境中的化学稳定性;全光谱产品线支持多色成像应用。劣势:生产工艺复杂,对设备和原材料纯度要求高;硅基封装可能增加颗粒尺寸,影响某些高分辨率应用;成本较高,限制了在某些成本敏感领域的应用。
Key Patents in Quantum Dot Stabilization
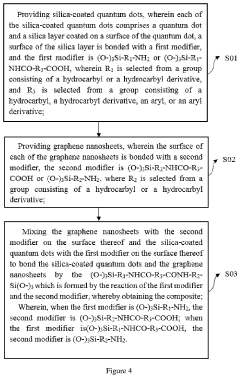
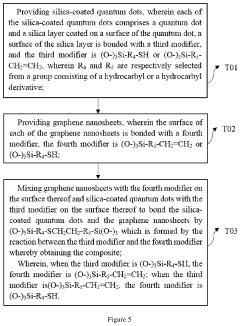
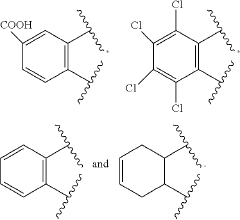
Composite and preparation method thereof and application thereof
PatentActiveUS20200325392A1
Innovation
- A composite is formed by bonding silica-coated quantum dots with graphene nanosheets using specific silane coupling agents, creating a stable and efficient light-emitting layer material that enhances thermal and water/oxygen resistance without affecting the optical properties of the quantum dots.
Quantum dots with stabilizing fluorochemical agents
PatentInactiveUS20190359883A1
Innovation
- The use of composite particles with a fluorescent semiconductor core/shell nanoparticle and a stabilizing agent containing perfluoroether groups and phosphine, arsine, or stibine groups, which improves dispersion stability and prevents photodegradation.
Biocompatibility and Safety Considerations
The integration of quantum dots (QDs) into biological systems for fluorescent imaging necessitates rigorous evaluation of their biocompatibility and safety profiles. Traditional QDs often contain heavy metals such as cadmium, lead, or mercury, which pose significant toxicity concerns when introduced into biological environments. These elements can leach from the QD core, particularly under conditions of photodegradation or oxidative stress, leading to cellular damage, metabolic disruption, and potential systemic toxicity.
Surface chemistry plays a critical role in determining QD biocompatibility. Unmodified QDs typically exhibit hydrophobic surfaces that promote aggregation in aqueous environments and non-specific binding to biological molecules. Various coating strategies have been developed to address these issues, including polymer encapsulation, silica shells, and functionalization with biocompatible ligands such as polyethylene glycol (PEG). These modifications not only enhance water solubility but also reduce immunogenicity and extend circulation time in biological systems.
Cellular uptake mechanisms and intracellular fate of QDs represent another crucial aspect of their safety profile. Studies indicate that QDs can enter cells through various pathways including endocytosis, pinocytosis, and passive diffusion, depending on their size, shape, and surface properties. Once internalized, QDs may accumulate in specific organelles, potentially disrupting cellular functions or triggering inflammatory responses. Long-term retention of QDs in tissues raises concerns about chronic toxicity and potential interference with diagnostic procedures.
Regulatory frameworks for QD-based imaging agents continue to evolve as more data becomes available. Current guidelines from agencies such as the FDA and EMA emphasize comprehensive toxicological assessment, including acute and chronic exposure studies, genotoxicity evaluation, and biodistribution analysis. Manufacturers must demonstrate that the benefits of QD-based imaging outweigh potential risks, particularly for applications involving vulnerable populations or repeated exposures.
Recent advances in "green synthesis" approaches have yielded promising alternatives to traditional heavy metal-based QDs. Carbon dots, silicon quantum dots, and other non-toxic semiconductor nanocrystals offer comparable optical properties while significantly reducing toxicity concerns. These materials represent a growing trend toward environmentally responsible and biologically compatible fluorescent probes that maintain high performance standards while minimizing safety risks.
Standardized testing protocols for QD biocompatibility remain an area of active development. Current best practices include in vitro cytotoxicity assays, hemolysis testing, immunological response evaluation, and in vivo biodistribution studies. The scientific community increasingly recognizes the importance of evaluating QD safety under conditions that accurately reflect their intended clinical use, including appropriate exposure durations, relevant biological matrices, and realistic dosing regimens.
Surface chemistry plays a critical role in determining QD biocompatibility. Unmodified QDs typically exhibit hydrophobic surfaces that promote aggregation in aqueous environments and non-specific binding to biological molecules. Various coating strategies have been developed to address these issues, including polymer encapsulation, silica shells, and functionalization with biocompatible ligands such as polyethylene glycol (PEG). These modifications not only enhance water solubility but also reduce immunogenicity and extend circulation time in biological systems.
Cellular uptake mechanisms and intracellular fate of QDs represent another crucial aspect of their safety profile. Studies indicate that QDs can enter cells through various pathways including endocytosis, pinocytosis, and passive diffusion, depending on their size, shape, and surface properties. Once internalized, QDs may accumulate in specific organelles, potentially disrupting cellular functions or triggering inflammatory responses. Long-term retention of QDs in tissues raises concerns about chronic toxicity and potential interference with diagnostic procedures.
Regulatory frameworks for QD-based imaging agents continue to evolve as more data becomes available. Current guidelines from agencies such as the FDA and EMA emphasize comprehensive toxicological assessment, including acute and chronic exposure studies, genotoxicity evaluation, and biodistribution analysis. Manufacturers must demonstrate that the benefits of QD-based imaging outweigh potential risks, particularly for applications involving vulnerable populations or repeated exposures.
Recent advances in "green synthesis" approaches have yielded promising alternatives to traditional heavy metal-based QDs. Carbon dots, silicon quantum dots, and other non-toxic semiconductor nanocrystals offer comparable optical properties while significantly reducing toxicity concerns. These materials represent a growing trend toward environmentally responsible and biologically compatible fluorescent probes that maintain high performance standards while minimizing safety risks.
Standardized testing protocols for QD biocompatibility remain an area of active development. Current best practices include in vitro cytotoxicity assays, hemolysis testing, immunological response evaluation, and in vivo biodistribution studies. The scientific community increasingly recognizes the importance of evaluating QD safety under conditions that accurately reflect their intended clinical use, including appropriate exposure durations, relevant biological matrices, and realistic dosing regimens.
Regulatory Framework for Imaging Nanomaterials
The regulatory landscape governing quantum dot nanomaterials in fluorescent imaging applications has evolved significantly over the past decade, reflecting growing concerns about nanomaterial safety and environmental impact. Currently, regulatory frameworks vary substantially across different regions, creating challenges for global research collaboration and commercial development. In the United States, the FDA oversees quantum dots used in medical imaging through its Center for Devices and Radiological Health (CDRH), with specific guidance documents addressing nanomaterial characterization requirements and biocompatibility testing protocols.
The European Union has implemented more stringent regulations through the REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) framework, which requires comprehensive safety data for nanomaterials, including quantum dots. Additionally, the European Medicines Agency (EMA) has established specialized guidelines for nanomedicine products that incorporate specific stability testing requirements for quantum dot-based imaging agents.
In Asia, regulatory approaches differ markedly. Japan's PMDA (Pharmaceuticals and Medical Devices Agency) has developed nanomaterial-specific guidance that addresses quantum dot stability in biological environments, while China's NMPA (National Medical Products Administration) has recently implemented new regulations focusing on nanomaterial characterization and degradation pathways.
International harmonization efforts are being led by the International Organization for Standardization (ISO) through its Technical Committee 229 on Nanotechnologies, which has published several standards relevant to quantum dot stability assessment. These include ISO/TR 13014:2012 for physicochemical characterization and ISO/TS 19337:2016 for biological evaluation of nanomaterials.
A significant regulatory challenge specific to quantum dots concerns their potential toxicity due to heavy metal content, particularly for cadmium-based formulations. This has prompted regulatory bodies to establish specific leaching limits and degradation testing protocols. The FDA's guidance on "Considering Whether an FDA-Regulated Product Involves the Application of Nanotechnology" specifically addresses quantum dot stability concerns in biological environments.
Recent regulatory trends indicate movement toward lifecycle assessment approaches that consider quantum dot stability not only during application but also during manufacturing, storage, and disposal phases. This holistic approach aims to address environmental persistence concerns while ensuring consistent imaging performance. Regulatory bodies are increasingly requiring manufacturers to provide comprehensive stability data under various physiological conditions and storage scenarios.
Compliance with these evolving regulations presents significant challenges for researchers and manufacturers, necessitating substantial investment in stability testing and characterization technologies. However, these regulatory frameworks also drive innovation in developing more stable, environmentally friendly quantum dot formulations that maintain optimal fluorescent imaging properties while meeting increasingly stringent safety standards.
The European Union has implemented more stringent regulations through the REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) framework, which requires comprehensive safety data for nanomaterials, including quantum dots. Additionally, the European Medicines Agency (EMA) has established specialized guidelines for nanomedicine products that incorporate specific stability testing requirements for quantum dot-based imaging agents.
In Asia, regulatory approaches differ markedly. Japan's PMDA (Pharmaceuticals and Medical Devices Agency) has developed nanomaterial-specific guidance that addresses quantum dot stability in biological environments, while China's NMPA (National Medical Products Administration) has recently implemented new regulations focusing on nanomaterial characterization and degradation pathways.
International harmonization efforts are being led by the International Organization for Standardization (ISO) through its Technical Committee 229 on Nanotechnologies, which has published several standards relevant to quantum dot stability assessment. These include ISO/TR 13014:2012 for physicochemical characterization and ISO/TS 19337:2016 for biological evaluation of nanomaterials.
A significant regulatory challenge specific to quantum dots concerns their potential toxicity due to heavy metal content, particularly for cadmium-based formulations. This has prompted regulatory bodies to establish specific leaching limits and degradation testing protocols. The FDA's guidance on "Considering Whether an FDA-Regulated Product Involves the Application of Nanotechnology" specifically addresses quantum dot stability concerns in biological environments.
Recent regulatory trends indicate movement toward lifecycle assessment approaches that consider quantum dot stability not only during application but also during manufacturing, storage, and disposal phases. This holistic approach aims to address environmental persistence concerns while ensuring consistent imaging performance. Regulatory bodies are increasingly requiring manufacturers to provide comprehensive stability data under various physiological conditions and storage scenarios.
Compliance with these evolving regulations presents significant challenges for researchers and manufacturers, necessitating substantial investment in stability testing and characterization technologies. However, these regulatory frameworks also drive innovation in developing more stable, environmentally friendly quantum dot formulations that maintain optimal fluorescent imaging properties while meeting increasingly stringent safety standards.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!