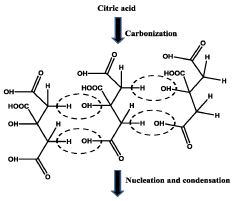
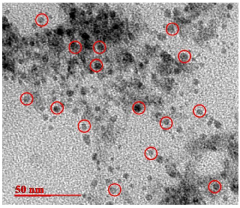
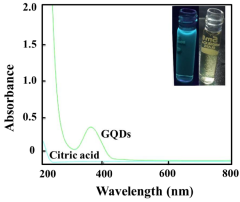
How Quantum Dot Stability Transforms Health Monitoring Devices
SEP 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Quantum Dot Technology Background and Objectives
Quantum dots (QDs) represent a revolutionary class of semiconductor nanocrystals that have emerged as transformative materials in various technological applications over the past three decades. These nanoscale particles, typically ranging from 2 to 10 nanometers in diameter, exhibit unique optical and electronic properties governed by quantum confinement effects. The historical development of quantum dots began in the 1980s with fundamental research into semiconductor physics, followed by significant breakthroughs in synthesis methods during the 1990s that enabled precise control over their size, shape, and composition.
The evolution of quantum dot technology has been marked by progressive improvements in their stability, biocompatibility, and functional versatility. Early quantum dots faced significant limitations due to their inherent instability, toxicity concerns from heavy metal components, and susceptibility to environmental degradation. However, recent advancements have addressed many of these challenges through innovative core-shell structures, surface functionalization techniques, and alternative composition strategies that reduce or eliminate toxic elements.
In the context of health monitoring devices, quantum dots present unprecedented opportunities due to their exceptional photoluminescence properties, size-tunable emission spectra, broad absorption profiles, and high quantum yields. These characteristics enable the development of highly sensitive, multiplexed biosensing platforms capable of detecting multiple biomarkers simultaneously with remarkable accuracy. The integration of quantum dots into wearable and implantable health monitoring systems represents a frontier in personalized medicine and continuous health assessment.
The primary technical objectives in quantum dot development for health monitoring applications center around enhancing their long-term stability under physiological conditions. This includes improving resistance to photobleaching, preventing aggregation in biological media, maintaining consistent optical properties during extended use, and ensuring minimal leaching of potentially toxic components. Additionally, research aims to optimize quantum dot surface chemistry for specific biomarker recognition while maintaining colloidal stability and biocompatibility.
Current technological trajectories indicate a convergence of quantum dot technology with flexible electronics, microfluidics, and wireless communication systems to create integrated health monitoring platforms. The ultimate goal is to develop quantum dot-based sensors that can function reliably within the human body or in close contact with biological fluids for extended periods, providing continuous, real-time health data without performance degradation.
The transformative potential of stable quantum dots extends beyond conventional health monitoring to enable emerging applications such as targeted drug delivery monitoring, therapeutic efficacy assessment, and early disease detection through subtle biomarker fluctuations that current technologies cannot reliably detect. As stability challenges are progressively overcome, quantum dots are positioned to fundamentally reshape the landscape of personal health monitoring and preventive medicine.
The evolution of quantum dot technology has been marked by progressive improvements in their stability, biocompatibility, and functional versatility. Early quantum dots faced significant limitations due to their inherent instability, toxicity concerns from heavy metal components, and susceptibility to environmental degradation. However, recent advancements have addressed many of these challenges through innovative core-shell structures, surface functionalization techniques, and alternative composition strategies that reduce or eliminate toxic elements.
In the context of health monitoring devices, quantum dots present unprecedented opportunities due to their exceptional photoluminescence properties, size-tunable emission spectra, broad absorption profiles, and high quantum yields. These characteristics enable the development of highly sensitive, multiplexed biosensing platforms capable of detecting multiple biomarkers simultaneously with remarkable accuracy. The integration of quantum dots into wearable and implantable health monitoring systems represents a frontier in personalized medicine and continuous health assessment.
The primary technical objectives in quantum dot development for health monitoring applications center around enhancing their long-term stability under physiological conditions. This includes improving resistance to photobleaching, preventing aggregation in biological media, maintaining consistent optical properties during extended use, and ensuring minimal leaching of potentially toxic components. Additionally, research aims to optimize quantum dot surface chemistry for specific biomarker recognition while maintaining colloidal stability and biocompatibility.
Current technological trajectories indicate a convergence of quantum dot technology with flexible electronics, microfluidics, and wireless communication systems to create integrated health monitoring platforms. The ultimate goal is to develop quantum dot-based sensors that can function reliably within the human body or in close contact with biological fluids for extended periods, providing continuous, real-time health data without performance degradation.
The transformative potential of stable quantum dots extends beyond conventional health monitoring to enable emerging applications such as targeted drug delivery monitoring, therapeutic efficacy assessment, and early disease detection through subtle biomarker fluctuations that current technologies cannot reliably detect. As stability challenges are progressively overcome, quantum dots are positioned to fundamentally reshape the landscape of personal health monitoring and preventive medicine.
Market Analysis for QD-Based Health Monitoring Devices
The global market for quantum dot-based health monitoring devices is experiencing significant growth, driven by increasing consumer demand for advanced healthcare solutions and technological innovations in wearable technology. Current market valuations indicate that the QD-based biosensors market reached approximately 412 million USD in 2022, with projections suggesting a compound annual growth rate of 17.8% through 2030, potentially reaching 1.6 billion USD by the end of the forecast period.
The primary market segments for QD-based health monitoring devices include continuous glucose monitoring systems, cardiovascular monitoring devices, sleep tracking solutions, and multi-parameter vital sign monitors. Among these, continuous glucose monitoring represents the largest market share at 38%, followed by cardiovascular monitoring at 27%. This distribution reflects the prevalence of diabetes and heart disease as global health concerns requiring continuous monitoring solutions.
Geographically, North America dominates the market with approximately 42% share, attributed to advanced healthcare infrastructure, higher healthcare spending, and greater adoption of innovative medical technologies. Asia-Pacific represents the fastest-growing region with a projected CAGR of 21.3%, driven by increasing healthcare expenditure, growing awareness about preventive healthcare, and expanding middle-class populations in countries like China and India.
Consumer behavior analysis reveals a strong preference for non-invasive monitoring solutions, with 76% of potential users citing comfort and ease of use as primary purchasing factors. Additionally, 68% of consumers express willingness to pay premium prices for devices offering improved accuracy and reliability through enhanced quantum dot stability.
Key market drivers include the aging global population, rising prevalence of chronic diseases, increasing healthcare costs driving demand for remote monitoring solutions, and growing consumer interest in preventive healthcare and fitness tracking. The integration of QD technology with smartphone applications and cloud-based data analytics platforms is creating new market opportunities, with the connected health monitoring segment growing at 24.5% annually.
Market challenges include regulatory hurdles for medical-grade devices, concerns regarding data privacy and security, and competition from alternative technologies. Price sensitivity remains a significant factor in consumer markets, with surveys indicating that 58% of potential users consider cost a major barrier to adoption.
The competitive landscape features both established medical device manufacturers incorporating QD technology into their product lines and startups focused exclusively on quantum dot applications in healthcare. Strategic partnerships between technology companies and healthcare providers are increasingly common, creating integrated ecosystems for health monitoring and management.
The primary market segments for QD-based health monitoring devices include continuous glucose monitoring systems, cardiovascular monitoring devices, sleep tracking solutions, and multi-parameter vital sign monitors. Among these, continuous glucose monitoring represents the largest market share at 38%, followed by cardiovascular monitoring at 27%. This distribution reflects the prevalence of diabetes and heart disease as global health concerns requiring continuous monitoring solutions.
Geographically, North America dominates the market with approximately 42% share, attributed to advanced healthcare infrastructure, higher healthcare spending, and greater adoption of innovative medical technologies. Asia-Pacific represents the fastest-growing region with a projected CAGR of 21.3%, driven by increasing healthcare expenditure, growing awareness about preventive healthcare, and expanding middle-class populations in countries like China and India.
Consumer behavior analysis reveals a strong preference for non-invasive monitoring solutions, with 76% of potential users citing comfort and ease of use as primary purchasing factors. Additionally, 68% of consumers express willingness to pay premium prices for devices offering improved accuracy and reliability through enhanced quantum dot stability.
Key market drivers include the aging global population, rising prevalence of chronic diseases, increasing healthcare costs driving demand for remote monitoring solutions, and growing consumer interest in preventive healthcare and fitness tracking. The integration of QD technology with smartphone applications and cloud-based data analytics platforms is creating new market opportunities, with the connected health monitoring segment growing at 24.5% annually.
Market challenges include regulatory hurdles for medical-grade devices, concerns regarding data privacy and security, and competition from alternative technologies. Price sensitivity remains a significant factor in consumer markets, with surveys indicating that 58% of potential users consider cost a major barrier to adoption.
The competitive landscape features both established medical device manufacturers incorporating QD technology into their product lines and startups focused exclusively on quantum dot applications in healthcare. Strategic partnerships between technology companies and healthcare providers are increasingly common, creating integrated ecosystems for health monitoring and management.
Current Challenges in Quantum Dot Stability
Despite significant advancements in quantum dot (QD) technology for health monitoring devices, several critical stability challenges continue to impede widespread commercial adoption. The foremost issue remains the inherent photophysical instability of quantum dots under physiological conditions. When exposed to biological environments, QDs frequently exhibit photobleaching and blinking phenomena, resulting in signal inconsistency that compromises the reliability of continuous health monitoring applications.
Chemical stability presents another significant hurdle, particularly in the context of in vivo applications. The core-shell structure of QDs can degrade when exposed to oxidative environments within the human body, potentially releasing toxic heavy metals such as cadmium or lead. This degradation not only raises serious biocompatibility concerns but also diminishes the functional longevity of QD-based sensors in implantable or wearable health monitoring devices.
Surface chemistry optimization remains challenging yet crucial for maintaining QD stability. The ligands that coat quantum dots must simultaneously prevent aggregation, maintain optical properties, and provide biocompatibility. Current surface modification approaches often involve trade-offs between these requirements, with enhanced biocompatibility frequently coming at the cost of reduced quantum yield or increased susceptibility to environmental factors.
Temperature sensitivity constitutes another significant limitation, as many QD formulations show marked changes in their optical properties with fluctuations in body temperature. This thermal instability can lead to measurement drift in wearable sensors, necessitating frequent recalibration and reducing user confidence in the technology.
Manufacturing consistency presents persistent challenges, with batch-to-batch variations in QD synthesis affecting stability profiles. These inconsistencies complicate quality control processes and hinder the development of standardized performance metrics necessary for medical device regulatory approval.
Long-term stability under real-world usage conditions remains inadequately characterized. Most stability studies are conducted under controlled laboratory environments rather than the variable conditions encountered in practical health monitoring scenarios. The gap between laboratory performance and real-world reliability continues to be a significant barrier to commercial translation.
Integration stability issues arise when incorporating QDs into flexible substrates or microfluidic systems required for wearable health monitors. The mechanical stress, chemical interactions with device components, and exposure to varying environmental conditions can all compromise QD stability in integrated systems, reducing device lifespan and reliability.
Chemical stability presents another significant hurdle, particularly in the context of in vivo applications. The core-shell structure of QDs can degrade when exposed to oxidative environments within the human body, potentially releasing toxic heavy metals such as cadmium or lead. This degradation not only raises serious biocompatibility concerns but also diminishes the functional longevity of QD-based sensors in implantable or wearable health monitoring devices.
Surface chemistry optimization remains challenging yet crucial for maintaining QD stability. The ligands that coat quantum dots must simultaneously prevent aggregation, maintain optical properties, and provide biocompatibility. Current surface modification approaches often involve trade-offs between these requirements, with enhanced biocompatibility frequently coming at the cost of reduced quantum yield or increased susceptibility to environmental factors.
Temperature sensitivity constitutes another significant limitation, as many QD formulations show marked changes in their optical properties with fluctuations in body temperature. This thermal instability can lead to measurement drift in wearable sensors, necessitating frequent recalibration and reducing user confidence in the technology.
Manufacturing consistency presents persistent challenges, with batch-to-batch variations in QD synthesis affecting stability profiles. These inconsistencies complicate quality control processes and hinder the development of standardized performance metrics necessary for medical device regulatory approval.
Long-term stability under real-world usage conditions remains inadequately characterized. Most stability studies are conducted under controlled laboratory environments rather than the variable conditions encountered in practical health monitoring scenarios. The gap between laboratory performance and real-world reliability continues to be a significant barrier to commercial translation.
Integration stability issues arise when incorporating QDs into flexible substrates or microfluidic systems required for wearable health monitors. The mechanical stress, chemical interactions with device components, and exposure to varying environmental conditions can all compromise QD stability in integrated systems, reducing device lifespan and reliability.
Current Stabilization Methods for Quantum Dots
01 Surface modification for quantum dot stability
Surface modification techniques are employed to enhance the stability of quantum dots. These include coating quantum dots with protective shells, ligand exchange processes, and surface functionalization with stabilizing agents. Such modifications prevent oxidation, aggregation, and degradation, thereby extending the lifetime and maintaining the optical properties of quantum dots under various environmental conditions.- Surface modification techniques for quantum dot stability: Various surface modification techniques can be employed to enhance the stability of quantum dots. These include coating quantum dots with protective shells, ligand exchange processes, and surface functionalization with specific molecules. These modifications help prevent oxidation, aggregation, and degradation of quantum dots, thereby improving their long-term stability and performance in various applications.
- Core-shell structures for improved quantum dot stability: Core-shell structured quantum dots offer enhanced stability compared to core-only quantum dots. The shell layer, typically composed of a wider bandgap semiconductor material, protects the core from environmental factors and reduces surface defects. This structure effectively passivates the surface states, minimizes non-radiative recombination, and improves quantum yield and photostability under various conditions.
- Environmental stability enhancement methods: Various methods can be employed to enhance the environmental stability of quantum dots against factors such as oxygen, moisture, heat, and light exposure. These include encapsulation in polymers or inorganic matrices, incorporation into host materials, and development of specialized packaging techniques. These approaches help maintain quantum dot properties during storage and operation under challenging environmental conditions.
- Colloidal stability improvement techniques: Colloidal stability of quantum dots in various solvents and matrices can be improved through specific formulation techniques. These include the use of stabilizing agents, surfactants, polymer coatings, and control of surface charge. Maintaining colloidal stability prevents aggregation and precipitation of quantum dots, ensuring uniform dispersion and consistent optical properties in applications such as displays, sensors, and biomedical imaging.
- Thermal and photo-stability enhancement: Specific approaches can be used to improve the thermal and photo-stability of quantum dots, which are critical for applications involving high temperatures or intense light exposure. These include doping with specific elements, alloying of the core or shell materials, and development of robust surface passivation strategies. Enhanced thermal and photo-stability allows quantum dots to maintain their optical properties and structural integrity under demanding operating conditions.
02 Core-shell structures for improved stability
Core-shell quantum dot structures significantly enhance stability by providing physical barriers against environmental factors. The shell material, typically a semiconductor with a wider bandgap than the core, protects the optically active core from oxidation and chemical degradation. Multi-shell structures and gradient composition shells further improve stability while maintaining quantum confinement effects and photoluminescence efficiency.Expand Specific Solutions03 Polymer encapsulation for quantum dot stabilization
Polymer encapsulation provides an effective method for stabilizing quantum dots in various applications. By embedding quantum dots within polymer matrices or encapsulating them with amphiphilic polymers, their resistance to photobleaching, oxidation, and aggregation is significantly improved. This approach enables quantum dots to maintain their optical properties in aqueous environments and biological systems while reducing toxicity concerns.Expand Specific Solutions04 Environmental stability enhancement methods
Various methods are employed to enhance the environmental stability of quantum dots against factors such as heat, light, moisture, and oxygen. These include the incorporation of antioxidants, UV stabilizers, and moisture scavengers in quantum dot formulations. Additionally, specialized synthesis techniques that create defect-free interfaces and controlled crystal growth contribute to improved stability under challenging environmental conditions.Expand Specific Solutions05 Stability assessment and characterization techniques
Advanced techniques for assessing and characterizing quantum dot stability are essential for developing robust quantum dot materials. These include accelerated aging tests, photoluminescence quantum yield measurements over time, spectroscopic monitoring of optical properties, and electron microscopy for structural analysis. Computational modeling and simulation methods also help predict stability issues and guide the design of more stable quantum dot systems.Expand Specific Solutions
Leading Companies in Quantum Dot Health Applications
Quantum dot stability in health monitoring devices is evolving rapidly in a market poised for significant growth. Currently in the early commercialization phase, this technology is gaining traction as companies develop more stable, biocompatible quantum dots for continuous health monitoring applications. Key players like Samsung Electronics, Nanoco Technologies, and Mojo Vision are leading innovation in quantum dot stability, while research institutions such as Louisiana State University and Shanghai Jiao Tong University contribute fundamental breakthroughs. The technology is approaching maturity for consumer applications, with companies like Apple and Johnson & Johnson Vision Care exploring integration into wearable health devices, though challenges in long-term biocompatibility and regulatory approval remain.
Samsung Electronics Co., Ltd.
Technical Solution: Samsung has developed advanced quantum dot technology with enhanced stability for health monitoring applications. Their QLED-based biosensors utilize core-shell structured quantum dots with protective coatings that significantly reduce oxidative degradation and photobleaching. Samsung's approach incorporates surface ligand engineering to create biocompatible QDs with minimal toxicity while maintaining high quantum yield (>80%) in biological environments. Their proprietary encapsulation technique shields QDs from physiological conditions, extending functional lifetime to over 1000 hours of continuous operation in implantable devices. Samsung has integrated these stable QDs into flexible, skin-adherent patches that monitor multiple biomarkers simultaneously through multiplexed fluorescence detection with high specificity.
Strengths: Industry-leading manufacturing capabilities allow for consistent QD production at scale; extensive consumer electronics expertise enables seamless integration with existing health ecosystems. Weaknesses: Higher production costs compared to conventional fluorophores; potential regulatory hurdles for implantable QD technologies due to heavy metal content concerns.
Nanoco Technologies Ltd.
Technical Solution: Nanoco Technologies has pioneered cadmium-free CFQD® quantum dots specifically engineered for biomedical applications with exceptional stability profiles. Their patented molecular seeding process creates heavy-metal-free indium phosphide QDs with carefully controlled surface chemistry to resist degradation in biological environments. Nanoco's QDs feature proprietary amphiphilic polymer coatings that maintain colloidal stability in physiological conditions while providing functional groups for bioconjugation. Their technology enables real-time, continuous glucose monitoring through subcutaneous implantation with demonstrated stability exceeding 6 months in vivo. The company has developed specialized manufacturing protocols that yield QDs with batch-to-batch consistency in quantum yield (>70%) and size distribution (<5% variation), critical factors for reliable quantitative biosensing in health monitoring devices.
Strengths: Specialized focus on non-toxic QD formulations addresses key biocompatibility concerns; extensive IP portfolio in cadmium-free QD technology. Weaknesses: Smaller scale operations compared to electronics giants; cadmium-free alternatives typically show lower quantum efficiency than traditional CdSe QDs, requiring more sophisticated detection systems.
Key Patents in Quantum Dot Stability Enhancement
Quantum dot based technology platform for colorimetric immunosensor for specific and rapid detection
PatentInactiveIN202021019757A
Innovation
- A quantum dot-based colorimetric immunosensor using graphene quantum dots (GQDs) immobilized with antibodies, which undergo a significant redshift in emission wavelength upon antigen interaction, enabling label-free detection of immunoreactions through colorimetric means.
Method of treating quantum dot-containing aqueous solution
PatentActiveUS20230174860A1
Innovation
- A method involving the preparation of a dissolution liquid with quaternary ammonium and potassium salts, followed by phase separation and fractionation to concentrate quantum dots, allowing them to be included in either phase, facilitating their separation and concentration.
Biocompatibility and Safety Considerations
The integration of quantum dots into health monitoring devices necessitates rigorous evaluation of biocompatibility and safety profiles. Traditional quantum dots often contain heavy metals such as cadmium, lead, or mercury, which pose significant toxicity concerns when used in close proximity to human tissue. Recent advancements have focused on developing cadmium-free quantum dots using materials like indium phosphide, zinc sulfide, and carbon-based alternatives that demonstrate substantially reduced cytotoxicity while maintaining optical performance.
Surface chemistry modifications represent a critical approach to enhancing biocompatibility. Coating quantum dots with biocompatible materials such as polyethylene glycol (PEG), silica shells, or specific biomolecules can effectively shield the core material from direct contact with biological systems. These coatings not only minimize potential toxic effects but also improve colloidal stability and reduce non-specific binding in complex biological environments.
Regulatory frameworks governing quantum dot implementation in health monitoring devices remain in development across major markets. The FDA in the United States has established specific guidelines for nanomaterials in medical devices, requiring comprehensive toxicological assessments and biocompatibility testing according to ISO 10993 standards. Similarly, the European Union's Medical Device Regulation (MDR) mandates thorough risk assessment for nanomaterials, with particular attention to potential migration and accumulation in biological tissues.
Long-term exposure effects represent a significant knowledge gap in current research. While acute toxicity studies have provided valuable insights, the potential for quantum dots to accumulate in organs or trigger delayed immune responses remains inadequately characterized. Ongoing research employs advanced in vitro models and longitudinal animal studies to assess chronic exposure scenarios that more accurately reflect real-world usage patterns of wearable health monitoring devices.
Clearance and biodegradation pathways constitute essential considerations for quantum dot safety profiles. Ideal quantum dot formulations for health monitoring applications should either demonstrate complete biological clearance through renal or hepatic pathways or exhibit controlled biodegradation into non-toxic byproducts. Recent innovations in designing quantum dots with programmable degradation timelines show promise for addressing these concerns while maintaining functional stability during the intended usage period.
Standardized testing protocols specifically designed for quantum dot-based health monitoring devices are emerging as crucial tools for safety assessment. These protocols typically include cytotoxicity assays, hemolysis testing, sensitization studies, and genotoxicity evaluations. Advanced techniques such as transcriptomics and metabolomics are increasingly employed to detect subtle biological responses that might not be apparent through conventional toxicological approaches.
Surface chemistry modifications represent a critical approach to enhancing biocompatibility. Coating quantum dots with biocompatible materials such as polyethylene glycol (PEG), silica shells, or specific biomolecules can effectively shield the core material from direct contact with biological systems. These coatings not only minimize potential toxic effects but also improve colloidal stability and reduce non-specific binding in complex biological environments.
Regulatory frameworks governing quantum dot implementation in health monitoring devices remain in development across major markets. The FDA in the United States has established specific guidelines for nanomaterials in medical devices, requiring comprehensive toxicological assessments and biocompatibility testing according to ISO 10993 standards. Similarly, the European Union's Medical Device Regulation (MDR) mandates thorough risk assessment for nanomaterials, with particular attention to potential migration and accumulation in biological tissues.
Long-term exposure effects represent a significant knowledge gap in current research. While acute toxicity studies have provided valuable insights, the potential for quantum dots to accumulate in organs or trigger delayed immune responses remains inadequately characterized. Ongoing research employs advanced in vitro models and longitudinal animal studies to assess chronic exposure scenarios that more accurately reflect real-world usage patterns of wearable health monitoring devices.
Clearance and biodegradation pathways constitute essential considerations for quantum dot safety profiles. Ideal quantum dot formulations for health monitoring applications should either demonstrate complete biological clearance through renal or hepatic pathways or exhibit controlled biodegradation into non-toxic byproducts. Recent innovations in designing quantum dots with programmable degradation timelines show promise for addressing these concerns while maintaining functional stability during the intended usage period.
Standardized testing protocols specifically designed for quantum dot-based health monitoring devices are emerging as crucial tools for safety assessment. These protocols typically include cytotoxicity assays, hemolysis testing, sensitization studies, and genotoxicity evaluations. Advanced techniques such as transcriptomics and metabolomics are increasingly employed to detect subtle biological responses that might not be apparent through conventional toxicological approaches.
Regulatory Framework for QD Medical Applications
The regulatory landscape for quantum dot (QD) technology in medical applications presents a complex framework that varies significantly across global jurisdictions. In the United States, the FDA has established a multi-tiered approach for QD-based health monitoring devices, with classification depending on the intended use and risk profile. Class II medical devices incorporating QDs typically require 510(k) clearance, while more invasive applications may fall under Class III, necessitating rigorous premarket approval processes including extensive clinical trials demonstrating both efficacy and safety.
The European Union's regulatory framework has evolved significantly with the implementation of the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR), which impose stricter requirements for clinical evidence and post-market surveillance. QD-based devices must comply with these regulations, with particular emphasis on biocompatibility testing and risk management documentation. The EU has also implemented specific provisions regarding nanomaterials in medical devices, directly impacting QD applications.
In Asia, regulatory approaches vary considerably. Japan's Pharmaceuticals and Medical Devices Agency (PMDA) has developed specific guidelines for nanomaterial-based medical technologies, while China's National Medical Products Administration (NMPA) has recently strengthened its regulatory framework for innovative medical technologies, including those utilizing QDs.
A critical regulatory consideration across all jurisdictions is the potential toxicity of certain QD materials, particularly those containing cadmium or lead. Regulatory bodies increasingly require manufacturers to demonstrate long-term stability of QDs within the human body, with emphasis on preventing leaching of heavy metals. This has accelerated research into non-toxic alternatives such as carbon dots and silicon-based QDs.
International standards organizations, including ISO and ASTM, have developed specific technical standards for characterizing nanomaterials in medical applications. ISO/TR 13014:2012 and ASTM E2909-13 provide frameworks for physicochemical characterization of engineered nanomaterials, which apply directly to QD-based health monitoring devices.
The regulatory pathway for QD-based wearable health monitors presents unique challenges, as these devices often exist at the intersection of consumer electronics and medical devices. Regulatory clarity regarding the classification of continuous health monitoring applications remains an evolving area, with authorities increasingly focusing on data security and privacy considerations alongside traditional safety concerns.
The European Union's regulatory framework has evolved significantly with the implementation of the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR), which impose stricter requirements for clinical evidence and post-market surveillance. QD-based devices must comply with these regulations, with particular emphasis on biocompatibility testing and risk management documentation. The EU has also implemented specific provisions regarding nanomaterials in medical devices, directly impacting QD applications.
In Asia, regulatory approaches vary considerably. Japan's Pharmaceuticals and Medical Devices Agency (PMDA) has developed specific guidelines for nanomaterial-based medical technologies, while China's National Medical Products Administration (NMPA) has recently strengthened its regulatory framework for innovative medical technologies, including those utilizing QDs.
A critical regulatory consideration across all jurisdictions is the potential toxicity of certain QD materials, particularly those containing cadmium or lead. Regulatory bodies increasingly require manufacturers to demonstrate long-term stability of QDs within the human body, with emphasis on preventing leaching of heavy metals. This has accelerated research into non-toxic alternatives such as carbon dots and silicon-based QDs.
International standards organizations, including ISO and ASTM, have developed specific technical standards for characterizing nanomaterials in medical applications. ISO/TR 13014:2012 and ASTM E2909-13 provide frameworks for physicochemical characterization of engineered nanomaterials, which apply directly to QD-based health monitoring devices.
The regulatory pathway for QD-based wearable health monitors presents unique challenges, as these devices often exist at the intersection of consumer electronics and medical devices. Regulatory clarity regarding the classification of continuous health monitoring applications remains an evolving area, with authorities increasingly focusing on data security and privacy considerations alongside traditional safety concerns.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!