Reducing Waste in Ethyl Acetate Synthesis Processes
JUN 27, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ethyl Acetate Synthesis Evolution and Objectives
Ethyl acetate synthesis has undergone significant evolution since its inception in the early 20th century. Initially, the process relied heavily on batch reactions, which were inefficient and produced considerable waste. The primary method involved the esterification of ethanol with acetic acid, catalyzed by sulfuric acid. This approach, while effective, was plagued by low yields and the generation of substantial byproducts.
As industrial demand for ethyl acetate grew, particularly in the paint and coating industries, there was a pressing need to improve the synthesis process. The 1960s saw the introduction of continuous flow reactors, which marked a significant leap in production efficiency. This advancement allowed for better control of reaction conditions and reduced the overall waste generated per unit of product.
The late 20th century brought about a paradigm shift with the development of reactive distillation techniques. This innovative approach combined reaction and separation steps in a single unit operation, dramatically reducing energy consumption and improving product purity. The integration of these processes not only enhanced yield but also significantly decreased the formation of unwanted byproducts.
Recent years have witnessed a surge in research focused on green chemistry principles applied to ethyl acetate synthesis. Efforts have been directed towards developing bio-based feedstocks, employing more environmentally friendly catalysts, and optimizing reaction conditions to minimize waste generation. The use of heterogeneous catalysts, such as ion-exchange resins, has gained traction due to their reusability and reduced corrosion issues compared to homogeneous acid catalysts.
The current technological landscape is characterized by a drive towards process intensification and the implementation of advanced control systems. These developments aim to further reduce waste, improve energy efficiency, and enhance the overall sustainability of ethyl acetate production. Emerging technologies, such as microreactor systems and membrane-assisted reactive separations, show promise in achieving these objectives.
Looking forward, the primary goals in ethyl acetate synthesis revolve around waste reduction, energy efficiency, and the transition to renewable resources. Researchers and industry professionals are exploring novel catalytic systems, including biocatalysts, that could potentially revolutionize the synthesis process. Additionally, there is a growing interest in developing closed-loop systems that can recycle byproducts and unreacted materials, further minimizing waste output.
As industrial demand for ethyl acetate grew, particularly in the paint and coating industries, there was a pressing need to improve the synthesis process. The 1960s saw the introduction of continuous flow reactors, which marked a significant leap in production efficiency. This advancement allowed for better control of reaction conditions and reduced the overall waste generated per unit of product.
The late 20th century brought about a paradigm shift with the development of reactive distillation techniques. This innovative approach combined reaction and separation steps in a single unit operation, dramatically reducing energy consumption and improving product purity. The integration of these processes not only enhanced yield but also significantly decreased the formation of unwanted byproducts.
Recent years have witnessed a surge in research focused on green chemistry principles applied to ethyl acetate synthesis. Efforts have been directed towards developing bio-based feedstocks, employing more environmentally friendly catalysts, and optimizing reaction conditions to minimize waste generation. The use of heterogeneous catalysts, such as ion-exchange resins, has gained traction due to their reusability and reduced corrosion issues compared to homogeneous acid catalysts.
The current technological landscape is characterized by a drive towards process intensification and the implementation of advanced control systems. These developments aim to further reduce waste, improve energy efficiency, and enhance the overall sustainability of ethyl acetate production. Emerging technologies, such as microreactor systems and membrane-assisted reactive separations, show promise in achieving these objectives.
Looking forward, the primary goals in ethyl acetate synthesis revolve around waste reduction, energy efficiency, and the transition to renewable resources. Researchers and industry professionals are exploring novel catalytic systems, including biocatalysts, that could potentially revolutionize the synthesis process. Additionally, there is a growing interest in developing closed-loop systems that can recycle byproducts and unreacted materials, further minimizing waste output.
Market Analysis for Sustainable Ethyl Acetate Production
The global market for sustainable ethyl acetate production is experiencing significant growth, driven by increasing environmental concerns and stringent regulations on industrial emissions. Ethyl acetate, a versatile solvent widely used in various industries, has traditionally been produced through processes that generate substantial waste and environmental impact. However, the shift towards sustainable production methods is reshaping the market landscape.
The demand for eco-friendly ethyl acetate is rising across multiple sectors, including paints and coatings, pharmaceuticals, food and beverages, and electronics. This surge is primarily attributed to the growing awareness among consumers and manufacturers about the importance of reducing carbon footprints and adopting green chemistry principles. As a result, companies are actively seeking sustainable alternatives to conventional ethyl acetate production methods.
Market analysis indicates that the Asia-Pacific region is emerging as a key player in sustainable ethyl acetate production. Countries like China, India, and Japan are investing heavily in research and development of green technologies. This regional dominance is expected to continue due to the presence of a large manufacturing base and supportive government policies promoting sustainable industrial practices.
In terms of market size, the sustainable ethyl acetate segment is projected to grow at a compound annual growth rate (CAGR) significantly higher than that of traditional production methods. This growth is fueled by the increasing adoption of bio-based feedstocks and the development of novel catalytic processes that minimize waste generation.
The competitive landscape is characterized by a mix of established chemical companies and innovative start-ups. Major players are focusing on strategic partnerships and collaborations to enhance their technological capabilities and expand their market presence. Additionally, there is a noticeable trend of vertical integration, with companies aiming to secure sustainable raw material sources to ensure a stable supply chain.
Pricing trends in the sustainable ethyl acetate market show a gradual decrease as production scales up and technologies mature. However, sustainable variants still command a premium over conventionally produced ethyl acetate. This price differential is expected to narrow in the coming years as economies of scale are achieved and production efficiencies improve.
Consumer preferences are shifting towards products with lower environmental impact, creating a pull effect in the market. End-users are increasingly willing to pay a premium for ethyl acetate produced through sustainable methods, recognizing the long-term benefits of reduced environmental liabilities and improved corporate image.
Regulatory support plays a crucial role in shaping the market dynamics. Governments worldwide are implementing stricter environmental regulations and offering incentives for adopting green technologies. These policies are acting as catalysts for innovation and investment in sustainable ethyl acetate production processes.
The demand for eco-friendly ethyl acetate is rising across multiple sectors, including paints and coatings, pharmaceuticals, food and beverages, and electronics. This surge is primarily attributed to the growing awareness among consumers and manufacturers about the importance of reducing carbon footprints and adopting green chemistry principles. As a result, companies are actively seeking sustainable alternatives to conventional ethyl acetate production methods.
Market analysis indicates that the Asia-Pacific region is emerging as a key player in sustainable ethyl acetate production. Countries like China, India, and Japan are investing heavily in research and development of green technologies. This regional dominance is expected to continue due to the presence of a large manufacturing base and supportive government policies promoting sustainable industrial practices.
In terms of market size, the sustainable ethyl acetate segment is projected to grow at a compound annual growth rate (CAGR) significantly higher than that of traditional production methods. This growth is fueled by the increasing adoption of bio-based feedstocks and the development of novel catalytic processes that minimize waste generation.
The competitive landscape is characterized by a mix of established chemical companies and innovative start-ups. Major players are focusing on strategic partnerships and collaborations to enhance their technological capabilities and expand their market presence. Additionally, there is a noticeable trend of vertical integration, with companies aiming to secure sustainable raw material sources to ensure a stable supply chain.
Pricing trends in the sustainable ethyl acetate market show a gradual decrease as production scales up and technologies mature. However, sustainable variants still command a premium over conventionally produced ethyl acetate. This price differential is expected to narrow in the coming years as economies of scale are achieved and production efficiencies improve.
Consumer preferences are shifting towards products with lower environmental impact, creating a pull effect in the market. End-users are increasingly willing to pay a premium for ethyl acetate produced through sustainable methods, recognizing the long-term benefits of reduced environmental liabilities and improved corporate image.
Regulatory support plays a crucial role in shaping the market dynamics. Governments worldwide are implementing stricter environmental regulations and offering incentives for adopting green technologies. These policies are acting as catalysts for innovation and investment in sustainable ethyl acetate production processes.
Current Challenges in Ethyl Acetate Synthesis Efficiency
The synthesis of ethyl acetate, a widely used solvent and intermediate in various industries, faces several efficiency challenges that contribute to waste generation. One of the primary issues is the equilibrium limitation in the esterification reaction between ethanol and acetic acid. This equilibrium constraint results in incomplete conversion, typically around 65-70%, leading to significant amounts of unreacted raw materials and the need for extensive separation processes.
Another major challenge is the formation of byproducts, particularly water, which not only reduces the yield but also complicates the purification process. The presence of water can lead to hydrolysis of the product, further decreasing the overall efficiency. Additionally, the azeotropic behavior of ethyl acetate with water and ethanol creates difficulties in achieving high-purity products through conventional distillation methods.
The energy-intensive nature of the traditional batch processes used in ethyl acetate production contributes to inefficiency and waste. These processes often require multiple heating and cooling cycles, resulting in high energy consumption and increased operational costs. Furthermore, the use of homogeneous acid catalysts, such as sulfuric acid, presents corrosion issues and necessitates neutralization steps, generating additional waste streams.
Catalyst deactivation and fouling represent another set of challenges in ethyl acetate synthesis. Over time, catalysts lose their activity, leading to decreased conversion rates and the need for frequent replacements or regenerations. This not only impacts the process efficiency but also generates solid waste in the form of spent catalysts.
The handling and storage of volatile organic compounds (VOCs) involved in the process pose environmental and safety concerns. Fugitive emissions from equipment leaks and storage tanks contribute to air pollution and product loss. Moreover, the flammability and toxicity of the reactants and products require stringent safety measures, which can sometimes conflict with efficiency goals.
Lastly, the variability in feedstock quality, particularly when using bio-based or recycled sources of ethanol or acetic acid, introduces additional challenges. Impurities in these feedstocks can affect reaction kinetics, catalyst performance, and product quality, necessitating more robust purification steps and potentially increasing waste generation.
Addressing these challenges requires a multifaceted approach, combining process intensification, advanced catalysis, and innovative separation technologies to significantly reduce waste and improve the overall efficiency of ethyl acetate synthesis processes.
Another major challenge is the formation of byproducts, particularly water, which not only reduces the yield but also complicates the purification process. The presence of water can lead to hydrolysis of the product, further decreasing the overall efficiency. Additionally, the azeotropic behavior of ethyl acetate with water and ethanol creates difficulties in achieving high-purity products through conventional distillation methods.
The energy-intensive nature of the traditional batch processes used in ethyl acetate production contributes to inefficiency and waste. These processes often require multiple heating and cooling cycles, resulting in high energy consumption and increased operational costs. Furthermore, the use of homogeneous acid catalysts, such as sulfuric acid, presents corrosion issues and necessitates neutralization steps, generating additional waste streams.
Catalyst deactivation and fouling represent another set of challenges in ethyl acetate synthesis. Over time, catalysts lose their activity, leading to decreased conversion rates and the need for frequent replacements or regenerations. This not only impacts the process efficiency but also generates solid waste in the form of spent catalysts.
The handling and storage of volatile organic compounds (VOCs) involved in the process pose environmental and safety concerns. Fugitive emissions from equipment leaks and storage tanks contribute to air pollution and product loss. Moreover, the flammability and toxicity of the reactants and products require stringent safety measures, which can sometimes conflict with efficiency goals.
Lastly, the variability in feedstock quality, particularly when using bio-based or recycled sources of ethanol or acetic acid, introduces additional challenges. Impurities in these feedstocks can affect reaction kinetics, catalyst performance, and product quality, necessitating more robust purification steps and potentially increasing waste generation.
Addressing these challenges requires a multifaceted approach, combining process intensification, advanced catalysis, and innovative separation technologies to significantly reduce waste and improve the overall efficiency of ethyl acetate synthesis processes.
Existing Waste Reduction Strategies in Synthesis Processes
01 Waste reduction in ethyl acetate synthesis
Various processes have been developed to reduce waste in ethyl acetate synthesis. These include optimizing reaction conditions, using catalysts to improve efficiency, and implementing recycling systems for unreacted materials. Such methods aim to minimize byproduct formation and increase overall yield, thereby reducing waste generation in the production process.- Waste reduction in ethyl acetate synthesis: Various processes have been developed to reduce waste in ethyl acetate synthesis. These include optimizing reaction conditions, using catalysts to improve efficiency, and implementing recycling systems for unreacted materials. Some methods focus on reducing byproduct formation and improving overall yield, thereby minimizing waste generation.
- Continuous flow processes for ethyl acetate production: Continuous flow processes have been implemented in ethyl acetate synthesis to improve efficiency and reduce waste. These processes often involve specialized reactor designs and control systems that allow for better heat and mass transfer, leading to higher yields and reduced byproduct formation. Some continuous processes also incorporate in-line purification steps to further minimize waste.
- Catalytic processes for ethyl acetate synthesis: Catalytic processes play a crucial role in improving the efficiency of ethyl acetate synthesis and reducing waste. Various catalysts, including heterogeneous and homogeneous types, have been developed to enhance reaction rates, improve selectivity, and allow for milder reaction conditions. Some catalytic processes also enable the use of alternative feedstocks or reaction pathways that generate less waste.
- Recycling and recovery systems in ethyl acetate production: Recycling and recovery systems are implemented in ethyl acetate production processes to minimize waste and improve overall efficiency. These systems often involve the separation and purification of unreacted raw materials, byproducts, and solvents, which are then reintroduced into the production process. Some advanced systems incorporate membrane technology or specialized distillation techniques for more effective separation and recovery.
- Green chemistry approaches for ethyl acetate synthesis: Green chemistry principles are being applied to ethyl acetate synthesis to reduce environmental impact and waste generation. These approaches include the use of renewable feedstocks, development of solvent-free or aqueous reaction systems, and implementation of atom-economical processes. Some green chemistry methods also focus on reducing energy consumption and improving the overall sustainability of the production process.
02 Utilization of waste products
Some processes focus on utilizing waste products generated during ethyl acetate synthesis. This includes converting byproducts into valuable chemicals or using them as raw materials for other industrial processes. By finding applications for waste streams, these methods aim to create a more circular and sustainable production system.Expand Specific Solutions03 Green synthesis methods
Environmentally friendly approaches to ethyl acetate synthesis have been developed to minimize waste generation. These methods often involve the use of renewable resources, bio-based catalysts, or alternative reaction pathways that produce fewer harmful byproducts. The goal is to create a more sustainable production process with reduced environmental impact.Expand Specific Solutions04 Continuous flow processes
Continuous flow processes for ethyl acetate synthesis have been implemented to improve efficiency and reduce waste. These systems allow for better control of reaction parameters, leading to higher yields and fewer side reactions. Additionally, they often incorporate in-line purification and recycling steps, further minimizing waste generation.Expand Specific Solutions05 Waste treatment and recovery
Various techniques have been developed for treating and recovering waste from ethyl acetate synthesis processes. These include advanced separation methods, such as membrane technology or selective adsorption, to isolate and purify valuable components from waste streams. Some processes also focus on the treatment of aqueous waste to meet environmental regulations and enable safe disposal or reuse.Expand Specific Solutions
Key Industry Players in Ethyl Acetate Manufacturing
The ethyl acetate synthesis process optimization market is in a mature stage, with established players and well-developed technologies. The global market size for ethyl acetate is substantial, driven by its widespread use in various industries. Technologically, the field is relatively mature, with ongoing efforts focused on improving efficiency and sustainability. Key players like Celanese International Corp., China Petroleum & Chemical Corp., and BASF Corp. are at the forefront of innovation, leveraging their extensive R&D capabilities to develop advanced synthesis processes. Emerging companies such as Anhui Ruibai New Material Co., Ltd. are also contributing to the competitive landscape, introducing novel approaches to waste reduction and process optimization. The industry is characterized by a mix of large, integrated chemical companies and specialized manufacturers, all striving to enhance their market position through technological advancements and sustainable practices.
Celanese International Corp.
Technical Solution: Celanese has developed an innovative process for ethyl acetate synthesis that significantly reduces waste. Their approach utilizes a reactive distillation technology, which combines reaction and separation in a single unit operation[1]. This process achieves higher conversion rates and selectivity, resulting in less byproduct formation. The company has also implemented advanced catalysts that operate at lower temperatures, reducing energy consumption and minimizing side reactions[3]. Additionally, Celanese has introduced a closed-loop solvent recovery system that recycles unreacted raw materials, further minimizing waste generation[5].
Strengths: High efficiency, reduced energy consumption, and improved product quality. Weaknesses: Potentially higher initial capital investment and complexity in process control.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has implemented a novel approach to reduce waste in ethyl acetate synthesis. Their method involves a two-stage reaction process with intermediate product separation[2]. This technique allows for better control of reaction conditions, reducing the formation of unwanted byproducts. Sinopec has also developed a proprietary catalyst that enhances selectivity towards ethyl acetate, minimizing side reactions[4]. The company utilizes advanced process intensification techniques, such as microreactor technology, which improves mass and heat transfer, leading to higher yields and reduced waste[6].
Strengths: Improved selectivity, enhanced process control, and scalability. Weaknesses: Potential challenges in catalyst regeneration and higher operational complexity.
Innovative Technologies for Ethyl Acetate Waste Minimization
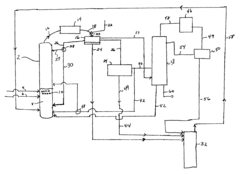
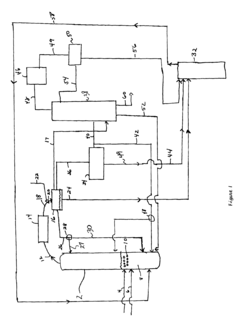
Reducing ethyl acetate concentration in recycle streams for ethanol production processes
PatentInactiveID201403455A
Innovation
- Hydrolysis of recycle streams to reduce ethyl acetate concentration in ethanol production processes.
- Converting ethyl acetate byproduct back into useful acetic acid and ethanol through hydrolysis.
- Integration of hydrolysis step into existing ethanol production process to manage byproduct accumulation.
Process improvement for continuous ethyl acetate production
PatentInactiveUS6768021B2
Innovation
- The process involves using a membrane separation unit to remove water from the condensed reaction stream, recycling the dried stream back into the production process, and employing an additional distillation zone to produce purified ethyl acetate with minimal acid content, thereby optimizing water management and increasing process capacity.
Environmental Impact Assessment of Production Methods
The environmental impact assessment of ethyl acetate production methods is crucial for understanding and mitigating the ecological footprint of this widely used industrial solvent. Traditional synthesis processes, particularly those involving esterification of ethanol and acetic acid, have been associated with significant waste generation and energy consumption. These methods often require excess reactants, produce byproducts, and necessitate energy-intensive separation processes, all of which contribute to environmental degradation.
Recent advancements in green chemistry have led to the development of more sustainable production routes for ethyl acetate. One such method involves the use of reactive distillation, which combines reaction and separation in a single unit operation. This approach significantly reduces energy requirements and minimizes waste generation by improving conversion rates and reducing the need for excess reactants. Additionally, the use of heterogeneous catalysts in this process further enhances its environmental performance by eliminating the need for homogeneous catalyst recovery and disposal.
Another promising approach is the utilization of biocatalysts, such as lipases, for ethyl acetate synthesis. Enzymatic processes operate under milder conditions, reducing energy consumption and the risk of side reactions. Furthermore, these biological catalysts are often reusable and biodegradable, aligning well with circular economy principles. However, the scalability and economic viability of biocatalytic processes for large-scale industrial production remain challenges that require further research and development.
Life cycle assessment (LCA) studies have been conducted to quantify the environmental impacts of various ethyl acetate production methods. These assessments typically consider factors such as global warming potential, acidification, eutrophication, and resource depletion. Results from these studies generally indicate that newer, more sustainable processes offer significant environmental benefits compared to traditional methods. For instance, reactive distillation has been shown to reduce carbon dioxide emissions by up to 30% and decrease overall energy consumption by 20-40% compared to conventional batch processes.
Water consumption and wastewater generation are also critical environmental considerations in ethyl acetate production. Traditional methods often require substantial amounts of water for washing and separation steps, leading to high volumes of contaminated wastewater. Advanced processes, such as those employing membrane separation technologies or solvent-free synthesis routes, have demonstrated potential for dramatically reducing water usage and wastewater generation, thus mitigating the strain on local water resources and treatment facilities.
In conclusion, the environmental impact assessment of ethyl acetate production methods reveals a clear trend towards more sustainable practices. While traditional processes continue to pose significant environmental challenges, emerging technologies offer promising solutions for waste reduction, energy efficiency, and overall ecological footprint minimization. Continued research and investment in these innovative approaches will be essential for further improving the environmental performance of ethyl acetate synthesis in the chemical industry.
Recent advancements in green chemistry have led to the development of more sustainable production routes for ethyl acetate. One such method involves the use of reactive distillation, which combines reaction and separation in a single unit operation. This approach significantly reduces energy requirements and minimizes waste generation by improving conversion rates and reducing the need for excess reactants. Additionally, the use of heterogeneous catalysts in this process further enhances its environmental performance by eliminating the need for homogeneous catalyst recovery and disposal.
Another promising approach is the utilization of biocatalysts, such as lipases, for ethyl acetate synthesis. Enzymatic processes operate under milder conditions, reducing energy consumption and the risk of side reactions. Furthermore, these biological catalysts are often reusable and biodegradable, aligning well with circular economy principles. However, the scalability and economic viability of biocatalytic processes for large-scale industrial production remain challenges that require further research and development.
Life cycle assessment (LCA) studies have been conducted to quantify the environmental impacts of various ethyl acetate production methods. These assessments typically consider factors such as global warming potential, acidification, eutrophication, and resource depletion. Results from these studies generally indicate that newer, more sustainable processes offer significant environmental benefits compared to traditional methods. For instance, reactive distillation has been shown to reduce carbon dioxide emissions by up to 30% and decrease overall energy consumption by 20-40% compared to conventional batch processes.
Water consumption and wastewater generation are also critical environmental considerations in ethyl acetate production. Traditional methods often require substantial amounts of water for washing and separation steps, leading to high volumes of contaminated wastewater. Advanced processes, such as those employing membrane separation technologies or solvent-free synthesis routes, have demonstrated potential for dramatically reducing water usage and wastewater generation, thus mitigating the strain on local water resources and treatment facilities.
In conclusion, the environmental impact assessment of ethyl acetate production methods reveals a clear trend towards more sustainable practices. While traditional processes continue to pose significant environmental challenges, emerging technologies offer promising solutions for waste reduction, energy efficiency, and overall ecological footprint minimization. Continued research and investment in these innovative approaches will be essential for further improving the environmental performance of ethyl acetate synthesis in the chemical industry.
Economic Feasibility of Waste Reduction Implementations
The economic feasibility of waste reduction implementations in ethyl acetate synthesis processes is a critical consideration for manufacturers seeking to optimize their operations. The primary focus is on evaluating the financial viability of various waste reduction strategies and their potential impact on the overall production costs and profitability.
One of the key factors in assessing economic feasibility is the initial investment required for implementing waste reduction technologies. This may include the purchase of new equipment, modification of existing processes, or the installation of advanced control systems. While these upfront costs can be substantial, they must be weighed against the long-term benefits and potential savings.
Operational cost savings are a significant driver for waste reduction initiatives. By minimizing waste, manufacturers can reduce raw material consumption, leading to lower procurement costs. Additionally, improved process efficiency often results in reduced energy consumption, further contributing to cost savings. The reduction in waste disposal expenses is another important economic benefit, as it not only lowers direct costs but also mitigates potential environmental liabilities.
The potential for increased product yield is a crucial aspect of the economic analysis. Waste reduction techniques often lead to higher conversion rates and improved product quality, resulting in a greater quantity of marketable product from the same input. This increased yield can significantly enhance the overall profitability of the ethyl acetate synthesis process.
Regulatory compliance is another economic consideration. As environmental regulations become more stringent, proactive waste reduction measures can help companies avoid costly fines and penalties. Moreover, compliance with environmental standards can improve a company's reputation, potentially leading to increased market share and customer loyalty.
The payback period for waste reduction implementations is a key metric in determining economic feasibility. This calculation takes into account the initial investment, projected cost savings, and potential revenue increases. A shorter payback period generally indicates a more attractive investment opportunity. However, it is essential to consider the long-term benefits beyond the payback period, as many waste reduction technologies continue to provide economic advantages throughout their operational life.
Market dynamics also play a role in the economic feasibility assessment. The volatility of raw material prices, energy costs, and ethyl acetate market prices can significantly impact the financial benefits of waste reduction strategies. Sensitivity analyses should be conducted to evaluate the robustness of the economic benefits under various market scenarios.
In conclusion, the economic feasibility of waste reduction implementations in ethyl acetate synthesis processes is multifaceted, requiring a comprehensive analysis of costs, benefits, and market factors. While the initial investment may be substantial, the potential for long-term cost savings, increased productivity, and improved environmental performance often makes waste reduction initiatives economically attractive for manufacturers in the chemical industry.
One of the key factors in assessing economic feasibility is the initial investment required for implementing waste reduction technologies. This may include the purchase of new equipment, modification of existing processes, or the installation of advanced control systems. While these upfront costs can be substantial, they must be weighed against the long-term benefits and potential savings.
Operational cost savings are a significant driver for waste reduction initiatives. By minimizing waste, manufacturers can reduce raw material consumption, leading to lower procurement costs. Additionally, improved process efficiency often results in reduced energy consumption, further contributing to cost savings. The reduction in waste disposal expenses is another important economic benefit, as it not only lowers direct costs but also mitigates potential environmental liabilities.
The potential for increased product yield is a crucial aspect of the economic analysis. Waste reduction techniques often lead to higher conversion rates and improved product quality, resulting in a greater quantity of marketable product from the same input. This increased yield can significantly enhance the overall profitability of the ethyl acetate synthesis process.
Regulatory compliance is another economic consideration. As environmental regulations become more stringent, proactive waste reduction measures can help companies avoid costly fines and penalties. Moreover, compliance with environmental standards can improve a company's reputation, potentially leading to increased market share and customer loyalty.
The payback period for waste reduction implementations is a key metric in determining economic feasibility. This calculation takes into account the initial investment, projected cost savings, and potential revenue increases. A shorter payback period generally indicates a more attractive investment opportunity. However, it is essential to consider the long-term benefits beyond the payback period, as many waste reduction technologies continue to provide economic advantages throughout their operational life.
Market dynamics also play a role in the economic feasibility assessment. The volatility of raw material prices, energy costs, and ethyl acetate market prices can significantly impact the financial benefits of waste reduction strategies. Sensitivity analyses should be conducted to evaluate the robustness of the economic benefits under various market scenarios.
In conclusion, the economic feasibility of waste reduction implementations in ethyl acetate synthesis processes is multifaceted, requiring a comprehensive analysis of costs, benefits, and market factors. While the initial investment may be substantial, the potential for long-term cost savings, increased productivity, and improved environmental performance often makes waste reduction initiatives economically attractive for manufacturers in the chemical industry.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!