Research on PEEK Polymer Properties for Regulatory Compliance
OCT 24, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
PEEK Polymer Development History and Research Objectives
Polyether ether ketone (PEEK) emerged in the late 1970s as a groundbreaking high-performance thermoplastic polymer, first commercialized by Imperial Chemical Industries (ICI) in 1978. The development of PEEK represented a significant advancement in polymer science, offering exceptional thermal stability, mechanical strength, and chemical resistance that far surpassed conventional polymers available at that time. The initial research focused primarily on aerospace and military applications where extreme operating conditions demanded superior material performance.
Throughout the 1980s and 1990s, PEEK underwent substantial refinement as researchers explored various synthesis methods to enhance its properties. The introduction of semi-crystalline variants and reinforced composites expanded its application potential. The polymer's unique combination of properties—including a glass transition temperature of approximately 143°C and melting point around 343°C—established it as an elite engineering thermoplastic capable of withstanding harsh environments.
The early 2000s marked a pivotal shift in PEEK research, with increasing focus on biomedical applications due to its biocompatibility and mechanical properties similar to human bone. This period saw significant advancements in processing techniques, including injection molding optimization and additive manufacturing compatibility, which broadened PEEK's industrial relevance across sectors.
Recent developments have concentrated on enhancing PEEK's regulatory compliance profiles, particularly for applications in healthcare, food contact, and environmentally sensitive industries. Research has intensified on understanding leachable compounds, degradation mechanisms, and long-term stability under various exposure conditions. The polymer's inherent flame retardancy without halogenated additives has positioned it favorably within increasingly stringent regulatory frameworks worldwide.
The current research objectives center on comprehensive characterization of PEEK's properties as they relate to regulatory standards across different jurisdictions. This includes investigating thermal degradation pathways, identifying potential migration compounds under various conditions, and establishing standardized testing protocols specific to PEEK-based materials. Additionally, research aims to quantify the polymer's environmental impact throughout its lifecycle, addressing growing concerns about sustainability and end-of-life management.
Another critical research goal involves developing enhanced analytical methods for detecting trace contaminants and characterizing structural changes in PEEK under different processing and application conditions. This work is essential for generating the robust scientific data required by regulatory bodies for approving PEEK in sensitive applications such as implantable medical devices, aerospace components, and food processing equipment.
The ultimate objective of current PEEK research is to establish a comprehensive property profile that aligns with evolving global regulatory frameworks while maintaining the polymer's exceptional performance characteristics. This balance between compliance and functionality represents the frontier of PEEK polymer development and will determine its future market expansion potential.
Throughout the 1980s and 1990s, PEEK underwent substantial refinement as researchers explored various synthesis methods to enhance its properties. The introduction of semi-crystalline variants and reinforced composites expanded its application potential. The polymer's unique combination of properties—including a glass transition temperature of approximately 143°C and melting point around 343°C—established it as an elite engineering thermoplastic capable of withstanding harsh environments.
The early 2000s marked a pivotal shift in PEEK research, with increasing focus on biomedical applications due to its biocompatibility and mechanical properties similar to human bone. This period saw significant advancements in processing techniques, including injection molding optimization and additive manufacturing compatibility, which broadened PEEK's industrial relevance across sectors.
Recent developments have concentrated on enhancing PEEK's regulatory compliance profiles, particularly for applications in healthcare, food contact, and environmentally sensitive industries. Research has intensified on understanding leachable compounds, degradation mechanisms, and long-term stability under various exposure conditions. The polymer's inherent flame retardancy without halogenated additives has positioned it favorably within increasingly stringent regulatory frameworks worldwide.
The current research objectives center on comprehensive characterization of PEEK's properties as they relate to regulatory standards across different jurisdictions. This includes investigating thermal degradation pathways, identifying potential migration compounds under various conditions, and establishing standardized testing protocols specific to PEEK-based materials. Additionally, research aims to quantify the polymer's environmental impact throughout its lifecycle, addressing growing concerns about sustainability and end-of-life management.
Another critical research goal involves developing enhanced analytical methods for detecting trace contaminants and characterizing structural changes in PEEK under different processing and application conditions. This work is essential for generating the robust scientific data required by regulatory bodies for approving PEEK in sensitive applications such as implantable medical devices, aerospace components, and food processing equipment.
The ultimate objective of current PEEK research is to establish a comprehensive property profile that aligns with evolving global regulatory frameworks while maintaining the polymer's exceptional performance characteristics. This balance between compliance and functionality represents the frontier of PEEK polymer development and will determine its future market expansion potential.
Market Analysis for PEEK Applications in Regulated Industries
The PEEK polymer market has experienced significant growth across regulated industries due to its exceptional properties and compliance with stringent regulatory standards. The global PEEK market was valued at approximately 721 million USD in 2021 and is projected to reach 1.2 billion USD by 2028, growing at a CAGR of 7.5% during the forecast period. This growth is primarily driven by increasing demand in aerospace, automotive, medical, and electronics industries where regulatory compliance is critical.
In the aerospace sector, PEEK applications have seen substantial adoption due to the material's ability to meet FAA and EASA requirements for flame retardancy, smoke emission, and toxicity. The aerospace industry accounts for roughly 18% of the total PEEK consumption, with applications in interior components, electrical insulation, and structural parts requiring high temperature resistance.
The medical device industry represents one of the fastest-growing segments for PEEK applications, with a growth rate exceeding 9% annually. This is attributed to PEEK's biocompatibility, sterilizability, and compliance with FDA, EU MDR, and ISO 10993 standards. Orthopedic implants, dental applications, and surgical instruments constitute the primary applications, with North America holding the largest market share at approximately 42%.
The automotive sector's adoption of PEEK is driven by increasingly stringent emissions and fuel efficiency regulations. As manufacturers seek lightweight alternatives to metal components, PEEK's compliance with automotive standards such as FMVSS, EPA requirements, and EU directives on end-of-life vehicles has positioned it as a premium material for under-hood components and transmission systems.
Regional analysis reveals that North America and Europe dominate the regulated PEEK market, collectively accounting for over 65% of global consumption. This is primarily due to stricter regulatory frameworks and higher adoption rates in medical and aerospace applications. However, the Asia-Pacific region, particularly China and Japan, is experiencing the fastest growth rate at approximately 8.5% annually, driven by expanding electronics manufacturing and increasing adoption of medical-grade PEEK.
Price sensitivity remains a significant market constraint, with PEEK commanding premium pricing compared to conventional polymers. The average price ranges from 85-120 USD per kilogram depending on grade and certification requirements, creating barriers to adoption in cost-sensitive applications despite regulatory advantages.
Market forecasts indicate that specialty grades of PEEK designed specifically for regulated industries will see the highest growth rates, with carbon-fiber reinforced and medical-grade PEEK expected to grow at 10% and 9.5% respectively through 2028.
In the aerospace sector, PEEK applications have seen substantial adoption due to the material's ability to meet FAA and EASA requirements for flame retardancy, smoke emission, and toxicity. The aerospace industry accounts for roughly 18% of the total PEEK consumption, with applications in interior components, electrical insulation, and structural parts requiring high temperature resistance.
The medical device industry represents one of the fastest-growing segments for PEEK applications, with a growth rate exceeding 9% annually. This is attributed to PEEK's biocompatibility, sterilizability, and compliance with FDA, EU MDR, and ISO 10993 standards. Orthopedic implants, dental applications, and surgical instruments constitute the primary applications, with North America holding the largest market share at approximately 42%.
The automotive sector's adoption of PEEK is driven by increasingly stringent emissions and fuel efficiency regulations. As manufacturers seek lightweight alternatives to metal components, PEEK's compliance with automotive standards such as FMVSS, EPA requirements, and EU directives on end-of-life vehicles has positioned it as a premium material for under-hood components and transmission systems.
Regional analysis reveals that North America and Europe dominate the regulated PEEK market, collectively accounting for over 65% of global consumption. This is primarily due to stricter regulatory frameworks and higher adoption rates in medical and aerospace applications. However, the Asia-Pacific region, particularly China and Japan, is experiencing the fastest growth rate at approximately 8.5% annually, driven by expanding electronics manufacturing and increasing adoption of medical-grade PEEK.
Price sensitivity remains a significant market constraint, with PEEK commanding premium pricing compared to conventional polymers. The average price ranges from 85-120 USD per kilogram depending on grade and certification requirements, creating barriers to adoption in cost-sensitive applications despite regulatory advantages.
Market forecasts indicate that specialty grades of PEEK designed specifically for regulated industries will see the highest growth rates, with carbon-fiber reinforced and medical-grade PEEK expected to grow at 10% and 9.5% respectively through 2028.
Current PEEK Properties and Compliance Challenges
Polyetheretherketone (PEEK) is a high-performance thermoplastic polymer characterized by exceptional mechanical properties, thermal stability, and chemical resistance. Currently, PEEK exhibits a tensile strength ranging from 90-100 MPa and a continuous service temperature of approximately 250°C, making it suitable for demanding applications in aerospace, automotive, and medical industries. However, these impressive properties are accompanied by significant regulatory compliance challenges that manufacturers must navigate.
The primary mechanical properties of PEEK include high strength-to-weight ratio, excellent fatigue resistance, and remarkable dimensional stability across a wide temperature range. Its elastic modulus typically ranges between 3.6-4.1 GPa, providing rigidity while maintaining some flexibility. These characteristics have positioned PEEK as a preferred material for critical components in various high-performance applications.
From a chemical perspective, PEEK demonstrates exceptional resistance to hydrolysis, organic and aqueous environments, and most industrial chemicals. This inherent chemical stability contributes to its biocompatibility, which is crucial for medical applications. However, this same stability presents challenges in processing and modification to meet specific regulatory requirements.
Current regulatory frameworks impose stringent requirements on PEEK materials, particularly in medical and aerospace applications. For medical-grade PEEK, compliance with ISO 10993 standards for biocompatibility is mandatory, requiring extensive testing for cytotoxicity, sensitization, and genotoxicity. The FDA's 510(k) clearance process adds another layer of complexity for PEEK components in medical devices.
In aerospace applications, PEEK materials must meet flame, smoke, and toxicity (FST) requirements as specified in FAR 25.853 and similar standards. The material's natural flame resistance (V-0 rating under UL94) is beneficial, but achieving consistent compliance across different formulations remains challenging.
Environmental regulations present additional hurdles. While PEEK is inherently free from halogens and other restricted substances under RoHS and REACH regulations, the additives and processing aids used in PEEK formulations may contain regulated substances. Manufacturers must carefully monitor and document the complete chemical composition of their PEEK products.
The recyclability of PEEK presents both an opportunity and a challenge. Its high melting point (approximately 343°C) makes conventional recycling difficult, yet its value incentivizes recovery and reprocessing. Regulatory frameworks increasingly emphasize circular economy principles, requiring manufacturers to develop effective end-of-life strategies for PEEK components.
Quality control represents another significant compliance challenge. The high processing temperatures required for PEEK (370-400°C) necessitate specialized equipment and strict process controls to ensure consistent properties. Batch-to-batch variation must be minimized to maintain regulatory compliance, particularly for critical applications where material properties directly impact safety and performance.
The primary mechanical properties of PEEK include high strength-to-weight ratio, excellent fatigue resistance, and remarkable dimensional stability across a wide temperature range. Its elastic modulus typically ranges between 3.6-4.1 GPa, providing rigidity while maintaining some flexibility. These characteristics have positioned PEEK as a preferred material for critical components in various high-performance applications.
From a chemical perspective, PEEK demonstrates exceptional resistance to hydrolysis, organic and aqueous environments, and most industrial chemicals. This inherent chemical stability contributes to its biocompatibility, which is crucial for medical applications. However, this same stability presents challenges in processing and modification to meet specific regulatory requirements.
Current regulatory frameworks impose stringent requirements on PEEK materials, particularly in medical and aerospace applications. For medical-grade PEEK, compliance with ISO 10993 standards for biocompatibility is mandatory, requiring extensive testing for cytotoxicity, sensitization, and genotoxicity. The FDA's 510(k) clearance process adds another layer of complexity for PEEK components in medical devices.
In aerospace applications, PEEK materials must meet flame, smoke, and toxicity (FST) requirements as specified in FAR 25.853 and similar standards. The material's natural flame resistance (V-0 rating under UL94) is beneficial, but achieving consistent compliance across different formulations remains challenging.
Environmental regulations present additional hurdles. While PEEK is inherently free from halogens and other restricted substances under RoHS and REACH regulations, the additives and processing aids used in PEEK formulations may contain regulated substances. Manufacturers must carefully monitor and document the complete chemical composition of their PEEK products.
The recyclability of PEEK presents both an opportunity and a challenge. Its high melting point (approximately 343°C) makes conventional recycling difficult, yet its value incentivizes recovery and reprocessing. Regulatory frameworks increasingly emphasize circular economy principles, requiring manufacturers to develop effective end-of-life strategies for PEEK components.
Quality control represents another significant compliance challenge. The high processing temperatures required for PEEK (370-400°C) necessitate specialized equipment and strict process controls to ensure consistent properties. Batch-to-batch variation must be minimized to maintain regulatory compliance, particularly for critical applications where material properties directly impact safety and performance.
Established Testing Methodologies for PEEK Regulatory Compliance
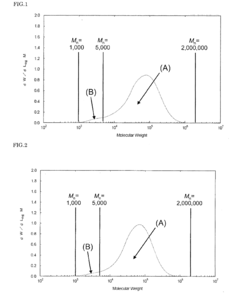
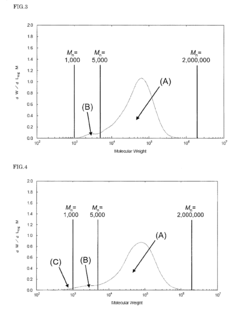
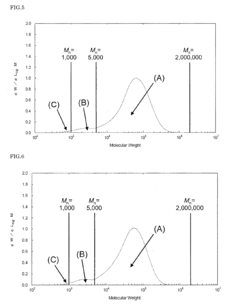
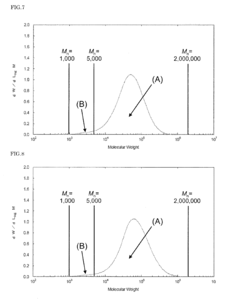
01 Thermal and mechanical properties of PEEK polymers
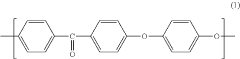
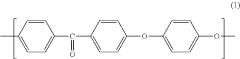
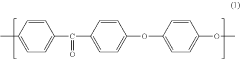
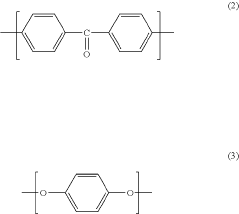
PEEK (Polyetheretherketone) polymers exhibit exceptional thermal stability and mechanical strength. They maintain their structural integrity at high temperatures, with a glass transition temperature around 143°C and melting point of approximately 343°C. PEEK materials demonstrate excellent mechanical properties including high tensile strength, stiffness, and impact resistance. These properties make PEEK suitable for applications requiring durability under extreme conditions.- Thermal and mechanical properties of PEEK polymers: PEEK (Polyetheretherketone) polymers exhibit exceptional thermal stability and mechanical strength. They maintain their structural integrity at high temperatures, with a glass transition temperature around 143°C and melting point of approximately 343°C. PEEK materials demonstrate excellent mechanical properties including high tensile strength, stiffness, and impact resistance. These properties make PEEK suitable for demanding applications in aerospace, automotive, and industrial sectors where exposure to extreme temperatures and mechanical stress is common.
- Chemical resistance and biocompatibility of PEEK: PEEK polymers possess outstanding chemical resistance to a wide range of solvents, acids, bases, and hydrocarbons. This resistance makes them ideal for applications involving harsh chemical environments. Additionally, PEEK demonstrates excellent biocompatibility, making it suitable for medical implants and devices. The material is inert, non-toxic, and does not cause adverse reactions when in contact with body tissues, allowing for long-term implantation in the human body.
- PEEK composite formulations and enhancements: PEEK polymers can be formulated with various fillers and reinforcements to enhance specific properties. Carbon fiber reinforced PEEK offers improved strength and stiffness while maintaining the base polymer's thermal resistance. Glass fiber reinforced variants provide enhanced dimensional stability and electrical insulation properties. Other additives such as graphene, ceramic particles, or metal powders can be incorporated to tailor electrical conductivity, wear resistance, or thermal conductivity while preserving the fundamental characteristics of PEEK.
- Processing methods and manufacturability of PEEK: PEEK polymers can be processed using various manufacturing techniques including injection molding, extrusion, compression molding, and additive manufacturing. The high melting point requires specialized equipment capable of reaching processing temperatures of 360-400°C. PEEK exhibits excellent dimensional stability during processing with minimal shrinkage. The material's flow characteristics can be modified through molecular weight adjustments or the addition of processing aids to improve manufacturability while maintaining the polymer's exceptional performance properties.
- Environmental durability and aging characteristics: PEEK polymers demonstrate exceptional resistance to environmental degradation, including UV radiation, moisture absorption, and hydrolysis. They maintain their mechanical and thermal properties even after prolonged exposure to harsh environments. The material shows minimal creep under sustained loading conditions and excellent fatigue resistance. PEEK's inherent flame retardancy, with a UL94 V-0 rating, combined with low smoke and toxic gas emission during combustion, makes it suitable for applications with stringent safety requirements.
02 Chemical resistance and biocompatibility of PEEK
PEEK polymers possess outstanding chemical resistance to a wide range of solvents, acids, and bases. They show minimal degradation when exposed to harsh chemical environments. Additionally, PEEK materials demonstrate excellent biocompatibility, making them suitable for medical implants and devices. Their inert nature prevents adverse reactions in biological environments, while their resistance to sterilization methods further enhances their suitability for medical applications.Expand Specific Solutions03 PEEK composite formulations and enhanced properties
PEEK can be formulated with various fillers and reinforcements to enhance specific properties. Carbon fiber reinforced PEEK offers improved strength-to-weight ratio and dimensional stability. Glass fiber reinforced variants provide enhanced mechanical properties and electrical insulation. Other additives can improve wear resistance, reduce friction, or add electrical conductivity while maintaining the base polymer's thermal stability and chemical resistance.Expand Specific Solutions04 Processing techniques for PEEK polymers
PEEK polymers can be processed using various techniques including injection molding, extrusion, and compression molding. The high melting point requires specialized equipment capable of reaching processing temperatures of 360-400°C. Proper drying before processing is essential to prevent hydrolytic degradation. Advanced techniques such as 3D printing of PEEK have been developed for complex geometries, though they require careful control of processing parameters to achieve optimal mechanical properties.Expand Specific Solutions05 Applications of PEEK based on property advantages
The exceptional properties of PEEK polymers make them suitable for demanding applications across multiple industries. In aerospace, PEEK components reduce weight while maintaining strength. In automotive applications, they withstand high temperatures in engine compartments. For medical devices, PEEK's biocompatibility enables use in implants and surgical instruments. In industrial settings, PEEK components offer longevity in harsh chemical environments and high-wear situations, providing cost benefits despite higher initial material costs.Expand Specific Solutions
Leading PEEK Manufacturers and Research Institutions
The PEEK polymer properties market is currently in a growth phase, with increasing regulatory compliance demands driving research and development. The global market size is expanding steadily, valued at approximately $1 billion with projected annual growth of 6-8%. Technologically, PEEK research has reached moderate maturity but continues to evolve, particularly in specialized applications. Leading players include Solvay Specialty Polymers and Victrex Manufacturing, who dominate with extensive patent portfolios and advanced manufacturing capabilities. Emerging competitors like Jilin Joinature Polymer and Nanjing Comptech Composites are gaining market share through specialized applications. Academic institutions such as Jilin University and Shanghai University collaborate with industry players to advance PEEK's properties for regulatory compliance in medical, aerospace, and automotive sectors.
Victrex Manufacturing Ltd.
Technical Solution: Victrex has developed advanced PEEK polymer formulations with enhanced regulatory compliance features. Their PEEK-OPTIMA™ polymer technology demonstrates exceptional chemical resistance, biocompatibility, and mechanical strength suitable for medical implants and aerospace applications. The company's research focuses on optimizing crystallinity control during processing to achieve specific mechanical properties while maintaining compliance with FDA, REACH, and other regulatory frameworks. Their proprietary manufacturing process ensures ultra-high purity PEEK with minimal extractables and leachables, critical for regulatory approval in medical and food contact applications. Victrex has also pioneered PEEK composite technologies that maintain the polymer's inherent flame retardancy and low smoke toxicity properties without requiring additional additives that might compromise regulatory status.
Strengths: Industry-leading expertise in medical-grade PEEK with extensive regulatory approvals; proprietary processing techniques that maintain polymer purity. Weaknesses: Higher cost compared to conventional polymers; limited customization options for specific regulatory environments without compromising core mechanical properties.
Ticona LLC
Technical Solution: Ticona (now part of Celanese) has developed VESTAKEEP® PEEK polymers with specific property profiles engineered for regulatory compliance in demanding applications. Their technology focuses on controlling molecular weight distribution and end-group chemistry to achieve optimal combinations of processability and mechanical performance while meeting stringent regulatory requirements. Ticona's research has established precise relationships between processing conditions and crystallinity development, allowing manufacturers to achieve consistent properties batch-to-batch - a critical factor for regulatory documentation. Their PEEK formulations demonstrate exceptional resistance to high-energy radiation sterilization while maintaining mechanical integrity, addressing key requirements for medical device approval. Ticona has also pioneered specialized testing methodologies for evaluating long-term chemical resistance under combined stress conditions, providing data essential for regulatory risk assessments in aerospace and oil/gas applications.
Strengths: Extensive experience in developing PEEK grades for highly regulated industries; strong technical documentation supporting regulatory submissions. Weaknesses: More limited portfolio of specialized grades compared to some competitors; relatively conservative approach to material innovation.
Critical Patents and Innovations in PEEK Property Enhancement
Polyether ether ketone, and method for purification of polymer material
PatentInactiveEP2208748A1
Innovation
- A polyether ether ketone with a specific molecular weight composition and a method for its purification using a water-soluble aprotonic solvent at elevated temperatures, followed by mixing with water under ambient pressure, effectively improving mold flow performance, mechanical properties, and thermal stability while reducing impurities and production costs.
Polyether ether ketone and method for producing polyether ether ketone
PatentActiveUS20230265244A1
Innovation
- A method involving the reaction of 4,4′-dichlorobenzophenone and hydroquinone under specific temperature conditions (260° C. to 320° C.) to produce a PEEK with a crystallization temperature Tc of 255° C. or more, while controlling fluorine and chlorine atom content, resulting in enhanced mechanical strength and reduced viscosity.
Regulatory Framework Analysis for PEEK Applications
The regulatory landscape governing PEEK (Polyetheretherketone) applications spans multiple jurisdictions and industries, creating a complex compliance environment for manufacturers and end-users. At the global level, organizations such as the International Organization for Standardization (ISO) have established standards like ISO 10993 for biocompatibility assessment, which is particularly relevant for medical-grade PEEK applications. These standards provide a framework for evaluating the biological safety of materials intended for contact with human tissues.
In the United States, the Food and Drug Administration (FDA) regulates PEEK materials used in medical devices through the 510(k) clearance process, requiring manufacturers to demonstrate substantial equivalence to legally marketed devices. The FDA has established specific guidance documents addressing polymer safety evaluation, with particular attention to leachables and extractables when PEEK is used in implantable devices or drug delivery systems.
The European Union's regulatory approach centers around the Medical Device Regulation (MDR) and the Registration, Evaluation, Authorization and Restriction of Chemicals (REACH) framework. Under MDR, PEEK-based medical devices must meet essential requirements for safety and performance, while REACH mandates registration and safety assessment of chemical substances, including polymers like PEEK when used in certain applications.
For aerospace and automotive applications, regulatory bodies such as the Federal Aviation Administration (FAA) and the European Aviation Safety Agency (EASA) have established certification requirements for materials used in critical components. These regulations focus on mechanical properties, thermal stability, and fire resistance characteristics of PEEK composites.
Industry-specific standards further refine compliance requirements. ASTM F2026 specifically addresses PEEK polymers intended for surgical implant applications, while ASTM D6262 provides standard test methods for determining the mechanical properties of unfilled and filled PEEK materials. These standards ensure consistency in material evaluation across different manufacturing processes and applications.
Environmental regulations increasingly impact PEEK applications, with directives such as RoHS (Restriction of Hazardous Substances) and WEEE (Waste Electrical and Electronic Equipment) in the EU affecting how PEEK-containing products are manufactured, used, and disposed of. Though PEEK itself is generally considered environmentally stable, additives and processing aids may fall under regulatory scrutiny.
Compliance documentation requirements vary by jurisdiction but typically include material safety data sheets (MSDS), technical data sheets, certificates of analysis, and in some cases, full material qualification reports. Manufacturers must maintain comprehensive records demonstrating adherence to applicable standards and regulations throughout the product lifecycle.
In the United States, the Food and Drug Administration (FDA) regulates PEEK materials used in medical devices through the 510(k) clearance process, requiring manufacturers to demonstrate substantial equivalence to legally marketed devices. The FDA has established specific guidance documents addressing polymer safety evaluation, with particular attention to leachables and extractables when PEEK is used in implantable devices or drug delivery systems.
The European Union's regulatory approach centers around the Medical Device Regulation (MDR) and the Registration, Evaluation, Authorization and Restriction of Chemicals (REACH) framework. Under MDR, PEEK-based medical devices must meet essential requirements for safety and performance, while REACH mandates registration and safety assessment of chemical substances, including polymers like PEEK when used in certain applications.
For aerospace and automotive applications, regulatory bodies such as the Federal Aviation Administration (FAA) and the European Aviation Safety Agency (EASA) have established certification requirements for materials used in critical components. These regulations focus on mechanical properties, thermal stability, and fire resistance characteristics of PEEK composites.
Industry-specific standards further refine compliance requirements. ASTM F2026 specifically addresses PEEK polymers intended for surgical implant applications, while ASTM D6262 provides standard test methods for determining the mechanical properties of unfilled and filled PEEK materials. These standards ensure consistency in material evaluation across different manufacturing processes and applications.
Environmental regulations increasingly impact PEEK applications, with directives such as RoHS (Restriction of Hazardous Substances) and WEEE (Waste Electrical and Electronic Equipment) in the EU affecting how PEEK-containing products are manufactured, used, and disposed of. Though PEEK itself is generally considered environmentally stable, additives and processing aids may fall under regulatory scrutiny.
Compliance documentation requirements vary by jurisdiction but typically include material safety data sheets (MSDS), technical data sheets, certificates of analysis, and in some cases, full material qualification reports. Manufacturers must maintain comprehensive records demonstrating adherence to applicable standards and regulations throughout the product lifecycle.
Sustainability Aspects of PEEK in Compliance-Critical Applications
Sustainability has become a critical factor in the evaluation and selection of materials for compliance-critical applications, with PEEK (Polyetheretherketone) emerging as a notable polymer in this context. The inherent properties of PEEK contribute significantly to its sustainability profile, including its exceptional durability, chemical resistance, and high-temperature stability, which collectively extend product lifecycles and reduce replacement frequency.
From a regulatory compliance perspective, PEEK demonstrates remarkable advantages in environmental sustainability. The polymer is halogen-free and produces minimal toxic emissions during processing or in case of fire, aligning with increasingly stringent global environmental regulations such as RoHS, REACH, and various clean air acts. This characteristic is particularly valuable in medical, aerospace, and food processing applications where material purity and emission control are paramount compliance requirements.
PEEK's recyclability presents both opportunities and challenges in sustainability frameworks. While theoretically recyclable, the high-performance nature of PEEK applications often means components are used in critical systems where material degradation concerns limit recycling potential. However, emerging technologies for mechanical and chemical recycling of PEEK are showing promise, with research indicating that recycled PEEK can retain up to 80-90% of virgin material properties under controlled processing conditions.
Energy efficiency considerations further enhance PEEK's sustainability credentials in compliance-sensitive sectors. The polymer's low thermal conductivity and excellent insulation properties contribute to energy conservation in operational systems. Additionally, the manufacturing process for PEEK, while energy-intensive initially, results in components that offer significant energy savings throughout their extended service life, creating a favorable lifecycle energy balance that meets emerging regulatory standards for carbon footprint reduction.
Biocompatibility represents another dimension of PEEK's sustainability profile, particularly relevant for medical device compliance. PEEK's inert nature and resistance to degradation in biological environments not only ensure regulatory compliance with ISO 10993 and FDA requirements but also contribute to sustainability by enabling the design of long-lasting implantable devices that reduce the need for revision surgeries and associated resource consumption.
Recent advancements in PEEK composites have further enhanced its sustainability aspects. Carbon fiber reinforced PEEK and other composite formulations offer improved strength-to-weight ratios, enabling lightweighting strategies that reduce fuel consumption in transportation applications while maintaining compliance with safety and performance regulations. These developments are particularly significant as regulatory frameworks increasingly incorporate lifecycle assessment criteria into compliance requirements.
From a regulatory compliance perspective, PEEK demonstrates remarkable advantages in environmental sustainability. The polymer is halogen-free and produces minimal toxic emissions during processing or in case of fire, aligning with increasingly stringent global environmental regulations such as RoHS, REACH, and various clean air acts. This characteristic is particularly valuable in medical, aerospace, and food processing applications where material purity and emission control are paramount compliance requirements.
PEEK's recyclability presents both opportunities and challenges in sustainability frameworks. While theoretically recyclable, the high-performance nature of PEEK applications often means components are used in critical systems where material degradation concerns limit recycling potential. However, emerging technologies for mechanical and chemical recycling of PEEK are showing promise, with research indicating that recycled PEEK can retain up to 80-90% of virgin material properties under controlled processing conditions.
Energy efficiency considerations further enhance PEEK's sustainability credentials in compliance-sensitive sectors. The polymer's low thermal conductivity and excellent insulation properties contribute to energy conservation in operational systems. Additionally, the manufacturing process for PEEK, while energy-intensive initially, results in components that offer significant energy savings throughout their extended service life, creating a favorable lifecycle energy balance that meets emerging regulatory standards for carbon footprint reduction.
Biocompatibility represents another dimension of PEEK's sustainability profile, particularly relevant for medical device compliance. PEEK's inert nature and resistance to degradation in biological environments not only ensure regulatory compliance with ISO 10993 and FDA requirements but also contribute to sustainability by enabling the design of long-lasting implantable devices that reduce the need for revision surgeries and associated resource consumption.
Recent advancements in PEEK composites have further enhanced its sustainability aspects. Carbon fiber reinforced PEEK and other composite formulations offer improved strength-to-weight ratios, enabling lightweighting strategies that reduce fuel consumption in transportation applications while maintaining compliance with safety and performance regulations. These developments are particularly significant as regulatory frameworks increasingly incorporate lifecycle assessment criteria into compliance requirements.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!