Solid State Battery Breakthrough and its Effect on Catalytic Processes
OCT 24, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Solid State Battery Evolution and Research Objectives
Solid state battery technology has evolved significantly over the past few decades, transitioning from conceptual research to increasingly viable commercial applications. The journey began in the 1970s with the discovery of solid electrolytes, but meaningful progress accelerated in the early 2000s when safety and energy density limitations of conventional lithium-ion batteries became apparent. This evolution has been characterized by persistent efforts to overcome key challenges including interfacial resistance, manufacturing scalability, and material stability.
The technological trajectory has seen three distinct phases: initial discovery of solid electrolyte materials, optimization of interfaces between electrodes and electrolytes, and the current phase focused on manufacturing scalability and cost reduction. Recent breakthroughs in ceramic and polymer-based solid electrolytes have demonstrated conductivity levels comparable to liquid electrolytes while offering superior safety profiles and potential for higher energy density.
Research objectives in this field are increasingly focused on understanding the fundamental mechanisms of ion transport across solid-solid interfaces, which represents the most significant barrier to performance optimization. Scientists aim to develop novel materials and architectures that can facilitate seamless ion movement while maintaining structural integrity during repeated charge-discharge cycles.
A particularly promising research direction involves the intersection of solid state battery technology with catalytic processes. The enhanced thermal stability of solid electrolytes creates opportunities for integration with catalytic systems that operate at elevated temperatures. This convergence could enable more efficient energy storage solutions for industrial applications where heat generation is inevitable.
Current research objectives also include developing solid state batteries capable of supporting rapid charging without degradation, extending cycle life beyond 1,000 cycles, and achieving energy densities exceeding 400 Wh/kg. These targets represent significant improvements over conventional lithium-ion technology and would enable transformative applications in transportation, grid storage, and portable electronics.
The environmental impact of solid state battery technology is another critical research focus. Scientists are investigating sustainable material sourcing, reduced reliance on rare earth elements, and designing for recyclability. These considerations are essential for ensuring that next-generation battery technologies contribute positively to global sustainability goals while delivering enhanced performance.
Interdisciplinary collaboration has become increasingly important, with materials scientists, electrochemists, and manufacturing engineers working together to address multifaceted challenges. This collaborative approach is essential for translating laboratory breakthroughs into commercially viable products that can be manufactured at scale with consistent quality and reasonable cost structures.
The technological trajectory has seen three distinct phases: initial discovery of solid electrolyte materials, optimization of interfaces between electrodes and electrolytes, and the current phase focused on manufacturing scalability and cost reduction. Recent breakthroughs in ceramic and polymer-based solid electrolytes have demonstrated conductivity levels comparable to liquid electrolytes while offering superior safety profiles and potential for higher energy density.
Research objectives in this field are increasingly focused on understanding the fundamental mechanisms of ion transport across solid-solid interfaces, which represents the most significant barrier to performance optimization. Scientists aim to develop novel materials and architectures that can facilitate seamless ion movement while maintaining structural integrity during repeated charge-discharge cycles.
A particularly promising research direction involves the intersection of solid state battery technology with catalytic processes. The enhanced thermal stability of solid electrolytes creates opportunities for integration with catalytic systems that operate at elevated temperatures. This convergence could enable more efficient energy storage solutions for industrial applications where heat generation is inevitable.
Current research objectives also include developing solid state batteries capable of supporting rapid charging without degradation, extending cycle life beyond 1,000 cycles, and achieving energy densities exceeding 400 Wh/kg. These targets represent significant improvements over conventional lithium-ion technology and would enable transformative applications in transportation, grid storage, and portable electronics.
The environmental impact of solid state battery technology is another critical research focus. Scientists are investigating sustainable material sourcing, reduced reliance on rare earth elements, and designing for recyclability. These considerations are essential for ensuring that next-generation battery technologies contribute positively to global sustainability goals while delivering enhanced performance.
Interdisciplinary collaboration has become increasingly important, with materials scientists, electrochemists, and manufacturing engineers working together to address multifaceted challenges. This collaborative approach is essential for translating laboratory breakthroughs into commercially viable products that can be manufactured at scale with consistent quality and reasonable cost structures.
Market Demand Analysis for Advanced Energy Storage Solutions
The global energy storage market is experiencing unprecedented growth, driven by the increasing adoption of renewable energy sources and the electrification of transportation. Advanced energy storage solutions, particularly solid-state batteries, are positioned at the forefront of this transformation. Current market projections indicate that the global advanced battery market will reach approximately $120 billion by 2027, with solid-state batteries expected to capture a significant portion of this growth as the technology matures.
The demand for solid-state batteries stems primarily from three key sectors: electric vehicles, consumer electronics, and grid-scale energy storage. In the electric vehicle segment, manufacturers are actively seeking battery technologies that offer higher energy density, faster charging capabilities, and enhanced safety profiles. Solid-state batteries, with their potential to deliver up to 2.5 times the energy density of conventional lithium-ion batteries, represent a compelling solution to range anxiety concerns that currently limit EV adoption.
Consumer electronics manufacturers are similarly motivated by the promise of longer device operation times and reduced form factors. The elimination of liquid electrolytes in solid-state designs addresses safety concerns related to thermal runaway, making these batteries particularly attractive for wearable technologies and mobile devices where proximity to users heightens safety considerations.
Grid-scale applications represent another substantial market opportunity, with utility companies increasingly deploying battery storage systems to balance load fluctuations from intermittent renewable energy sources. The extended cycle life and improved safety characteristics of solid-state technologies align perfectly with the requirements of these stationary applications.
Regional analysis reveals varying degrees of market readiness. Asia-Pacific currently leads in manufacturing capacity development, with Japan and South Korea hosting several advanced pilot production facilities. North America and Europe are focusing heavily on research and development, with substantial public and private investments flowing into solid-state battery startups and research institutions.
The catalytic processes affected by solid-state battery breakthroughs extend beyond the energy storage sector itself. Manufacturing processes for electrode materials and solid electrolytes require novel catalytic approaches, creating demand for specialized materials and process technologies. Additionally, the recycling and end-of-life management of these batteries will necessitate the development of new catalytic processes to efficiently recover valuable materials.
Market analysts predict that the transition to solid-state technology will accelerate significantly once production costs decrease to within 20% of conventional lithium-ion batteries, a threshold expected to be reached within the next 5-7 years based on current development trajectories.
The demand for solid-state batteries stems primarily from three key sectors: electric vehicles, consumer electronics, and grid-scale energy storage. In the electric vehicle segment, manufacturers are actively seeking battery technologies that offer higher energy density, faster charging capabilities, and enhanced safety profiles. Solid-state batteries, with their potential to deliver up to 2.5 times the energy density of conventional lithium-ion batteries, represent a compelling solution to range anxiety concerns that currently limit EV adoption.
Consumer electronics manufacturers are similarly motivated by the promise of longer device operation times and reduced form factors. The elimination of liquid electrolytes in solid-state designs addresses safety concerns related to thermal runaway, making these batteries particularly attractive for wearable technologies and mobile devices where proximity to users heightens safety considerations.
Grid-scale applications represent another substantial market opportunity, with utility companies increasingly deploying battery storage systems to balance load fluctuations from intermittent renewable energy sources. The extended cycle life and improved safety characteristics of solid-state technologies align perfectly with the requirements of these stationary applications.
Regional analysis reveals varying degrees of market readiness. Asia-Pacific currently leads in manufacturing capacity development, with Japan and South Korea hosting several advanced pilot production facilities. North America and Europe are focusing heavily on research and development, with substantial public and private investments flowing into solid-state battery startups and research institutions.
The catalytic processes affected by solid-state battery breakthroughs extend beyond the energy storage sector itself. Manufacturing processes for electrode materials and solid electrolytes require novel catalytic approaches, creating demand for specialized materials and process technologies. Additionally, the recycling and end-of-life management of these batteries will necessitate the development of new catalytic processes to efficiently recover valuable materials.
Market analysts predict that the transition to solid-state technology will accelerate significantly once production costs decrease to within 20% of conventional lithium-ion batteries, a threshold expected to be reached within the next 5-7 years based on current development trajectories.
Current Challenges in Solid State Battery Technology
Despite significant advancements in solid-state battery technology, several critical challenges continue to impede widespread commercialization and integration with catalytic processes. The most persistent obstacle remains the solid-state electrolyte-electrode interface, where high impedance creates substantial resistance to ion transfer. This interface problem manifests as capacity degradation during cycling and limits power density capabilities, making solid-state batteries currently unsuitable for high-power applications where catalytic processes are often employed.
Material compatibility issues present another significant hurdle. The chemical and mechanical interactions between solid electrolytes and electrode materials frequently lead to unwanted side reactions and degradation pathways. These interactions are particularly problematic when considering integration with catalytic systems, as catalysts are inherently designed to accelerate chemical reactions, potentially exacerbating degradation mechanisms at interfaces.
Manufacturing scalability remains underdeveloped, with current production techniques for solid-state batteries being predominantly laboratory-focused and cost-prohibitive. The precision required for multilayer assembly of solid components presents formidable engineering challenges, especially when considering the need for consistent performance across large-format batteries necessary for industrial catalytic applications.
Temperature sensitivity constitutes another major limitation. Many promising solid electrolyte materials only achieve adequate ionic conductivity at elevated temperatures, creating operational constraints that conflict with the temperature requirements of many catalytic processes. Conversely, some solid electrolytes suffer from thermal stability issues at higher temperatures, limiting their application in high-temperature catalytic environments.
Mechanical stress management during cycling represents a persistent engineering challenge. Volume changes during charging and discharging create mechanical stresses that can lead to contact loss between solid components, resulting in capacity fade and potential safety hazards. These mechanical integrity issues become particularly critical when batteries must withstand vibration and thermal cycling in industrial catalytic settings.
The development of suitable cathode materials compatible with solid electrolytes continues to lag behind anode development. Current cathode formulations often demonstrate limited capacity, poor rate capability, or inadequate cycle life when paired with solid electrolytes. This imbalance in component development creates a bottleneck for overall battery performance, particularly in applications requiring high energy density alongside catalytic functionality.
Finally, fundamental understanding of ion transport mechanisms at solid-solid interfaces remains incomplete. This knowledge gap hampers rational design approaches for next-generation materials and interfaces that could potentially overcome current limitations and enable effective integration with catalytic processes. Addressing these challenges requires coordinated research efforts spanning materials science, electrochemistry, and manufacturing engineering.
Material compatibility issues present another significant hurdle. The chemical and mechanical interactions between solid electrolytes and electrode materials frequently lead to unwanted side reactions and degradation pathways. These interactions are particularly problematic when considering integration with catalytic systems, as catalysts are inherently designed to accelerate chemical reactions, potentially exacerbating degradation mechanisms at interfaces.
Manufacturing scalability remains underdeveloped, with current production techniques for solid-state batteries being predominantly laboratory-focused and cost-prohibitive. The precision required for multilayer assembly of solid components presents formidable engineering challenges, especially when considering the need for consistent performance across large-format batteries necessary for industrial catalytic applications.
Temperature sensitivity constitutes another major limitation. Many promising solid electrolyte materials only achieve adequate ionic conductivity at elevated temperatures, creating operational constraints that conflict with the temperature requirements of many catalytic processes. Conversely, some solid electrolytes suffer from thermal stability issues at higher temperatures, limiting their application in high-temperature catalytic environments.
Mechanical stress management during cycling represents a persistent engineering challenge. Volume changes during charging and discharging create mechanical stresses that can lead to contact loss between solid components, resulting in capacity fade and potential safety hazards. These mechanical integrity issues become particularly critical when batteries must withstand vibration and thermal cycling in industrial catalytic settings.
The development of suitable cathode materials compatible with solid electrolytes continues to lag behind anode development. Current cathode formulations often demonstrate limited capacity, poor rate capability, or inadequate cycle life when paired with solid electrolytes. This imbalance in component development creates a bottleneck for overall battery performance, particularly in applications requiring high energy density alongside catalytic functionality.
Finally, fundamental understanding of ion transport mechanisms at solid-solid interfaces remains incomplete. This knowledge gap hampers rational design approaches for next-generation materials and interfaces that could potentially overcome current limitations and enable effective integration with catalytic processes. Addressing these challenges requires coordinated research efforts spanning materials science, electrochemistry, and manufacturing engineering.
Existing Solid State Battery Architectures and Catalytic Interfaces
01 Catalytic materials for solid-state battery electrodes
Various catalytic materials can be incorporated into solid-state battery electrodes to enhance electrochemical performance. These catalysts facilitate ion transport at the electrode-electrolyte interface, improve reaction kinetics, and enhance the overall efficiency of the battery. The catalytic materials can include transition metals, metal oxides, and composite structures that are strategically designed to optimize the electrochemical processes within the solid-state battery system.- Catalytic materials for solid-state battery electrodes: Various catalytic materials can be incorporated into solid-state battery electrodes to enhance electrochemical performance. These catalysts facilitate ion transport at interfaces, improve reaction kinetics, and enhance the overall efficiency of the battery. Specific catalytic materials include transition metal oxides, noble metals, and composite structures that can significantly reduce interfacial resistance and improve cycling stability in solid-state battery systems.
- Interface engineering with catalytic layers: Interface engineering involves the application of catalytic layers between solid electrolytes and electrodes to improve contact and reduce resistance. These catalytic interlayers promote ion transfer across interfaces, mitigate degradation mechanisms, and enhance the electrochemical stability of solid-state batteries. The engineered interfaces with catalytic properties can significantly improve battery performance by facilitating smoother ion transport and reducing interfacial impedance.
- Solid electrolyte synthesis with catalytic processes: Catalytic processes are employed in the synthesis of solid electrolytes to enhance ionic conductivity and mechanical properties. These processes involve the use of catalysts to control crystallization, grain growth, and phase formation during solid electrolyte preparation. The resulting solid electrolytes exhibit improved ionic conductivity, better mechanical integrity, and enhanced compatibility with electrode materials, leading to superior battery performance.
- Catalytic conversion processes for solid-state battery materials: Catalytic conversion processes are utilized to transform precursor materials into active components for solid-state batteries. These processes involve the use of catalysts to facilitate chemical transformations under milder conditions, resulting in materials with optimized properties. The catalytic conversion approach enables the synthesis of novel electrode and electrolyte materials with tailored structures and compositions that enhance energy density, power capability, and cycling stability.
- In-situ catalytic processes during battery operation: In-situ catalytic processes occur during battery operation to maintain performance and extend cycle life. These processes involve catalytic materials that continuously facilitate electrode reactions, prevent dendrite formation, and mitigate degradation mechanisms during charging and discharging cycles. The incorporation of in-situ catalysts helps maintain interfacial stability, promotes uniform ion distribution, and enables more efficient energy conversion throughout the battery's operational lifetime.
02 Interface engineering using catalytic processes
Interface engineering employs catalytic processes to improve the contact between solid electrolytes and electrodes in solid-state batteries. These processes help reduce interfacial resistance, enhance ion transport across boundaries, and stabilize the electrode-electrolyte interface during cycling. Catalytic treatments can modify surface properties, create favorable reaction pathways, and mitigate degradation mechanisms that typically occur at these critical interfaces.Expand Specific Solutions03 Solid electrolyte synthesis via catalytic methods
Catalytic methods are employed in the synthesis of solid electrolytes to achieve desired crystalline structures, phase purity, and ionic conductivity. These processes can reduce synthesis temperatures, shorten reaction times, and improve the homogeneity of the resulting materials. The catalytic synthesis approaches enable the production of high-performance solid electrolytes with optimized properties for enhanced battery performance, including superior ionic conductivity and mechanical stability.Expand Specific Solutions04 Catalytic conversion processes for electrode materials
Catalytic conversion processes are utilized to transform precursor materials into high-performance electrode components for solid-state batteries. These processes can control particle morphology, size distribution, and surface characteristics of active materials. By employing specific catalysts during material synthesis, manufacturers can enhance the electrochemical properties of electrode materials, including capacity, rate capability, and cycle life, while also improving their compatibility with solid electrolytes.Expand Specific Solutions05 In-situ catalytic processes during battery operation
In-situ catalytic processes occur during solid-state battery operation to facilitate electrochemical reactions and maintain performance over extended cycling. These processes can involve the formation of catalytic intermediates, surface reconstruction phenomena, or activation of specific reaction pathways. Understanding and controlling these in-situ catalytic mechanisms is crucial for developing solid-state batteries with improved cycling stability, reduced degradation, and enhanced safety characteristics.Expand Specific Solutions
Leading Companies and Research Institutions in Solid State Battery Development
The solid-state battery market is currently in an early growth phase, characterized by significant R&D investments but limited commercial deployment. Market size is projected to expand rapidly, reaching approximately $6-8 billion by 2030, driven by automotive applications and consumer electronics. Technologically, solid-state batteries remain in the pre-commercialization stage, with key players at different maturity levels. Samsung, Toyota, and Panasonic lead in patent portfolios, while specialized companies like Sakti3 and Nextech Batteries focus on innovative materials. Academic-industry partnerships are accelerating development, with Kyushu University, MIT, and Fraunhofer-Gesellschaft collaborating with manufacturers like CATL and Murata. The integration with catalytic processes represents an emerging frontier that could enhance energy efficiency and battery performance.
Samsung Electronics Co., Ltd.
Technical Solution: Samsung Electronics has developed an innovative solid-state battery technology utilizing a composite solid electrolyte system that combines ceramic and polymer materials to optimize both ionic conductivity and mechanical flexibility[1]. Their approach addresses the critical interface challenges between electrodes and electrolytes through a gradient composition strategy that minimizes resistance and enhances ion transport[2]. Samsung's solid-state batteries incorporate nano-engineered catalytic layers at electrode interfaces that facilitate ion transfer and improve overall electrochemical performance. The company has demonstrated prototype cells with energy densities exceeding 900 Wh/L, significantly higher than conventional lithium-ion batteries[3]. Samsung's technology integrates advanced manufacturing techniques including dry deposition methods that eliminate the need for traditional liquid-based processes, resulting in more environmentally friendly production. Their solid-state battery design specifically enhances catalytic processes at the electrode-electrolyte interface, improving reaction kinetics and overall battery efficiency[4]. Samsung has also developed proprietary coating technologies that stabilize the solid electrolyte interface during cycling, addressing one of the key degradation mechanisms in solid-state systems.
Strengths: Exceptional energy density, improved thermal stability allowing operation across wider temperature ranges, compatibility with existing manufacturing infrastructure, and enhanced safety characteristics. Weaknesses: Challenges with mechanical stress during cycling leading to potential micro-crack formation, higher initial production costs compared to conventional batteries, and limited demonstration of long-term cycling stability at commercial scale.
SAMSUNG SDI CO LTD
Technical Solution: Samsung SDI has developed a hybrid solid-state battery technology that combines the benefits of both ceramic and polymer electrolytes. Their approach utilizes a composite solid electrolyte system with proprietary additives that enhance ionic conductivity while maintaining mechanical integrity[1]. The company's solid-state battery design features a unique multi-layered structure that optimizes the interface between electrodes and electrolyte, significantly reducing resistance and improving energy efficiency[2]. Samsung SDI has integrated catalytic nanoparticles within their electrode structures that facilitate faster ion transport and enhance electrochemical reactions at the electrode-electrolyte interface. Their manufacturing process employs advanced deposition techniques that enable precise control of layer thickness and uniformity, critical factors for solid-state battery performance[3]. The company has demonstrated prototype cells with energy densities approaching 1,000 Wh/L and fast-charging capabilities that allow 80% charge in approximately 20 minutes. Samsung SDI's solid-state technology also incorporates innovative thermal management systems that maintain optimal operating conditions for catalytic processes within the battery, enhancing overall performance and longevity[4].
Strengths: High energy density, excellent thermal stability, enhanced safety profile with non-flammable components, and compatibility with existing manufacturing infrastructure. Weaknesses: Challenges with interface stability during extended cycling, higher production costs compared to conventional lithium-ion batteries, and sensitivity to environmental conditions that can affect catalytic efficiency.
Key Patents and Scientific Breakthroughs in Solid Electrolyte Materials
Cermet electrode for solid state and lithium ion batteries
PatentPendingUS20200388854A1
Innovation
- A porous ceramic-metal (cermet) cathode is developed, where a metallic material acts as a binder and conductive additive, providing mechanical integrity and interconnected porosity to accommodate liquid, gel, or polymer electrolytes, and is free of conventional binders and conductive carbon, enhancing the cathode's mechanical strength and stability.
Impregnated sintered solid state composite electrode, solid state battery, and methods of preparation
PatentInactiveUS20150056520A1
Innovation
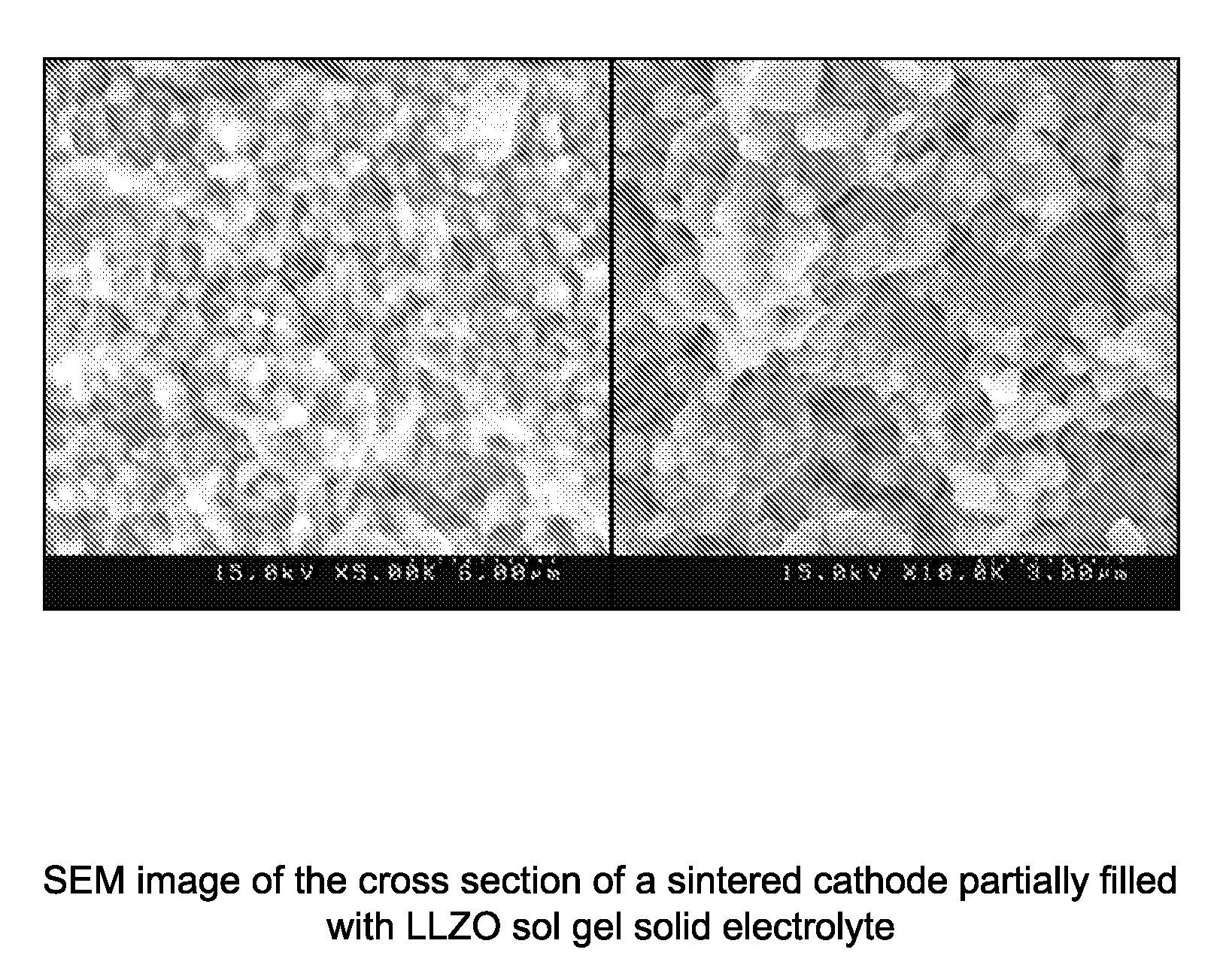
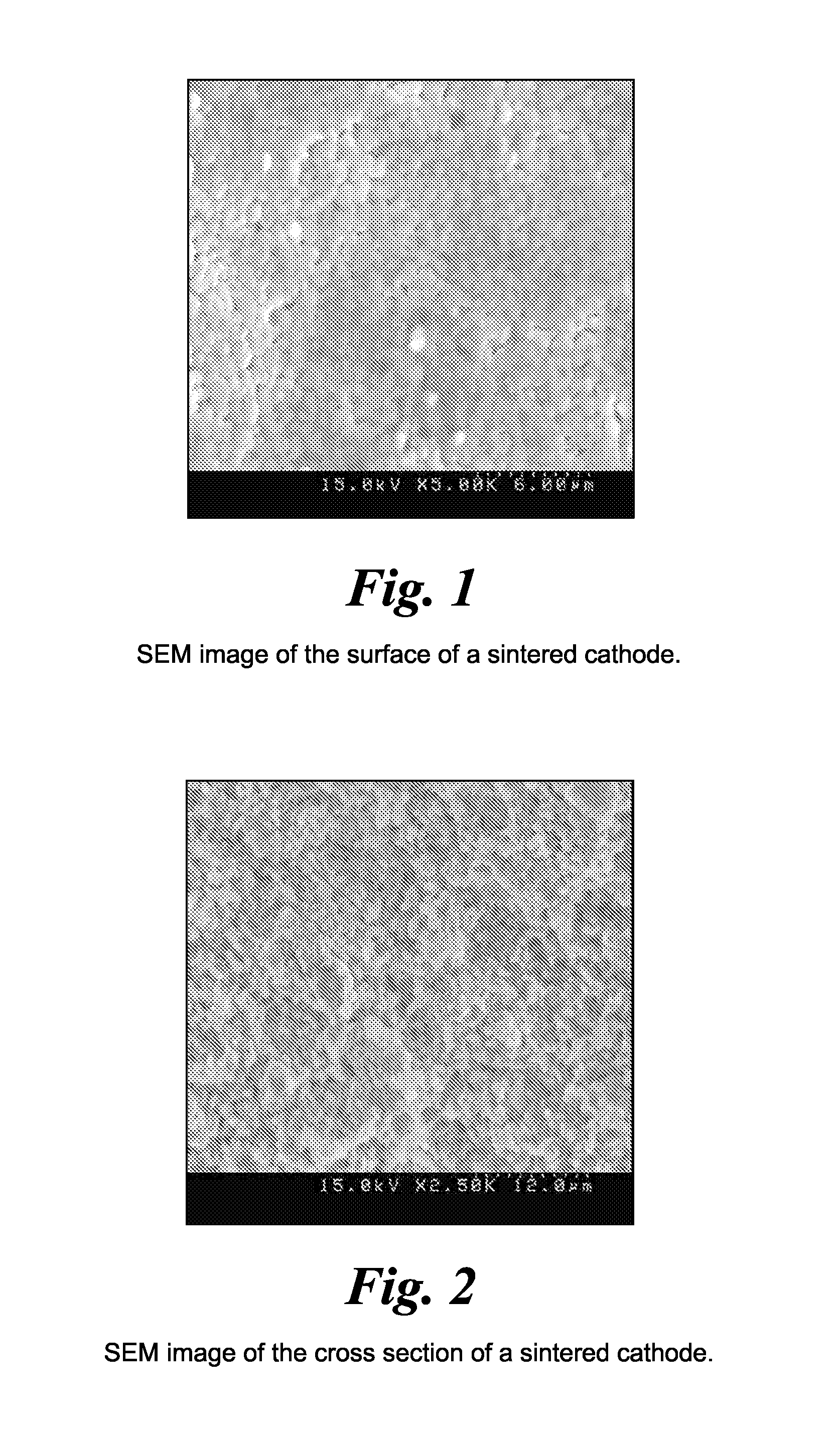
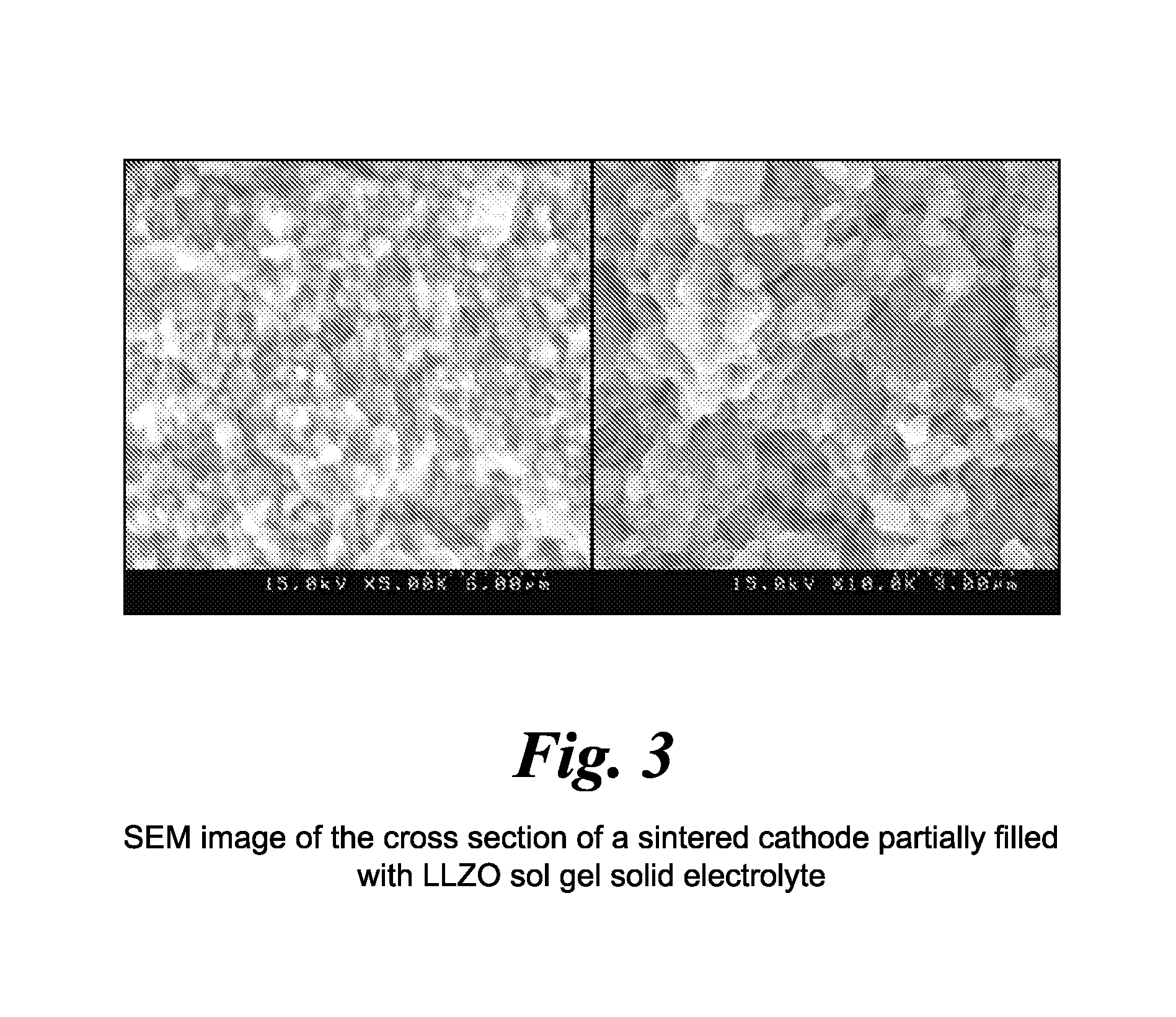
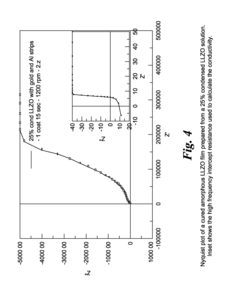
- A method involving the formation of sintered porous cathode pellets, impregnation with a liquid precursor of an inorganic amorphous ionically conductive solid electrolyte, and curing to create a composite cathode with enhanced ionic pathways, using materials like amorphous lithium lanthanum zirconium oxide (LLZO), which reduces shrinkage and improves conductivity.
Environmental Impact and Sustainability Considerations
The advancement of solid-state battery technology represents a significant step toward more sustainable energy storage solutions with far-reaching environmental implications. Unlike conventional lithium-ion batteries that utilize liquid electrolytes, solid-state batteries eliminate the need for toxic and flammable components, substantially reducing the risk of environmental contamination from leakage or thermal runaway incidents. This fundamental design difference translates to a significantly improved environmental safety profile throughout the battery lifecycle.
From a resource perspective, solid-state battery technology offers promising pathways to reduce dependence on critical raw materials. Current research directions include the development of catalytic processes that enable more efficient use of rare earth elements and transition metals, potentially alleviating supply chain pressures on environmentally sensitive mining operations. The integration of these advanced catalytic processes with solid-state battery manufacturing could establish more sustainable material cycles, particularly important as global demand for energy storage continues to escalate.
Life cycle assessment studies indicate that solid-state batteries may achieve a 15-30% reduction in carbon footprint compared to conventional lithium-ion technologies when accounting for manufacturing, use, and end-of-life phases. This improvement stems primarily from extended service life, enhanced energy density, and the elimination of certain energy-intensive production steps associated with liquid electrolyte preparation and handling.
The catalytic processes enabled by solid-state battery breakthroughs extend beyond the batteries themselves, potentially transforming adjacent industries. For instance, more efficient catalytic converters utilizing similar materials and technologies could significantly reduce emissions from remaining internal combustion engines during the transition to electrification. Additionally, industrial chemical processes could benefit from these advanced catalytic materials, reducing energy requirements and waste generation across manufacturing sectors.
End-of-life management represents another critical sustainability dimension where solid-state technology offers advantages. The absence of liquid components simplifies recycling processes, potentially increasing recovery rates for valuable materials. Emerging recycling technologies specifically designed for solid-state batteries demonstrate recovery efficiencies exceeding 90% for key elements, compared to 50-70% for conventional batteries, creating a more circular material economy.
Water conservation benefits also merit consideration, as solid-state manufacturing processes typically require significantly less water than conventional battery production. This advantage becomes increasingly important as battery manufacturing scales up in regions facing water scarcity challenges, potentially reducing freshwater consumption by millions of gallons annually per gigawatt-hour of production capacity.
From a resource perspective, solid-state battery technology offers promising pathways to reduce dependence on critical raw materials. Current research directions include the development of catalytic processes that enable more efficient use of rare earth elements and transition metals, potentially alleviating supply chain pressures on environmentally sensitive mining operations. The integration of these advanced catalytic processes with solid-state battery manufacturing could establish more sustainable material cycles, particularly important as global demand for energy storage continues to escalate.
Life cycle assessment studies indicate that solid-state batteries may achieve a 15-30% reduction in carbon footprint compared to conventional lithium-ion technologies when accounting for manufacturing, use, and end-of-life phases. This improvement stems primarily from extended service life, enhanced energy density, and the elimination of certain energy-intensive production steps associated with liquid electrolyte preparation and handling.
The catalytic processes enabled by solid-state battery breakthroughs extend beyond the batteries themselves, potentially transforming adjacent industries. For instance, more efficient catalytic converters utilizing similar materials and technologies could significantly reduce emissions from remaining internal combustion engines during the transition to electrification. Additionally, industrial chemical processes could benefit from these advanced catalytic materials, reducing energy requirements and waste generation across manufacturing sectors.
End-of-life management represents another critical sustainability dimension where solid-state technology offers advantages. The absence of liquid components simplifies recycling processes, potentially increasing recovery rates for valuable materials. Emerging recycling technologies specifically designed for solid-state batteries demonstrate recovery efficiencies exceeding 90% for key elements, compared to 50-70% for conventional batteries, creating a more circular material economy.
Water conservation benefits also merit consideration, as solid-state manufacturing processes typically require significantly less water than conventional battery production. This advantage becomes increasingly important as battery manufacturing scales up in regions facing water scarcity challenges, potentially reducing freshwater consumption by millions of gallons annually per gigawatt-hour of production capacity.
Manufacturing Scalability and Cost Analysis
The manufacturing scalability of solid-state batteries represents a critical challenge in transitioning from laboratory breakthroughs to commercial viability. Current production methods for solid-state batteries remain largely confined to small-scale laboratory settings, with significant hurdles in scaling to mass production. The complex multi-layer structure of solid-state batteries demands precision manufacturing techniques that are difficult to implement at industrial scales without compromising performance or safety.
Cost analysis reveals that solid-state batteries currently carry a substantial premium compared to conventional lithium-ion batteries, with production costs estimated at 2-3 times higher per kWh. This cost differential stems primarily from expensive raw materials, particularly solid electrolytes, and specialized manufacturing equipment requirements. The high-purity materials needed for solid electrolytes and the precision assembly processes contribute significantly to the elevated costs.
Material sourcing presents another dimension of the scalability challenge. Many solid-state battery designs incorporate elements with limited global supply chains, such as lithium, cobalt, and specialized ceramic materials. The concentration of these resources in specific geographic regions introduces supply chain vulnerabilities that could impede large-scale manufacturing efforts.
Process optimization represents a promising avenue for improving manufacturing scalability. Innovations in continuous processing techniques, rather than batch production, could substantially reduce manufacturing time and costs. Additionally, advances in automated precision assembly and quality control systems are essential for maintaining consistent performance across mass-produced units.
The integration of solid-state battery production with existing manufacturing infrastructure presents both opportunities and challenges. Leveraging modified versions of current lithium-ion battery production lines could accelerate scalability, though significant retooling would be necessary. Industry analysts project that achieving cost parity with conventional batteries will require production volumes exceeding 10 GWh annually, a scale that demands substantial capital investment.
The catalytic processes affected by solid-state battery technology may benefit from these manufacturing challenges being addressed. As production scales and costs decrease, the application of solid-state batteries in catalytic research and industrial processes becomes more economically viable, potentially accelerating innovations in energy-efficient chemical transformations and electrochemical reactions.
Cost analysis reveals that solid-state batteries currently carry a substantial premium compared to conventional lithium-ion batteries, with production costs estimated at 2-3 times higher per kWh. This cost differential stems primarily from expensive raw materials, particularly solid electrolytes, and specialized manufacturing equipment requirements. The high-purity materials needed for solid electrolytes and the precision assembly processes contribute significantly to the elevated costs.
Material sourcing presents another dimension of the scalability challenge. Many solid-state battery designs incorporate elements with limited global supply chains, such as lithium, cobalt, and specialized ceramic materials. The concentration of these resources in specific geographic regions introduces supply chain vulnerabilities that could impede large-scale manufacturing efforts.
Process optimization represents a promising avenue for improving manufacturing scalability. Innovations in continuous processing techniques, rather than batch production, could substantially reduce manufacturing time and costs. Additionally, advances in automated precision assembly and quality control systems are essential for maintaining consistent performance across mass-produced units.
The integration of solid-state battery production with existing manufacturing infrastructure presents both opportunities and challenges. Leveraging modified versions of current lithium-ion battery production lines could accelerate scalability, though significant retooling would be necessary. Industry analysts project that achieving cost parity with conventional batteries will require production volumes exceeding 10 GWh annually, a scale that demands substantial capital investment.
The catalytic processes affected by solid-state battery technology may benefit from these manufacturing challenges being addressed. As production scales and costs decrease, the application of solid-state batteries in catalytic research and industrial processes becomes more economically viable, potentially accelerating innovations in energy-efficient chemical transformations and electrochemical reactions.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!