Solid State Battery Breakthrough in Healthcare Applications
OCT 24, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Solid State Battery Evolution and Healthcare Goals
Solid state batteries represent a significant evolution in energy storage technology, emerging from decades of research into safer and more efficient alternatives to traditional lithium-ion batteries. The journey began in the 1970s with initial explorations into solid electrolytes, but substantial progress has only materialized in the last decade. This acceleration has been driven by increasing demands for higher energy density, improved safety profiles, and longer operational lifespans across multiple industries, with healthcare applications showing particular promise.
The evolution of solid state battery technology has been marked by progressive improvements in electrolyte materials, from early ceramic compounds to advanced polymer-ceramic composites that offer superior ion conductivity while maintaining structural integrity. These advancements have addressed historical limitations in power density and operational temperature ranges that previously restricted practical applications in medical devices.
In the healthcare context, solid state batteries aim to revolutionize medical device functionality through several key objectives. Primary among these is the development of implantable medical devices with significantly extended operational lifespans, potentially eliminating the need for replacement surgeries that carry inherent risks and costs. Current pacemakers and neurostimulators typically require replacement every 5-10 years, creating a substantial burden on patients and healthcare systems.
Another critical goal is the miniaturization of medical devices while maintaining or improving power output. This would enable less invasive implantation procedures and expand the range of conditions that can be addressed through implantable technology. The reduced form factor could particularly benefit applications in neurological monitoring and stimulation, where space constraints are significant.
Safety represents perhaps the most crucial objective in healthcare applications. Unlike conventional batteries, solid state technology eliminates liquid electrolytes that pose leakage and flammability risks. This safety profile is essential for implantable devices where battery failure could have catastrophic consequences for patients.
Biocompatibility advancements constitute another key goal, with researchers working to develop encapsulation materials and electrolyte compositions that minimize foreign body responses and tissue inflammation. This includes exploration of bioresorbable battery components that could potentially be absorbed safely by the body after their functional lifespan.
The technological trajectory suggests convergence toward flexible solid state batteries that can conform to anatomical structures, further expanding application possibilities in wearable health monitors and implantable therapeutic devices. This flexibility, combined with improved energy density, positions solid state batteries as a transformative technology for next-generation healthcare solutions.
The evolution of solid state battery technology has been marked by progressive improvements in electrolyte materials, from early ceramic compounds to advanced polymer-ceramic composites that offer superior ion conductivity while maintaining structural integrity. These advancements have addressed historical limitations in power density and operational temperature ranges that previously restricted practical applications in medical devices.
In the healthcare context, solid state batteries aim to revolutionize medical device functionality through several key objectives. Primary among these is the development of implantable medical devices with significantly extended operational lifespans, potentially eliminating the need for replacement surgeries that carry inherent risks and costs. Current pacemakers and neurostimulators typically require replacement every 5-10 years, creating a substantial burden on patients and healthcare systems.
Another critical goal is the miniaturization of medical devices while maintaining or improving power output. This would enable less invasive implantation procedures and expand the range of conditions that can be addressed through implantable technology. The reduced form factor could particularly benefit applications in neurological monitoring and stimulation, where space constraints are significant.
Safety represents perhaps the most crucial objective in healthcare applications. Unlike conventional batteries, solid state technology eliminates liquid electrolytes that pose leakage and flammability risks. This safety profile is essential for implantable devices where battery failure could have catastrophic consequences for patients.
Biocompatibility advancements constitute another key goal, with researchers working to develop encapsulation materials and electrolyte compositions that minimize foreign body responses and tissue inflammation. This includes exploration of bioresorbable battery components that could potentially be absorbed safely by the body after their functional lifespan.
The technological trajectory suggests convergence toward flexible solid state batteries that can conform to anatomical structures, further expanding application possibilities in wearable health monitors and implantable therapeutic devices. This flexibility, combined with improved energy density, positions solid state batteries as a transformative technology for next-generation healthcare solutions.
Market Demand Analysis for Medical-Grade Batteries
The healthcare sector is witnessing unprecedented demand for advanced battery technologies, particularly solid-state batteries, driven by the proliferation of wearable medical devices, implantable medical devices (IMDs), and portable diagnostic equipment. The global medical battery market was valued at $1.84 billion in 2021 and is projected to reach $3.6 billion by 2028, growing at a CAGR of 8.2% during the forecast period. Solid-state batteries are positioned to capture a significant portion of this growth due to their superior safety profile and performance characteristics.
Patient monitoring devices represent the largest application segment, accounting for approximately 32% of the medical battery market. The increasing prevalence of chronic diseases such as diabetes, cardiovascular disorders, and neurological conditions has accelerated the adoption of continuous glucose monitors, cardiac monitors, and neurostimulation devices, all requiring reliable, long-lasting power sources.
The implantable medical device segment demonstrates the highest growth potential, with demand increasing by 12.4% annually. Pacemakers, defibrillators, cochlear implants, and drug delivery systems require batteries with exceptional reliability, biocompatibility, and longevity. Traditional lithium-ion batteries with liquid electrolytes pose safety risks such as leakage and thermal runaway, creating a critical need for solid-state alternatives that eliminate these concerns.
Healthcare providers are increasingly demanding batteries with specific performance characteristics: extended cycle life exceeding 5,000 cycles, operational longevity of 10+ years for implantable devices, rapid charging capabilities, and enhanced safety features. A survey of medical device manufacturers revealed that 78% consider battery performance a critical factor in product development, with 64% actively seeking alternatives to conventional lithium-ion technologies.
Regional analysis indicates North America currently dominates the medical battery market with 42% share, followed by Europe (28%) and Asia-Pacific (22%). However, the Asia-Pacific region is expected to exhibit the fastest growth rate due to expanding healthcare infrastructure and increasing medical device manufacturing capabilities in countries like China, Japan, and South Korea.
The COVID-19 pandemic has accelerated market demand for portable medical equipment and remote patient monitoring systems, creating additional pressure for battery innovation. Telemedicine platforms experienced 154% growth in 2020, driving demand for reliable power sources in home healthcare devices.
Regulatory considerations are significantly influencing market dynamics, with FDA and EU MDR requirements imposing stringent safety standards for batteries in medical applications. These regulations favor solid-state technologies that eliminate risks associated with electrolyte leakage and thermal events, providing manufacturers with potential regulatory advantages and faster approval pathways.
Patient monitoring devices represent the largest application segment, accounting for approximately 32% of the medical battery market. The increasing prevalence of chronic diseases such as diabetes, cardiovascular disorders, and neurological conditions has accelerated the adoption of continuous glucose monitors, cardiac monitors, and neurostimulation devices, all requiring reliable, long-lasting power sources.
The implantable medical device segment demonstrates the highest growth potential, with demand increasing by 12.4% annually. Pacemakers, defibrillators, cochlear implants, and drug delivery systems require batteries with exceptional reliability, biocompatibility, and longevity. Traditional lithium-ion batteries with liquid electrolytes pose safety risks such as leakage and thermal runaway, creating a critical need for solid-state alternatives that eliminate these concerns.
Healthcare providers are increasingly demanding batteries with specific performance characteristics: extended cycle life exceeding 5,000 cycles, operational longevity of 10+ years for implantable devices, rapid charging capabilities, and enhanced safety features. A survey of medical device manufacturers revealed that 78% consider battery performance a critical factor in product development, with 64% actively seeking alternatives to conventional lithium-ion technologies.
Regional analysis indicates North America currently dominates the medical battery market with 42% share, followed by Europe (28%) and Asia-Pacific (22%). However, the Asia-Pacific region is expected to exhibit the fastest growth rate due to expanding healthcare infrastructure and increasing medical device manufacturing capabilities in countries like China, Japan, and South Korea.
The COVID-19 pandemic has accelerated market demand for portable medical equipment and remote patient monitoring systems, creating additional pressure for battery innovation. Telemedicine platforms experienced 154% growth in 2020, driving demand for reliable power sources in home healthcare devices.
Regulatory considerations are significantly influencing market dynamics, with FDA and EU MDR requirements imposing stringent safety standards for batteries in medical applications. These regulations favor solid-state technologies that eliminate risks associated with electrolyte leakage and thermal events, providing manufacturers with potential regulatory advantages and faster approval pathways.
Current Challenges in Healthcare Battery Technology
Healthcare battery technology currently faces several critical challenges that impede the advancement of medical devices and applications. Traditional lithium-ion batteries used in healthcare settings suffer from significant safety concerns, including the risk of thermal runaway and potential leakage of toxic electrolytes. These safety issues are particularly problematic in implantable medical devices where battery failure could lead to severe patient harm or necessitate invasive replacement procedures.
Energy density limitations represent another major challenge, as current battery technologies struggle to provide sufficient power for advanced medical devices while maintaining a compact form factor. This constraint particularly affects implantable devices such as pacemakers, neurostimulators, and drug delivery systems, where size restrictions are paramount but energy demands are increasing with device functionality.
Longevity issues plague existing healthcare batteries, with most requiring replacement every 5-10 years, necessitating additional surgical procedures that increase patient risk and healthcare costs. The cycle life degradation of conventional batteries is accelerated in the warm, moist environment of the human body, further reducing their effective lifespan in implantable applications.
Biocompatibility remains a significant hurdle, as conventional battery materials can trigger immune responses or inflammation when placed within or in close contact with human tissue. The potential for toxic material leakage presents ongoing concerns for long-term implantable devices, requiring extensive encapsulation that adds bulk and complexity.
Temperature sensitivity of current battery technologies creates reliability issues in healthcare applications, where maintaining consistent performance across varying body temperatures and external environments is essential for patient safety. Performance fluctuations due to temperature changes can compromise critical medical functions.
Manufacturing complexity and cost factors limit the widespread adoption of advanced battery technologies in healthcare settings. Current production processes for medical-grade batteries involve stringent quality control and specialized materials that significantly increase costs, making cutting-edge devices less accessible to broader patient populations.
Regulatory hurdles present additional challenges, as healthcare batteries must meet exceptionally high safety and reliability standards before receiving approval for clinical use. The extensive testing and validation required for novel battery technologies creates substantial delays in bringing innovative medical devices to market, slowing the overall pace of healthcare technology advancement.
Energy density limitations represent another major challenge, as current battery technologies struggle to provide sufficient power for advanced medical devices while maintaining a compact form factor. This constraint particularly affects implantable devices such as pacemakers, neurostimulators, and drug delivery systems, where size restrictions are paramount but energy demands are increasing with device functionality.
Longevity issues plague existing healthcare batteries, with most requiring replacement every 5-10 years, necessitating additional surgical procedures that increase patient risk and healthcare costs. The cycle life degradation of conventional batteries is accelerated in the warm, moist environment of the human body, further reducing their effective lifespan in implantable applications.
Biocompatibility remains a significant hurdle, as conventional battery materials can trigger immune responses or inflammation when placed within or in close contact with human tissue. The potential for toxic material leakage presents ongoing concerns for long-term implantable devices, requiring extensive encapsulation that adds bulk and complexity.
Temperature sensitivity of current battery technologies creates reliability issues in healthcare applications, where maintaining consistent performance across varying body temperatures and external environments is essential for patient safety. Performance fluctuations due to temperature changes can compromise critical medical functions.
Manufacturing complexity and cost factors limit the widespread adoption of advanced battery technologies in healthcare settings. Current production processes for medical-grade batteries involve stringent quality control and specialized materials that significantly increase costs, making cutting-edge devices less accessible to broader patient populations.
Regulatory hurdles present additional challenges, as healthcare batteries must meet exceptionally high safety and reliability standards before receiving approval for clinical use. The extensive testing and validation required for novel battery technologies creates substantial delays in bringing innovative medical devices to market, slowing the overall pace of healthcare technology advancement.
Current Solid State Battery Solutions for Healthcare
01 Electrolyte materials for solid state batteries
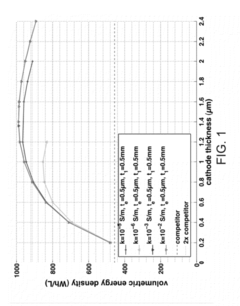
Various electrolyte materials are used in solid state batteries to improve ionic conductivity and battery performance. These include solid polymer electrolytes, ceramic electrolytes, and composite electrolytes that combine different materials to achieve optimal properties. Advanced electrolyte formulations help overcome challenges related to interfacial resistance and enable faster ion transport between electrodes, resulting in higher energy density and improved safety characteristics.- Solid-state electrolyte compositions: Solid-state batteries utilize specialized electrolyte compositions that enable ion transport without liquid components. These electrolytes typically include ceramic materials, polymer matrices, or composite structures that provide high ionic conductivity while maintaining mechanical stability. Advanced formulations may incorporate sulfide-based, oxide-based, or phosphate-based materials that enhance lithium-ion transport while preventing dendrite formation. These solid electrolytes are crucial for improving battery safety and energy density compared to conventional liquid electrolyte systems.
- Interface engineering for solid-state batteries: Interface engineering focuses on optimizing the contact between solid electrolytes and electrodes to reduce resistance and improve ion transfer. This involves developing specialized coatings, buffer layers, or gradient structures that mitigate interfacial impedance issues. Techniques include surface modification of active materials, incorporation of interlayers, and specialized processing methods to ensure intimate contact between components. Effective interface engineering is essential for maximizing power density and cycle life in solid-state battery systems.
- Cathode and anode materials for solid-state batteries: Specialized electrode materials are developed specifically for solid-state battery architectures. These materials must be compatible with solid electrolytes and maintain structural integrity during cycling. High-capacity cathode materials may include lithium-rich layered oxides, sulfur-based compounds, or advanced phospho-olivines, while anodes often utilize lithium metal, silicon-based composites, or carbon structures with tailored porosity. The electrode formulations are designed to minimize volume changes during cycling and facilitate ion transport across the solid-solid interfaces.
- Manufacturing processes for solid-state batteries: Novel manufacturing techniques are essential for producing high-performance solid-state batteries at scale. These processes include specialized deposition methods for thin-film electrolytes, advanced sintering techniques for ceramic components, and innovative assembly approaches that ensure proper layer stacking and interfacial contact. Cold sintering, tape casting, and pressure-assisted methods are employed to create dense, defect-free structures. Manufacturing innovations focus on reducing processing temperatures, improving throughput, and ensuring consistent quality across large-format cells.
- Cell design and architecture for solid-state batteries: Innovative cell designs optimize the performance of solid-state battery systems through structural engineering. These designs include bipolar configurations, 3D architectures, and specialized housing that accommodates the unique properties of solid components. Cell architectures may incorporate pressure management systems to maintain interfacial contact, thermal management features, and novel current collector designs. Advanced packaging solutions address challenges related to mechanical stress during cycling while maximizing volumetric energy density and facilitating efficient manufacturing and assembly.
02 Electrode design and interface engineering
Innovative electrode designs and interface engineering techniques are crucial for solid state batteries. These approaches focus on optimizing the contact between electrodes and solid electrolytes to minimize resistance and enhance ion transfer. Methods include specialized coating techniques, gradient structures, and novel manufacturing processes that create stable interfaces while accommodating volume changes during cycling, ultimately leading to improved battery performance and longevity.Expand Specific Solutions03 Manufacturing processes for solid state batteries
Advanced manufacturing techniques are being developed specifically for solid state batteries to address challenges in mass production. These include novel deposition methods, sintering processes, and assembly techniques that enable the creation of thin, uniform layers with excellent contact between components. Innovations in manufacturing aim to reduce costs, increase production efficiency, and ensure consistent quality while maintaining the performance advantages of solid state technology.Expand Specific Solutions04 Cathode and anode materials for enhanced performance
Research on electrode materials for solid state batteries focuses on developing high-capacity cathodes and anodes that are compatible with solid electrolytes. This includes lithium metal anodes, silicon-based composites, and high-voltage cathode materials that can deliver superior energy density. These materials are engineered to maintain structural stability during cycling and to form stable interfaces with solid electrolytes, addressing key challenges in solid state battery technology.Expand Specific Solutions05 Safety and thermal management systems
Solid state batteries incorporate advanced safety and thermal management systems to prevent thermal runaway and enhance operational stability. These include protective layers, temperature monitoring systems, and structural designs that minimize the risk of short circuits. The inherent safety advantages of solid electrolytes are further enhanced through engineering solutions that manage heat distribution and protect against mechanical stress, making these batteries suitable for demanding applications including electric vehicles.Expand Specific Solutions
Key Industry Players in Medical Battery Innovation
Solid State Battery technology in healthcare applications is currently in an early growth phase, with significant R&D investment but limited commercial deployment. The market is projected to expand rapidly as healthcare devices increasingly demand safer, longer-lasting power solutions. Leading players include QuantumScape, developing high-energy density batteries; Toyota and Honda, integrating solid-state technology into medical devices; and Wildcat Discovery Technologies, accelerating materials discovery. Academic-industry partnerships are forming between institutions like Harvard, Michigan State University, and Oxford with companies like IBM and Novartis. The technology is approaching commercial viability, with companies like Murata Manufacturing and Sakti3 making significant progress in miniaturization for implantable medical devices, though widespread adoption remains 3-5 years away.
Murata Manufacturing Co. Ltd.
Technical Solution: Murata has developed a specialized solid-state battery technology for healthcare applications based on their expertise in ceramic components and electronic materials. Their approach utilizes a proprietary ceramic electrolyte system that enables stable operation at body temperature while maintaining high safety standards essential for medical devices. For healthcare applications, Murata has engineered ultra-thin solid-state batteries (thickness <0.5mm) that can be integrated directly into flexible medical patches and wearable health monitors. Their manufacturing process leverages advanced ceramic processing techniques to create batteries with exceptional dimensional stability and resistance to physical deformation. Murata's solid-state batteries feature a unique layered structure that enables customization of voltage and capacity parameters to match specific medical device requirements. The company has also developed specialized battery management systems that optimize performance in variable-load healthcare applications, such as continuous glucose monitors and smart drug delivery systems, where power demands fluctuate based on monitoring and transmission schedules.
Strengths: Exceptional miniaturization capabilities ideal for wearable healthcare devices; expertise in ceramic materials translates to high-quality solid electrolytes; flexible form factors enable integration into adhesive medical patches; established manufacturing infrastructure. Weaknesses: Lower energy density compared to some competing solid-state technologies; primarily focused on external wearable applications rather than implantable devices; higher unit costs in small production volumes.
Toyota Motor Corp.
Technical Solution: Toyota has adapted its automotive solid-state battery technology for healthcare applications through its medical technology division. Their approach utilizes sulfide-based solid electrolytes that enable high ionic conductivity comparable to liquid electrolytes while eliminating safety risks associated with flammable components. For healthcare applications, Toyota has developed specialized small-format batteries with energy densities exceeding 400 Wh/kg while maintaining strict safety parameters required for medical devices. Their manufacturing process incorporates clean room production standards that meet medical-grade requirements for contaminant control. Toyota's solid-state batteries feature a proprietary interface engineering approach that minimizes resistance between electrodes and electrolyte, enabling high power delivery capabilities necessary for therapeutic devices like portable defibrillators and powered surgical tools. The batteries demonstrate exceptional thermal stability, operating safely across a temperature range of -20°C to 60°C without performance degradation, making them suitable for both storage in varied clinical environments and close contact with patients.
Strengths: Leverages Toyota's extensive experience in solid-state battery development; high energy density in compact form factors; excellent thermal stability across wide temperature ranges; manufacturing capabilities that meet medical-grade standards. Weaknesses: Relatively new entrant to healthcare-specific applications; technology primarily adapted from automotive applications rather than purpose-built for medical use; limited deployment in commercial healthcare products to date.
Critical Patents and Research in Medical Battery Technology
Monolithically integrated thin-film solid state lithium battery device having multiple layers of lithium electrochemical cells
PatentActiveUS20120058380A1
Innovation
- A method and device for fabricating a solid-state thin-film battery using a prismatic multilayer structure with specific layer thicknesses and materials, including a substrate, cathode and anode current collectors, electrolyte, and barrier layers, optimized through numerical techniques for enhanced energy density and stability.
Impregnated sintered solid state composite electrode, solid state battery, and methods of preparation
PatentInactiveUS20150056520A1
Innovation
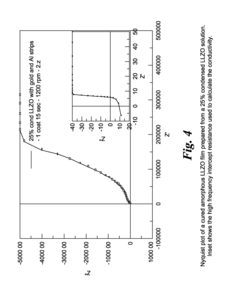
- A method involving the formation of sintered porous cathode pellets, impregnation with a liquid precursor of an inorganic amorphous ionically conductive solid electrolyte, and curing to create a composite cathode with enhanced ionic pathways, using materials like amorphous lithium lanthanum zirconium oxide (LLZO), which reduces shrinkage and improves conductivity.
Biocompatibility and Safety Considerations
The integration of solid-state batteries into healthcare applications necessitates rigorous assessment of biocompatibility and safety considerations. Unlike conventional lithium-ion batteries with liquid electrolytes that pose leakage and flammability risks, solid-state batteries utilize solid electrolytes that significantly reduce these hazards. However, their implementation in medical devices requires comprehensive evaluation against stringent biocompatibility standards, including ISO 10993 series and FDA guidelines for implantable devices.
Material selection represents a critical factor in ensuring biocompatibility. Ceramic and polymer-based solid electrolytes must undergo extensive testing for cytotoxicity, sensitization, irritation, and systemic toxicity. Recent research indicates that glass-ceramic electrolytes containing lithium phosphorus oxynitride (LiPON) demonstrate promising biocompatibility profiles when properly encapsulated, while polymer-based electrolytes utilizing polyethylene oxide (PEO) show minimal adverse tissue reactions in preliminary studies.
Encapsulation technologies play a vital role in isolating battery components from biological environments. Advanced hermetic sealing techniques using biocompatible materials such as medical-grade titanium, ceramic-to-metal seals, and specialized polymeric coatings have demonstrated effectiveness in preventing material leaching and tissue interaction. Multi-layered encapsulation approaches combining different materials have shown superior performance in long-term implantation scenarios.
Thermal management represents another crucial safety consideration. While solid-state batteries generally operate at lower temperatures than conventional batteries, localized heating during charging cycles must remain within biologically acceptable parameters (typically below 2°C above normal body temperature) to prevent tissue damage. Recent innovations in thermal regulation systems utilizing heat-dissipating materials and intelligent charging algorithms have shown promising results in maintaining safe operating temperatures.
Long-term stability and degradation pathways require thorough investigation before widespread clinical adoption. Accelerated aging studies suggest that properly engineered solid-state batteries can maintain structural integrity and performance characteristics beyond 5-7 years in simulated biological environments. However, potential degradation products and their biological interactions remain an active area of research, particularly for newer electrolyte compositions.
Regulatory pathways for solid-state battery-powered medical devices are evolving, with agencies developing specialized testing protocols that address the unique characteristics of these power sources. Manufacturers must demonstrate comprehensive safety data through in vitro, in vivo, and computational modeling approaches to satisfy regulatory requirements, with particular emphasis on failure mode analysis and long-term biocompatibility assessment.
Material selection represents a critical factor in ensuring biocompatibility. Ceramic and polymer-based solid electrolytes must undergo extensive testing for cytotoxicity, sensitization, irritation, and systemic toxicity. Recent research indicates that glass-ceramic electrolytes containing lithium phosphorus oxynitride (LiPON) demonstrate promising biocompatibility profiles when properly encapsulated, while polymer-based electrolytes utilizing polyethylene oxide (PEO) show minimal adverse tissue reactions in preliminary studies.
Encapsulation technologies play a vital role in isolating battery components from biological environments. Advanced hermetic sealing techniques using biocompatible materials such as medical-grade titanium, ceramic-to-metal seals, and specialized polymeric coatings have demonstrated effectiveness in preventing material leaching and tissue interaction. Multi-layered encapsulation approaches combining different materials have shown superior performance in long-term implantation scenarios.
Thermal management represents another crucial safety consideration. While solid-state batteries generally operate at lower temperatures than conventional batteries, localized heating during charging cycles must remain within biologically acceptable parameters (typically below 2°C above normal body temperature) to prevent tissue damage. Recent innovations in thermal regulation systems utilizing heat-dissipating materials and intelligent charging algorithms have shown promising results in maintaining safe operating temperatures.
Long-term stability and degradation pathways require thorough investigation before widespread clinical adoption. Accelerated aging studies suggest that properly engineered solid-state batteries can maintain structural integrity and performance characteristics beyond 5-7 years in simulated biological environments. However, potential degradation products and their biological interactions remain an active area of research, particularly for newer electrolyte compositions.
Regulatory pathways for solid-state battery-powered medical devices are evolving, with agencies developing specialized testing protocols that address the unique characteristics of these power sources. Manufacturers must demonstrate comprehensive safety data through in vitro, in vivo, and computational modeling approaches to satisfy regulatory requirements, with particular emphasis on failure mode analysis and long-term biocompatibility assessment.
Regulatory Framework for Medical Device Batteries
The regulatory landscape for medical device batteries, particularly solid-state batteries in healthcare applications, is complex and multifaceted. In the United States, the FDA regulates medical device batteries under the Medical Device Regulations (21 CFR Part 820), with specific requirements for safety, performance, and risk management. Solid-state batteries must comply with these regulations, which include extensive testing for biocompatibility, electrical safety, and thermal stability.
In Europe, the Medical Device Regulation (MDR 2017/745) and the In Vitro Diagnostic Regulation (IVDR 2017/746) govern medical devices, including their power sources. These regulations emphasize risk classification, post-market surveillance, and technical documentation. Solid-state batteries integrated into medical devices must meet these stringent requirements, with particular attention to safety and performance characteristics.
International standards play a crucial role in regulatory compliance. IEC 60601-1 addresses electrical safety requirements for medical equipment, while ISO 13485 focuses on quality management systems. For implantable devices, ISO 14708 provides specific guidelines on power sources, including batteries. These standards are continuously evolving to accommodate emerging technologies like solid-state batteries.
The regulatory pathway for solid-state batteries in healthcare applications typically involves premarket approval or clearance processes. In the US, this may include 510(k) clearance or Premarket Approval (PMA), depending on the device classification. The regulatory burden increases with the risk level of the device, with implantable devices facing the most rigorous scrutiny.
Battery-specific regulations address unique concerns such as thermal runaway prevention, leakage protection, and end-of-life management. For solid-state batteries, regulators are developing new frameworks that consider their distinct characteristics, including higher energy density and reduced fire risk compared to traditional lithium-ion batteries.
Environmental regulations also impact medical device batteries. The EU's Restriction of Hazardous Substances (RoHS) Directive and Battery Directive impose restrictions on certain materials and mandate recycling programs. Similar regulations exist globally, requiring manufacturers to consider the entire lifecycle of their battery technologies.
Regulatory harmonization efforts, such as the Medical Device Single Audit Program (MDSAP), aim to streamline compliance across multiple jurisdictions. However, significant regional variations persist, creating challenges for global market access of solid-state battery-powered medical devices.
In Europe, the Medical Device Regulation (MDR 2017/745) and the In Vitro Diagnostic Regulation (IVDR 2017/746) govern medical devices, including their power sources. These regulations emphasize risk classification, post-market surveillance, and technical documentation. Solid-state batteries integrated into medical devices must meet these stringent requirements, with particular attention to safety and performance characteristics.
International standards play a crucial role in regulatory compliance. IEC 60601-1 addresses electrical safety requirements for medical equipment, while ISO 13485 focuses on quality management systems. For implantable devices, ISO 14708 provides specific guidelines on power sources, including batteries. These standards are continuously evolving to accommodate emerging technologies like solid-state batteries.
The regulatory pathway for solid-state batteries in healthcare applications typically involves premarket approval or clearance processes. In the US, this may include 510(k) clearance or Premarket Approval (PMA), depending on the device classification. The regulatory burden increases with the risk level of the device, with implantable devices facing the most rigorous scrutiny.
Battery-specific regulations address unique concerns such as thermal runaway prevention, leakage protection, and end-of-life management. For solid-state batteries, regulators are developing new frameworks that consider their distinct characteristics, including higher energy density and reduced fire risk compared to traditional lithium-ion batteries.
Environmental regulations also impact medical device batteries. The EU's Restriction of Hazardous Substances (RoHS) Directive and Battery Directive impose restrictions on certain materials and mandate recycling programs. Similar regulations exist globally, requiring manufacturers to consider the entire lifecycle of their battery technologies.
Regulatory harmonization efforts, such as the Medical Device Single Audit Program (MDSAP), aim to streamline compliance across multiple jurisdictions. However, significant regional variations persist, creating challenges for global market access of solid-state battery-powered medical devices.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!