Why Solid State Battery Breakthrough Innovates the Pharmaceutical Industry?
OCT 24, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Solid State Battery Evolution and Pharmaceutical Applications
The evolution of solid-state batteries represents a significant technological leap from traditional lithium-ion batteries, characterized by the replacement of liquid electrolytes with solid materials. This transformation began in the early 2000s with rudimentary designs and has accelerated dramatically in the past decade. The trajectory has moved from laboratory prototypes with limited capacity to increasingly sophisticated systems offering enhanced energy density, safety, and longevity.
Solid-state battery technology has evolved through several distinct phases. Initially, researchers focused on overcoming fundamental materials science challenges, particularly the ionic conductivity limitations of solid electrolytes. By 2015, breakthroughs in ceramic and polymer-based electrolytes enabled the first viable prototypes. The period from 2018 to 2022 saw significant advancements in manufacturing scalability and interface engineering, reducing production costs while improving performance metrics.
The pharmaceutical industry stands to benefit substantially from these developments. Traditional pharmaceutical manufacturing and research facilities rely heavily on uninterrupted power supplies and precise temperature control systems. Solid-state batteries offer unprecedented stability for critical laboratory equipment and sensitive pharmaceutical processes, minimizing the risk of power fluctuations that could compromise research integrity or production quality.
Beyond power stability, solid-state batteries enable the miniaturization of medical devices used in pharmaceutical research and patient care. Their higher energy density supports the development of implantable drug delivery systems with extended operational lifespans, facilitating long-term studies and treatments that were previously impractical. This capability transforms how pharmaceutical companies approach drug efficacy testing and chronic condition management.
The enhanced safety profile of solid-state batteries—particularly their non-flammability and resistance to thermal runaway—makes them ideal for pharmaceutical environments where chemical safety is paramount. This characteristic enables the integration of power sources directly into sensitive testing equipment without risk of contamination or adverse reactions with pharmaceutical compounds.
Most recently, the development of flexible solid-state batteries has opened new frontiers in wearable health monitoring devices. These technologies allow pharmaceutical companies to collect unprecedented volumes of real-world patient data during clinical trials, enhancing the quality of drug development research and enabling more personalized medication regimens based on continuous physiological monitoring.
As manufacturing scales and costs decrease, we anticipate solid-state batteries will become ubiquitous throughout pharmaceutical infrastructure by 2030, fundamentally altering how medications are developed, tested, and delivered to patients.
Solid-state battery technology has evolved through several distinct phases. Initially, researchers focused on overcoming fundamental materials science challenges, particularly the ionic conductivity limitations of solid electrolytes. By 2015, breakthroughs in ceramic and polymer-based electrolytes enabled the first viable prototypes. The period from 2018 to 2022 saw significant advancements in manufacturing scalability and interface engineering, reducing production costs while improving performance metrics.
The pharmaceutical industry stands to benefit substantially from these developments. Traditional pharmaceutical manufacturing and research facilities rely heavily on uninterrupted power supplies and precise temperature control systems. Solid-state batteries offer unprecedented stability for critical laboratory equipment and sensitive pharmaceutical processes, minimizing the risk of power fluctuations that could compromise research integrity or production quality.
Beyond power stability, solid-state batteries enable the miniaturization of medical devices used in pharmaceutical research and patient care. Their higher energy density supports the development of implantable drug delivery systems with extended operational lifespans, facilitating long-term studies and treatments that were previously impractical. This capability transforms how pharmaceutical companies approach drug efficacy testing and chronic condition management.
The enhanced safety profile of solid-state batteries—particularly their non-flammability and resistance to thermal runaway—makes them ideal for pharmaceutical environments where chemical safety is paramount. This characteristic enables the integration of power sources directly into sensitive testing equipment without risk of contamination or adverse reactions with pharmaceutical compounds.
Most recently, the development of flexible solid-state batteries has opened new frontiers in wearable health monitoring devices. These technologies allow pharmaceutical companies to collect unprecedented volumes of real-world patient data during clinical trials, enhancing the quality of drug development research and enabling more personalized medication regimens based on continuous physiological monitoring.
As manufacturing scales and costs decrease, we anticipate solid-state batteries will become ubiquitous throughout pharmaceutical infrastructure by 2030, fundamentally altering how medications are developed, tested, and delivered to patients.
Market Demand Analysis for Advanced Battery Technologies in Healthcare
The healthcare sector is witnessing a significant transformation in its operational infrastructure, with advanced battery technologies emerging as critical components. The market for these technologies, particularly solid-state batteries, is experiencing robust growth driven by several key factors. Current market analysis indicates that the global medical battery market is expanding at a compound annual growth rate exceeding 8%, with solid-state technologies positioned to capture an increasing share of this growth.
Healthcare facilities worldwide are facing mounting pressure to enhance operational efficiency while reducing environmental impact. This has created substantial demand for energy storage solutions that offer longer life cycles, improved safety profiles, and reduced maintenance requirements. Solid-state batteries address these needs directly, presenting a compelling value proposition for healthcare providers seeking to modernize their infrastructure and reduce long-term operational costs.
Pharmaceutical manufacturing and research facilities represent a particularly promising market segment. These environments require uninterrupted power supply for sensitive equipment and processes, with even momentary disruptions potentially resulting in significant financial losses. The enhanced stability and reliability of solid-state batteries make them ideally suited for these applications, driving adoption across the pharmaceutical value chain.
Portable and implantable medical devices constitute another high-growth market segment. The aging global population and increasing prevalence of chronic conditions have accelerated demand for wearable health monitoring systems and implantable therapeutic devices. These applications require batteries that combine high energy density with absolute safety assurances – precisely the characteristics that solid-state technology delivers.
Market research indicates that healthcare providers are increasingly prioritizing sustainability in their procurement decisions. Traditional battery technologies present environmental challenges throughout their lifecycle, from resource extraction to disposal. Solid-state alternatives offer reduced environmental impact through longer service life, fewer replacement cycles, and potentially less hazardous materials, aligning with the sector's growing sustainability commitments.
Regional market analysis reveals varying adoption rates, with North America and Europe leading in implementation of advanced battery technologies in healthcare settings. However, the Asia-Pacific region is projected to demonstrate the highest growth rate over the next five years, driven by rapid healthcare infrastructure development and substantial investments in pharmaceutical manufacturing capacity.
Customer feedback from early adopters highlights several key value drivers, including reduced downtime, decreased maintenance requirements, and enhanced safety profiles. These benefits translate directly to operational cost savings and risk reduction, strengthening the business case for wider adoption across the healthcare ecosystem.
Healthcare facilities worldwide are facing mounting pressure to enhance operational efficiency while reducing environmental impact. This has created substantial demand for energy storage solutions that offer longer life cycles, improved safety profiles, and reduced maintenance requirements. Solid-state batteries address these needs directly, presenting a compelling value proposition for healthcare providers seeking to modernize their infrastructure and reduce long-term operational costs.
Pharmaceutical manufacturing and research facilities represent a particularly promising market segment. These environments require uninterrupted power supply for sensitive equipment and processes, with even momentary disruptions potentially resulting in significant financial losses. The enhanced stability and reliability of solid-state batteries make them ideally suited for these applications, driving adoption across the pharmaceutical value chain.
Portable and implantable medical devices constitute another high-growth market segment. The aging global population and increasing prevalence of chronic conditions have accelerated demand for wearable health monitoring systems and implantable therapeutic devices. These applications require batteries that combine high energy density with absolute safety assurances – precisely the characteristics that solid-state technology delivers.
Market research indicates that healthcare providers are increasingly prioritizing sustainability in their procurement decisions. Traditional battery technologies present environmental challenges throughout their lifecycle, from resource extraction to disposal. Solid-state alternatives offer reduced environmental impact through longer service life, fewer replacement cycles, and potentially less hazardous materials, aligning with the sector's growing sustainability commitments.
Regional market analysis reveals varying adoption rates, with North America and Europe leading in implementation of advanced battery technologies in healthcare settings. However, the Asia-Pacific region is projected to demonstrate the highest growth rate over the next five years, driven by rapid healthcare infrastructure development and substantial investments in pharmaceutical manufacturing capacity.
Customer feedback from early adopters highlights several key value drivers, including reduced downtime, decreased maintenance requirements, and enhanced safety profiles. These benefits translate directly to operational cost savings and risk reduction, strengthening the business case for wider adoption across the healthcare ecosystem.
Technical Challenges and Current Limitations in Pharmaceutical Energy Storage
The pharmaceutical industry faces significant challenges in energy storage systems that directly impact product quality, operational efficiency, and environmental sustainability. Current energy storage solutions predominantly rely on traditional lithium-ion batteries which present several critical limitations in pharmaceutical applications. These batteries suffer from thermal instability issues, posing potential fire hazards in sensitive manufacturing environments where flammable materials are often present. This safety concern necessitates extensive protective measures that increase operational costs and facility complexity.
Energy density constraints represent another major limitation, as pharmaceutical equipment often requires compact yet powerful energy sources for portable diagnostic tools, temperature-controlled supply chains, and automated manufacturing systems. Traditional batteries fail to deliver sufficient power longevity within the space constraints of modern pharmaceutical equipment, creating bottlenecks in operational efficiency.
Pharmaceutical manufacturing demands precise temperature control, yet conventional batteries experience significant performance degradation in extreme temperature environments. This limitation particularly affects cold chain logistics crucial for vaccine and biological product distribution, where temperature excursions can compromise product integrity and efficacy.
The industry also struggles with battery lifespan issues, as frequent charging cycles in continuous manufacturing operations lead to accelerated degradation. This results in higher replacement costs and increased downtime for critical equipment. Additionally, the chemical composition of conventional batteries includes toxic materials that pose contamination risks in sterile pharmaceutical environments, necessitating stringent isolation protocols.
Regulatory compliance presents another significant hurdle, as energy storage systems in pharmaceutical applications must meet rigorous standards from multiple regulatory bodies. Current battery technologies often require extensive validation processes to ensure they meet these complex requirements, extending development timelines and increasing costs.
From a sustainability perspective, traditional batteries contain environmentally harmful materials and their manufacturing processes generate substantial carbon footprints. This conflicts with the pharmaceutical industry's increasing focus on sustainable operations and corporate environmental responsibility commitments.
The integration challenges between existing battery management systems and pharmaceutical equipment control systems create additional technical barriers. These interoperability issues often result in suboptimal performance, data collection limitations, and increased system complexity that hampers innovation in automated pharmaceutical processes.
These multifaceted limitations collectively create a significant technology gap that impedes advancement in pharmaceutical manufacturing, research, and distribution systems, highlighting the urgent need for innovative energy storage solutions specifically designed for pharmaceutical applications.
Energy density constraints represent another major limitation, as pharmaceutical equipment often requires compact yet powerful energy sources for portable diagnostic tools, temperature-controlled supply chains, and automated manufacturing systems. Traditional batteries fail to deliver sufficient power longevity within the space constraints of modern pharmaceutical equipment, creating bottlenecks in operational efficiency.
Pharmaceutical manufacturing demands precise temperature control, yet conventional batteries experience significant performance degradation in extreme temperature environments. This limitation particularly affects cold chain logistics crucial for vaccine and biological product distribution, where temperature excursions can compromise product integrity and efficacy.
The industry also struggles with battery lifespan issues, as frequent charging cycles in continuous manufacturing operations lead to accelerated degradation. This results in higher replacement costs and increased downtime for critical equipment. Additionally, the chemical composition of conventional batteries includes toxic materials that pose contamination risks in sterile pharmaceutical environments, necessitating stringent isolation protocols.
Regulatory compliance presents another significant hurdle, as energy storage systems in pharmaceutical applications must meet rigorous standards from multiple regulatory bodies. Current battery technologies often require extensive validation processes to ensure they meet these complex requirements, extending development timelines and increasing costs.
From a sustainability perspective, traditional batteries contain environmentally harmful materials and their manufacturing processes generate substantial carbon footprints. This conflicts with the pharmaceutical industry's increasing focus on sustainable operations and corporate environmental responsibility commitments.
The integration challenges between existing battery management systems and pharmaceutical equipment control systems create additional technical barriers. These interoperability issues often result in suboptimal performance, data collection limitations, and increased system complexity that hampers innovation in automated pharmaceutical processes.
These multifaceted limitations collectively create a significant technology gap that impedes advancement in pharmaceutical manufacturing, research, and distribution systems, highlighting the urgent need for innovative energy storage solutions specifically designed for pharmaceutical applications.
Current Implementation Solutions for Energy Storage in Pharmaceutical Devices
01 Electrolyte materials for solid state batteries
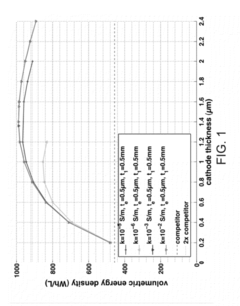
Various electrolyte materials are used in solid state batteries to improve ionic conductivity and battery performance. These include solid polymer electrolytes, ceramic electrolytes, and composite electrolytes that combine different materials to achieve optimal properties. Advanced electrolyte formulations help overcome challenges related to interfacial resistance and enable faster ion transport between electrodes, resulting in higher energy density and improved safety characteristics.- Solid-state electrolyte compositions: Solid-state batteries utilize specialized electrolyte compositions that enable ion transport without liquid components. These electrolytes typically include ceramic materials, polymer matrices, or composite structures that provide high ionic conductivity while maintaining mechanical stability. Advanced formulations may incorporate sulfide-based, oxide-based, or phosphate-based materials that enhance lithium-ion transport while preventing dendrite formation. These solid electrolytes are crucial for improving battery safety and energy density compared to conventional liquid electrolyte systems.
- Interface engineering for solid-state batteries: Interface engineering focuses on optimizing the contact between solid electrolytes and electrodes to reduce resistance and improve ion transfer. This involves developing specialized coatings, buffer layers, or gradient structures that mitigate interfacial impedance issues. Techniques include surface modification of active materials, incorporation of interfacial stabilizers, and creation of engineered interphases that accommodate volume changes during cycling. These approaches are essential for addressing one of the primary challenges in solid-state battery technology: maintaining stable and efficient interfaces throughout battery operation.
- Manufacturing methods for solid-state batteries: Advanced manufacturing techniques are being developed to produce solid-state batteries at scale. These include dry processing methods, cold sintering, tape casting, and various deposition techniques that enable the creation of thin, uniform layers with good interfacial contact. Novel approaches focus on reducing processing temperatures, improving layer adhesion, and developing scalable assembly processes that maintain the integrity of sensitive components. Manufacturing innovations also address challenges related to pressure application during assembly and the creation of defect-free interfaces between battery components.
- Electrode designs for solid-state batteries: Specialized electrode designs are critical for solid-state battery performance. These include structured cathodes and anodes that maximize active material utilization while maintaining good contact with the solid electrolyte. Innovations focus on composite electrodes that blend active materials with solid electrolytes, conductive additives, and binders to create mechanically robust structures with efficient ion and electron transport pathways. Advanced designs may incorporate three-dimensional architectures, gradient compositions, or hierarchical structures that optimize energy density while accommodating the unique challenges of all-solid-state configurations.
- Safety and performance enhancements: Solid-state batteries incorporate various features to enhance safety and performance metrics. These include thermal management systems, pressure regulation mechanisms, and protective structures that prevent short circuits and mechanical failures. Advanced designs focus on improving cycle life through stress-relieving architectures, self-healing components, and degradation-resistant materials. Additional innovations address fast charging capabilities, low-temperature performance, and methods to prevent capacity fade over extended cycling. These enhancements collectively address the key advantages that solid-state technology offers over conventional lithium-ion batteries.
02 Electrode design and interface engineering
Innovative electrode designs and interface engineering techniques are crucial for solid state batteries. These approaches focus on optimizing the contact between solid electrolytes and electrode materials to reduce resistance and improve charge transfer. Methods include surface coating of active materials, creation of gradient interfaces, and development of composite electrodes that maintain good mechanical contact during cycling, thereby enhancing overall battery performance and cycle life.Expand Specific Solutions03 Manufacturing processes for solid state batteries
Advanced manufacturing techniques are being developed specifically for solid state batteries to address challenges in production scaling and cost reduction. These include novel deposition methods for thin film electrolytes, pressure-assisted sintering processes, and roll-to-roll fabrication techniques. Innovations in manufacturing aim to create defect-free interfaces between battery components while enabling mass production capabilities that can compete with conventional liquid electrolyte batteries.Expand Specific Solutions04 Thermal management and safety features
Solid state batteries incorporate advanced thermal management systems and safety features to prevent thermal runaway and enhance operational stability. These designs include heat dissipation structures, temperature monitoring systems, and inherently safer materials that resist combustion. The absence of flammable liquid electrolytes significantly improves safety, while specialized battery management systems help maintain optimal operating temperatures across a wider range of environmental conditions.Expand Specific Solutions05 Integration of solid state batteries in applications
Solid state batteries are being designed for integration into various applications including electric vehicles, portable electronics, and grid storage systems. These designs focus on form factor flexibility, scalability, and compatibility with existing systems. Innovations include modular battery packs, specialized cell configurations for specific applications, and integration with power management systems that can take advantage of the unique characteristics of solid state technology to deliver improved performance in real-world use cases.Expand Specific Solutions
Key Industry Players in Solid State Battery and Pharmaceutical Sectors
The solid-state battery market is currently in an early growth phase, characterized by significant R&D investment but limited commercial deployment. With a projected market size exceeding $6 billion by 2030, this technology represents a transformative opportunity for pharmaceutical applications through improved medical devices and drug delivery systems. Companies like Wildcat Discovery Technologies and PolyPlus Battery are pioneering materials innovation, while established players including Toyota, Honda, and Medtronic are developing application-specific solutions. Academic-industry partnerships involving institutions like Michigan State University and University of California are accelerating technological maturity. The competitive landscape features both specialized battery developers (Sila Nanotechnologies, Solid Power) and diversified corporations seeking to integrate this technology into pharmaceutical delivery systems and implantable medical devices.
Wildcat Discovery Technologies, Inc.
Technical Solution: Wildcat Discovery Technologies has developed a high-throughput experimentation platform specifically for accelerating solid-state battery materials discovery, with direct applications to pharmaceutical delivery systems. Their technology combines robotic synthesis and testing capabilities with AI-driven materials prediction to rapidly identify and optimize novel solid electrolytes and electrode materials. This approach has led to the discovery of proprietary solid electrolyte compositions with ionic conductivities exceeding 10 mS/cm at room temperature, comparable to liquid electrolytes but without safety risks. For pharmaceutical applications, Wildcat has focused on developing solid-state batteries that maintain stable performance in biological environments and can be miniaturized for implantable drug delivery systems. Their materials discovery platform has identified solid electrolytes that are both biocompatible and stable in contact with bodily fluids, addressing a critical challenge for implantable medical devices. The company's solid-state batteries feature extended cycle life and calendar life, essential for long-term pharmaceutical delivery applications where device replacement is invasive and costly.
Strengths: High-throughput discovery platform enables rapid iteration and optimization of materials specifically for pharmaceutical applications. Focus on biocompatible materials addresses regulatory requirements for medical applications. Weaknesses: As primarily a materials discovery company, full battery integration and manufacturing may require partnerships. Some of their most promising materials are still in development phases rather than full commercial deployment.
Sakti3, Inc.
Technical Solution: Sakti3 has developed a proprietary solid-state battery technology based on thin-film deposition methods that creates batteries with energy densities approaching 1000 Wh/L, nearly double that of conventional lithium-ion batteries. Their approach uses vacuum deposition techniques to create ultra-thin layers of solid electrolyte and electrode materials, eliminating liquid components entirely. This technology has significant implications for pharmaceutical applications, particularly in the realm of miniaturized medical devices and advanced drug delivery systems. The solid-state nature of these batteries eliminates leakage risks, while their high energy density enables smaller form factors for implantable drug delivery devices. Sakti3's manufacturing process also allows for custom form factors, enabling batteries to be designed around pharmaceutical delivery requirements rather than forcing device designs to accommodate standard battery shapes. The company's batteries demonstrate excellent performance at body temperature, making them particularly suitable for wearable and implantable pharmaceutical delivery systems.
Strengths: Extremely high energy density enables miniaturization of pharmaceutical delivery devices. Manufacturing process allows for customized form factors to meet specific medical device requirements. Weaknesses: Vacuum deposition manufacturing techniques may face scaling challenges for mass production. Higher initial costs compared to conventional battery technologies may limit adoption in cost-sensitive applications.
Core Patents and Research in Solid State Battery for Medical Applications
Monolithically integrated thin-film solid state lithium battery device having multiple layers of lithium electrochemical cells
PatentActiveUS20120058380A1
Innovation
- A method and device for fabricating a solid-state thin-film battery using a prismatic multilayer structure with specific layer thicknesses and materials, including a substrate, cathode and anode current collectors, electrolyte, and barrier layers, optimized through numerical techniques for enhanced energy density and stability.
Impregnated sintered solid state composite electrode, solid state battery, and methods of preparation
PatentInactiveUS20150056520A1
Innovation
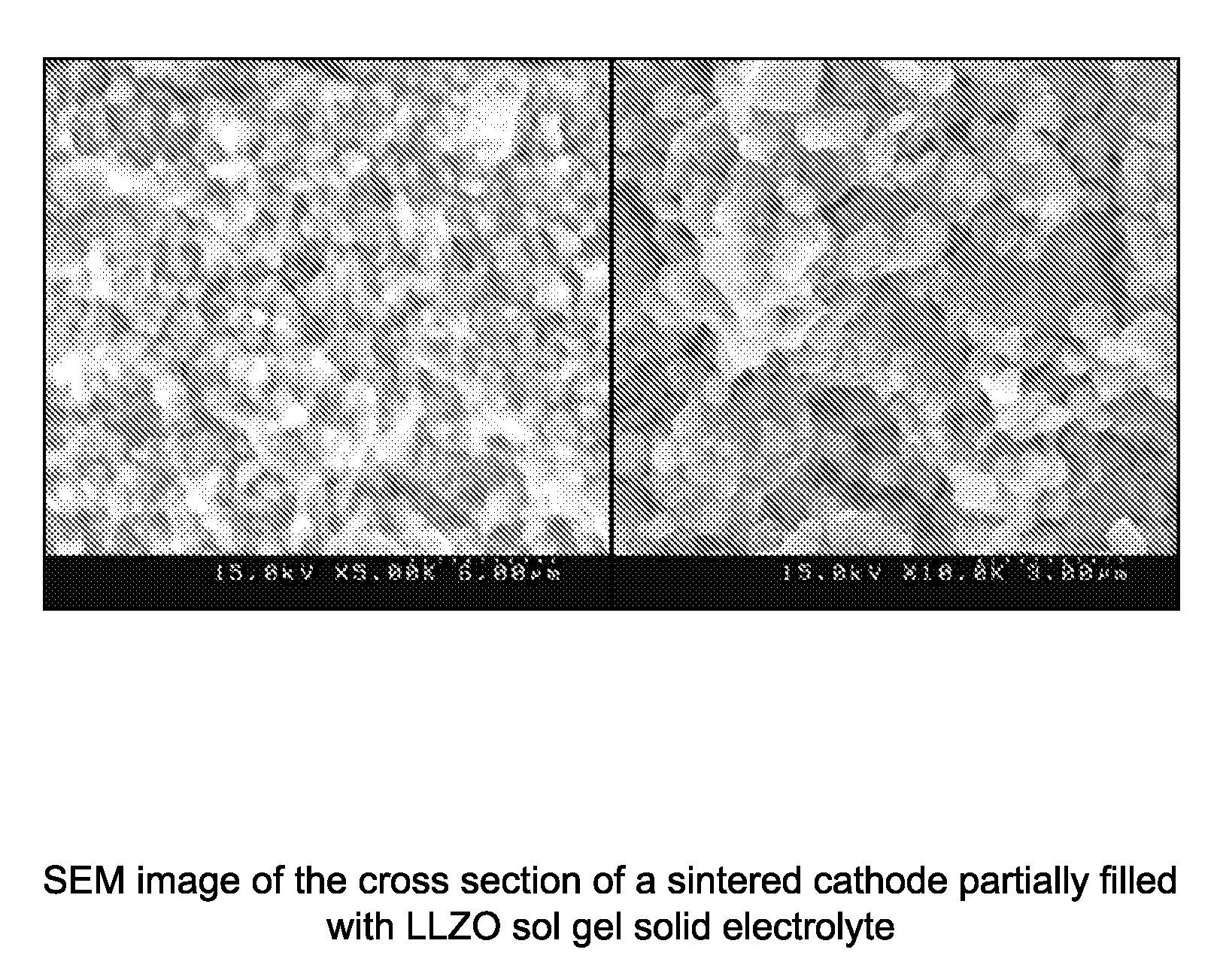
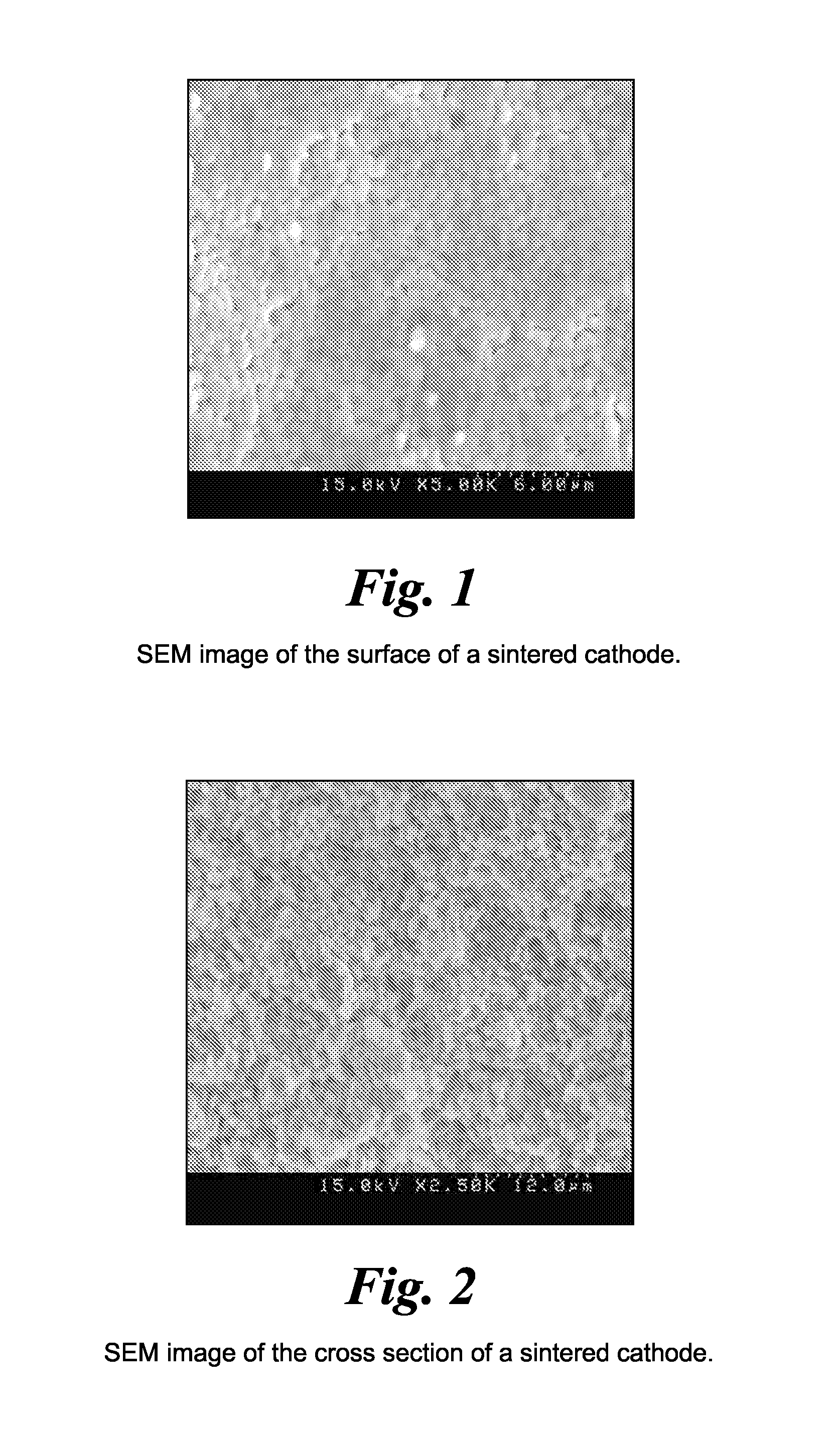
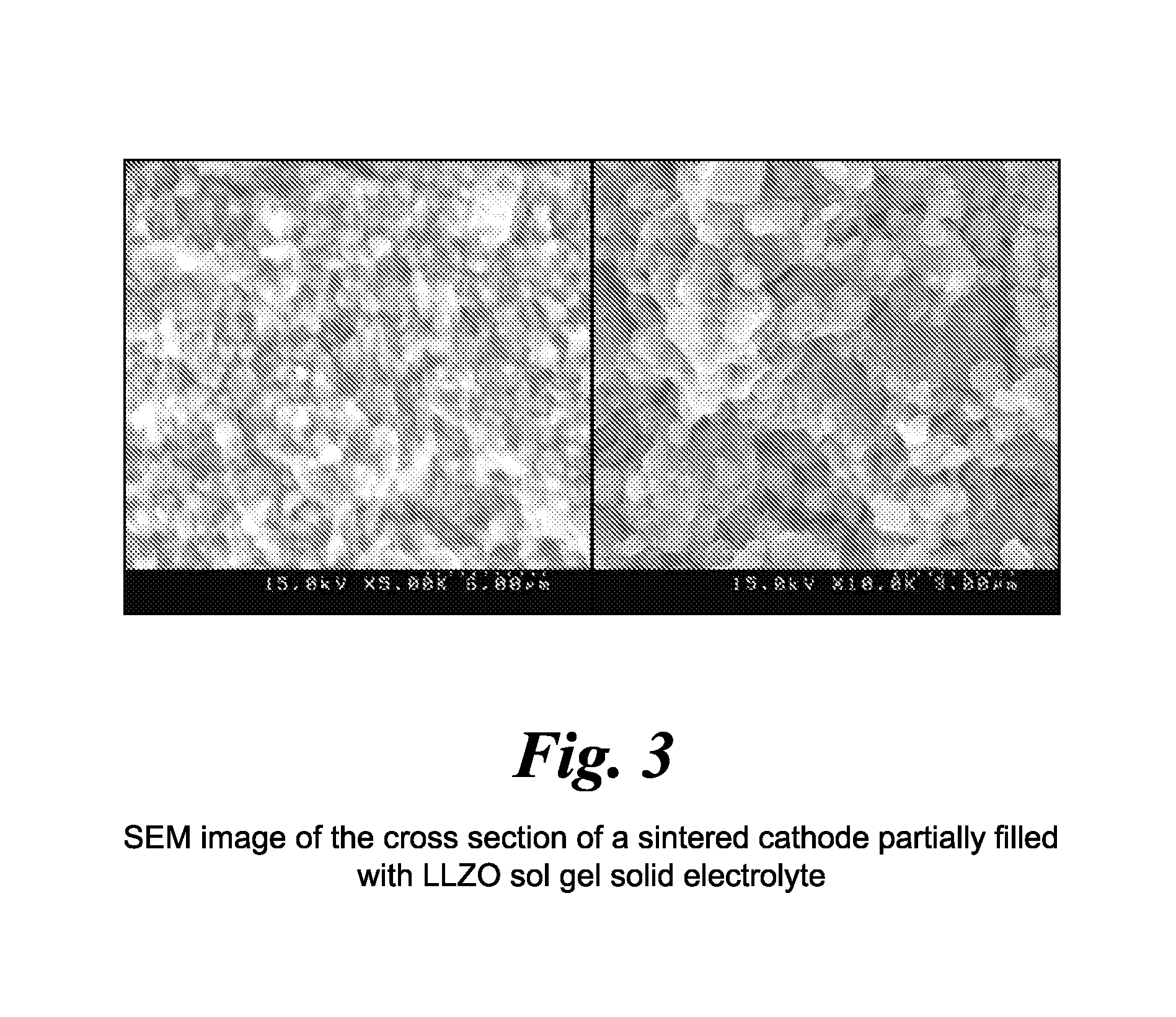
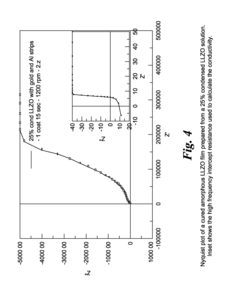
- A method involving the formation of sintered porous cathode pellets, impregnation with a liquid precursor of an inorganic amorphous ionically conductive solid electrolyte, and curing to create a composite cathode with enhanced ionic pathways, using materials like amorphous lithium lanthanum zirconium oxide (LLZO), which reduces shrinkage and improves conductivity.
Regulatory Framework for Medical-Grade Battery Technologies
The regulatory landscape for medical-grade battery technologies represents a complex framework that significantly impacts the adoption of solid-state batteries in pharmaceutical applications. The FDA's Center for Devices and Radiological Health (CDRH) maintains stringent requirements for battery-powered medical devices, with particular emphasis on safety, reliability, and biocompatibility when these technologies interface with human tissues.
Medical-grade batteries must comply with ISO 13485 standards for quality management systems and IEC 60601 for electrical medical equipment safety. For implantable devices powered by solid-state batteries, the regulatory bar rises substantially, requiring extensive pre-clinical and clinical testing under IDE (Investigational Device Exemption) protocols before market approval consideration.
The European Union's Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) impose additional requirements for battery-powered medical technologies, including comprehensive technical documentation and post-market surveillance systems. These regulations specifically address battery longevity, thermal management, and failure mode analysis—areas where solid-state batteries demonstrate significant advantages over traditional lithium-ion technologies.
Regulatory bodies increasingly recognize the potential benefits of solid-state batteries for pharmaceutical applications, particularly their enhanced safety profile with non-flammable electrolytes. This has led to the development of expedited review pathways for breakthrough technologies that demonstrate substantial improvements in patient safety. The FDA's Breakthrough Devices Program and the EU's Innovation Pathway provide mechanisms for accelerated assessment of novel battery technologies that address unmet medical needs.
Environmental regulations also influence the medical battery landscape. The EU's Restriction of Hazardous Substances (RoHS) directive and Battery Directive establish strict guidelines for material composition and end-of-life management. Solid-state batteries, with their reduced reliance on toxic materials and improved recyclability, align well with these sustainability-focused regulatory frameworks.
International harmonization efforts through the International Medical Device Regulators Forum (IMDRF) are working to standardize requirements for emerging battery technologies across global markets. This initiative aims to reduce regulatory barriers while maintaining rigorous safety standards, potentially accelerating the adoption of solid-state batteries in pharmaceutical applications worldwide.
Regulatory compliance costs remain a significant consideration for manufacturers. The extensive testing and documentation required for medical-grade batteries can extend development timelines by 2-3 years compared to consumer applications, with associated costs often exceeding $5-10 million for complex implantable systems.
Medical-grade batteries must comply with ISO 13485 standards for quality management systems and IEC 60601 for electrical medical equipment safety. For implantable devices powered by solid-state batteries, the regulatory bar rises substantially, requiring extensive pre-clinical and clinical testing under IDE (Investigational Device Exemption) protocols before market approval consideration.
The European Union's Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) impose additional requirements for battery-powered medical technologies, including comprehensive technical documentation and post-market surveillance systems. These regulations specifically address battery longevity, thermal management, and failure mode analysis—areas where solid-state batteries demonstrate significant advantages over traditional lithium-ion technologies.
Regulatory bodies increasingly recognize the potential benefits of solid-state batteries for pharmaceutical applications, particularly their enhanced safety profile with non-flammable electrolytes. This has led to the development of expedited review pathways for breakthrough technologies that demonstrate substantial improvements in patient safety. The FDA's Breakthrough Devices Program and the EU's Innovation Pathway provide mechanisms for accelerated assessment of novel battery technologies that address unmet medical needs.
Environmental regulations also influence the medical battery landscape. The EU's Restriction of Hazardous Substances (RoHS) directive and Battery Directive establish strict guidelines for material composition and end-of-life management. Solid-state batteries, with their reduced reliance on toxic materials and improved recyclability, align well with these sustainability-focused regulatory frameworks.
International harmonization efforts through the International Medical Device Regulators Forum (IMDRF) are working to standardize requirements for emerging battery technologies across global markets. This initiative aims to reduce regulatory barriers while maintaining rigorous safety standards, potentially accelerating the adoption of solid-state batteries in pharmaceutical applications worldwide.
Regulatory compliance costs remain a significant consideration for manufacturers. The extensive testing and documentation required for medical-grade batteries can extend development timelines by 2-3 years compared to consumer applications, with associated costs often exceeding $5-10 million for complex implantable systems.
Environmental Impact and Sustainability of Solid State Batteries in Healthcare
The integration of solid state battery technology into healthcare systems represents a significant advancement in environmental sustainability. Traditional lithium-ion batteries used in medical devices contain liquid electrolytes that pose environmental hazards when improperly disposed of, potentially leaching toxic chemicals into soil and water systems. Solid state batteries eliminate these liquid components, substantially reducing the risk of environmental contamination and offering a more ecologically responsible alternative for medical equipment.
Healthcare facilities generate considerable electronic waste, with battery disposal representing a significant environmental challenge. Solid state batteries demonstrate superior longevity, with some prototypes showing 2-3 times the lifespan of conventional batteries. This extended operational life directly translates to reduced waste generation in medical settings, decreasing the environmental footprint of healthcare operations while simultaneously reducing disposal costs.
The manufacturing process for solid state batteries also presents environmental advantages. Production typically requires fewer toxic materials and solvents compared to conventional battery manufacturing. Recent advancements in solid electrolyte production have reduced energy requirements by approximately 30%, according to research from the National Renewable Energy Laboratory, further diminishing the carbon footprint associated with battery production for medical applications.
Pharmaceutical cold chain management represents another area where solid state batteries offer substantial sustainability benefits. Temperature-controlled pharmaceutical storage and transportation systems currently rely heavily on energy-intensive cooling mechanisms. Solid state batteries provide more efficient and reliable power sources for these systems, reducing energy consumption by an estimated 25% while improving temperature stability for sensitive medications.
The recyclability of solid state batteries further enhances their environmental profile in healthcare applications. Their simplified composition, lacking liquid components and utilizing more stable materials, facilitates more efficient recycling processes. Early studies indicate recovery rates of up to 90% for key materials like lithium and cobalt, significantly higher than the 50-60% typically achieved with conventional batteries used in medical devices.
As healthcare systems worldwide increasingly prioritize sustainability initiatives, solid state battery technology aligns perfectly with environmental stewardship goals. The reduced toxicity, extended lifespan, improved manufacturing efficiency, and enhanced recyclability collectively position solid state batteries as an environmentally responsible power solution for next-generation medical devices and pharmaceutical management systems.
Healthcare facilities generate considerable electronic waste, with battery disposal representing a significant environmental challenge. Solid state batteries demonstrate superior longevity, with some prototypes showing 2-3 times the lifespan of conventional batteries. This extended operational life directly translates to reduced waste generation in medical settings, decreasing the environmental footprint of healthcare operations while simultaneously reducing disposal costs.
The manufacturing process for solid state batteries also presents environmental advantages. Production typically requires fewer toxic materials and solvents compared to conventional battery manufacturing. Recent advancements in solid electrolyte production have reduced energy requirements by approximately 30%, according to research from the National Renewable Energy Laboratory, further diminishing the carbon footprint associated with battery production for medical applications.
Pharmaceutical cold chain management represents another area where solid state batteries offer substantial sustainability benefits. Temperature-controlled pharmaceutical storage and transportation systems currently rely heavily on energy-intensive cooling mechanisms. Solid state batteries provide more efficient and reliable power sources for these systems, reducing energy consumption by an estimated 25% while improving temperature stability for sensitive medications.
The recyclability of solid state batteries further enhances their environmental profile in healthcare applications. Their simplified composition, lacking liquid components and utilizing more stable materials, facilitates more efficient recycling processes. Early studies indicate recovery rates of up to 90% for key materials like lithium and cobalt, significantly higher than the 50-60% typically achieved with conventional batteries used in medical devices.
As healthcare systems worldwide increasingly prioritize sustainability initiatives, solid state battery technology aligns perfectly with environmental stewardship goals. The reduced toxicity, extended lifespan, improved manufacturing efficiency, and enhanced recyclability collectively position solid state batteries as an environmentally responsible power solution for next-generation medical devices and pharmaceutical management systems.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!