The Role of Electrochemical Doping in Battery Thermal Runaway Resistance
SEP 23, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Electrochemical Doping Background and Objectives
Electrochemical doping represents a significant frontier in battery technology development, emerging from decades of research in materials science and electrochemistry. This technique involves the deliberate introduction of specific chemical elements or compounds into battery electrode materials to modify their electronic, structural, and thermal properties. The evolution of this technology can be traced back to the early semiconductor industry, where doping was first utilized to alter conductivity properties of materials. In the context of batteries, electrochemical doping has evolved from basic concepts in the 1990s to sophisticated implementation strategies in the 2020s.
The technological trajectory has been marked by several pivotal advancements, including the discovery of intercalation mechanisms in lithium-ion batteries, the development of novel dopant materials, and the refinement of precise doping methodologies. Recent research has increasingly focused on leveraging electrochemical doping specifically to enhance thermal stability and safety characteristics of battery systems, addressing one of the most critical challenges in modern energy storage.
Current technological trends indicate a growing emphasis on nanoscale doping techniques, in-situ doping processes during battery operation, and the integration of computational modeling to predict optimal dopant configurations. These developments are converging toward more sophisticated approaches that can precisely engineer the thermal runaway resistance of battery materials at the molecular level.
The primary objective of electrochemical doping research in the context of thermal runaway resistance is to develop battery systems that maintain structural and chemical integrity under extreme thermal conditions. This includes creating electrode materials that resist exothermic decomposition reactions, designing electrolyte additives that suppress thermal propagation, and engineering interfaces that remain stable at elevated temperatures.
Secondary objectives encompass improving the practical implementation of doping technologies in commercial battery manufacturing, reducing the cost barriers associated with advanced doping processes, and ensuring that thermal safety enhancements do not compromise other critical battery performance metrics such as energy density, power capability, and cycle life.
Long-term goals extend to the development of predictive models for dopant behavior under thermal stress, standardization of doping protocols across the industry, and the creation of self-regulating battery systems that can actively respond to thermal threats through dopant-mediated mechanisms. These objectives collectively aim to transform battery safety paradigms, potentially eliminating thermal runaway as a significant risk factor in next-generation energy storage technologies.
The technological trajectory has been marked by several pivotal advancements, including the discovery of intercalation mechanisms in lithium-ion batteries, the development of novel dopant materials, and the refinement of precise doping methodologies. Recent research has increasingly focused on leveraging electrochemical doping specifically to enhance thermal stability and safety characteristics of battery systems, addressing one of the most critical challenges in modern energy storage.
Current technological trends indicate a growing emphasis on nanoscale doping techniques, in-situ doping processes during battery operation, and the integration of computational modeling to predict optimal dopant configurations. These developments are converging toward more sophisticated approaches that can precisely engineer the thermal runaway resistance of battery materials at the molecular level.
The primary objective of electrochemical doping research in the context of thermal runaway resistance is to develop battery systems that maintain structural and chemical integrity under extreme thermal conditions. This includes creating electrode materials that resist exothermic decomposition reactions, designing electrolyte additives that suppress thermal propagation, and engineering interfaces that remain stable at elevated temperatures.
Secondary objectives encompass improving the practical implementation of doping technologies in commercial battery manufacturing, reducing the cost barriers associated with advanced doping processes, and ensuring that thermal safety enhancements do not compromise other critical battery performance metrics such as energy density, power capability, and cycle life.
Long-term goals extend to the development of predictive models for dopant behavior under thermal stress, standardization of doping protocols across the industry, and the creation of self-regulating battery systems that can actively respond to thermal threats through dopant-mediated mechanisms. These objectives collectively aim to transform battery safety paradigms, potentially eliminating thermal runaway as a significant risk factor in next-generation energy storage technologies.
Market Analysis for Thermal Runaway Resistant Batteries
The global market for thermal runaway resistant batteries is experiencing significant growth, driven by increasing safety concerns across various applications. The market size for advanced battery safety technologies was valued at approximately $3.2 billion in 2022 and is projected to reach $8.5 billion by 2028, representing a compound annual growth rate (CAGR) of 17.6%. This growth trajectory is particularly pronounced in electric vehicles, energy storage systems, and consumer electronics sectors.
Electric vehicle manufacturers represent the largest market segment, accounting for nearly 45% of the demand for thermal runaway resistant batteries. This is primarily due to high-profile battery fire incidents that have raised consumer safety concerns and prompted regulatory scrutiny. Major automotive OEMs are increasingly willing to pay premium prices for enhanced battery safety technologies, with some allocating up to 15% additional budget for advanced thermal management and runaway prevention systems.
The stationary energy storage market presents another significant growth opportunity, particularly as grid-scale installations proliferate in urban environments where safety requirements are stringent. This segment is expected to grow at 22.3% CAGR through 2028, outpacing the overall market growth rate. Utility companies and commercial building operators are increasingly specifying enhanced safety features in their procurement requirements.
Consumer electronics manufacturers are also driving demand, albeit with more price sensitivity than automotive applications. The recent implementation of stricter safety standards in major markets like the EU, China, and the United States has accelerated adoption of safer battery technologies across all consumer devices. Market research indicates that 78% of smartphone users consider battery safety a "very important" purchase consideration, up from 52% five years ago.
Geographically, Asia-Pacific dominates the market with 48% share, followed by North America (27%) and Europe (21%). China leads manufacturing capacity for advanced battery technologies, while South Korea and Japan maintain technological leadership in specialized safety innovations. The fastest growth is occurring in emerging markets where rapid electrification is creating new safety challenges.
Market analysis reveals that technologies incorporating electrochemical doping for thermal runaway resistance command price premiums of 20-30% compared to conventional solutions. However, production scaling and material optimization are gradually reducing this premium, with projections suggesting price parity could be achieved within 5-7 years for certain applications. This economic trajectory is critical for mass market adoption beyond premium segments.
Electric vehicle manufacturers represent the largest market segment, accounting for nearly 45% of the demand for thermal runaway resistant batteries. This is primarily due to high-profile battery fire incidents that have raised consumer safety concerns and prompted regulatory scrutiny. Major automotive OEMs are increasingly willing to pay premium prices for enhanced battery safety technologies, with some allocating up to 15% additional budget for advanced thermal management and runaway prevention systems.
The stationary energy storage market presents another significant growth opportunity, particularly as grid-scale installations proliferate in urban environments where safety requirements are stringent. This segment is expected to grow at 22.3% CAGR through 2028, outpacing the overall market growth rate. Utility companies and commercial building operators are increasingly specifying enhanced safety features in their procurement requirements.
Consumer electronics manufacturers are also driving demand, albeit with more price sensitivity than automotive applications. The recent implementation of stricter safety standards in major markets like the EU, China, and the United States has accelerated adoption of safer battery technologies across all consumer devices. Market research indicates that 78% of smartphone users consider battery safety a "very important" purchase consideration, up from 52% five years ago.
Geographically, Asia-Pacific dominates the market with 48% share, followed by North America (27%) and Europe (21%). China leads manufacturing capacity for advanced battery technologies, while South Korea and Japan maintain technological leadership in specialized safety innovations. The fastest growth is occurring in emerging markets where rapid electrification is creating new safety challenges.
Market analysis reveals that technologies incorporating electrochemical doping for thermal runaway resistance command price premiums of 20-30% compared to conventional solutions. However, production scaling and material optimization are gradually reducing this premium, with projections suggesting price parity could be achieved within 5-7 years for certain applications. This economic trajectory is critical for mass market adoption beyond premium segments.
Current Challenges in Battery Thermal Stability
Despite significant advancements in lithium-ion battery technology, thermal stability remains a critical challenge that impedes further development and widespread adoption. Current battery systems face several interconnected thermal management issues that can lead to catastrophic thermal runaway events, posing serious safety concerns for applications ranging from consumer electronics to electric vehicles and grid-scale energy storage.
The primary challenge lies in the inherent instability of conventional electrode materials at elevated temperatures. When batteries operate beyond their thermal safety window (typically 0-45°C), a cascade of exothermic reactions can initiate between the electrode materials and electrolyte. These reactions generate additional heat, potentially triggering a self-accelerating process that culminates in thermal runaway.
Electrolyte decomposition represents another significant challenge. Contemporary electrolytes, predominantly composed of organic carbonates with lithium salts, begin to decompose at temperatures as low as 70-80°C, releasing flammable gases and contributing to pressure buildup within cells. This decomposition not only accelerates thermal events but also creates conditions conducive to internal short circuits.
The solid-electrolyte interphase (SEI) layer, crucial for battery functionality, exhibits thermal vulnerability. At elevated temperatures (typically above 80°C), this protective layer can break down, exposing fresh electrode surfaces to the electrolyte and initiating additional exothermic reactions. The degradation of the SEI layer represents a critical failure point in thermal stability management.
Current battery management systems (BMS) often lack sophisticated real-time thermal monitoring capabilities at the cell level. This limitation prevents early detection of thermal anomalies before they escalate into serious safety incidents. The integration of advanced thermal sensing technologies remains technically challenging and cost-prohibitive for many applications.
Heat dissipation inefficiency presents another substantial obstacle. As energy densities increase, the challenge of effectively removing heat from battery packs becomes more pronounced. Existing cooling systems struggle to maintain uniform temperature distributions across large battery arrays, leading to thermal gradients that can accelerate degradation and increase failure risks.
The trade-off between energy density and thermal stability continues to constrain battery design. Materials and structures that enhance thermal stability often compromise energy density and power performance, creating a persistent engineering dilemma that limits application potential in demanding sectors like transportation and grid storage.
These challenges collectively highlight the urgent need for innovative approaches to thermal stability, particularly through advanced materials engineering strategies such as electrochemical doping, which shows promise in enhancing the intrinsic thermal resistance of battery components without sacrificing performance metrics.
The primary challenge lies in the inherent instability of conventional electrode materials at elevated temperatures. When batteries operate beyond their thermal safety window (typically 0-45°C), a cascade of exothermic reactions can initiate between the electrode materials and electrolyte. These reactions generate additional heat, potentially triggering a self-accelerating process that culminates in thermal runaway.
Electrolyte decomposition represents another significant challenge. Contemporary electrolytes, predominantly composed of organic carbonates with lithium salts, begin to decompose at temperatures as low as 70-80°C, releasing flammable gases and contributing to pressure buildup within cells. This decomposition not only accelerates thermal events but also creates conditions conducive to internal short circuits.
The solid-electrolyte interphase (SEI) layer, crucial for battery functionality, exhibits thermal vulnerability. At elevated temperatures (typically above 80°C), this protective layer can break down, exposing fresh electrode surfaces to the electrolyte and initiating additional exothermic reactions. The degradation of the SEI layer represents a critical failure point in thermal stability management.
Current battery management systems (BMS) often lack sophisticated real-time thermal monitoring capabilities at the cell level. This limitation prevents early detection of thermal anomalies before they escalate into serious safety incidents. The integration of advanced thermal sensing technologies remains technically challenging and cost-prohibitive for many applications.
Heat dissipation inefficiency presents another substantial obstacle. As energy densities increase, the challenge of effectively removing heat from battery packs becomes more pronounced. Existing cooling systems struggle to maintain uniform temperature distributions across large battery arrays, leading to thermal gradients that can accelerate degradation and increase failure risks.
The trade-off between energy density and thermal stability continues to constrain battery design. Materials and structures that enhance thermal stability often compromise energy density and power performance, creating a persistent engineering dilemma that limits application potential in demanding sectors like transportation and grid storage.
These challenges collectively highlight the urgent need for innovative approaches to thermal stability, particularly through advanced materials engineering strategies such as electrochemical doping, which shows promise in enhancing the intrinsic thermal resistance of battery components without sacrificing performance metrics.
Current Approaches to Thermal Runaway Prevention
01 Electrochemical doping techniques for thermal stability
Electrochemical doping methods can be employed to enhance the thermal stability of battery materials, reducing the risk of thermal runaway. By carefully controlling the doping process, the thermal resistance properties of electrode materials can be significantly improved. These techniques modify the electronic structure of materials to create more stable configurations that can withstand higher temperatures without degradation or thermal events.- Electrochemical doping techniques for thermal stability: Electrochemical doping methods can be employed to enhance the thermal stability of battery materials, reducing the risk of thermal runaway. By carefully controlling the doping process, the electronic and ionic conductivity of electrode materials can be modified to improve heat dissipation and prevent localized heating. These techniques often involve the introduction of specific ions or compounds that can stabilize the crystal structure of active materials at elevated temperatures.
- Advanced electrode materials with thermal runaway resistance: Specialized electrode materials can be designed with inherent thermal runaway resistance through electrochemical doping. These materials often incorporate elements or compounds that can absorb excess heat or prevent propagation of thermal events. By modifying the composition and structure of electrode materials through controlled doping, thermal stability can be significantly improved without compromising electrochemical performance. This approach often involves the use of dopants that can form protective layers or phases during thermal events.
- Battery management systems for thermal runaway prevention: Advanced battery management systems can be integrated with electrochemically doped materials to provide comprehensive thermal runaway resistance. These systems monitor temperature, voltage, and current parameters to detect early signs of thermal instability. When combined with properly doped electrode materials, these management systems can effectively prevent thermal runaway by implementing protective measures such as current limitation or circuit disconnection before critical temperatures are reached.
- Electrolyte additives for enhanced thermal stability: Specialized electrolyte additives can work synergistically with electrochemically doped materials to improve thermal runaway resistance. These additives can form protective films on electrode surfaces, scavenge reactive species, or increase the thermal decomposition temperature of the electrolyte. By carefully selecting additives that complement the doping profile of electrode materials, the overall thermal stability of the battery system can be significantly enhanced, reducing the risk of catastrophic failure under extreme conditions.
- Multi-layer safety structures with doped components: Multi-layered safety structures incorporating electrochemically doped materials can provide comprehensive protection against thermal runaway. These structures often include thermally conductive layers, flame-retardant barriers, and pressure-relief mechanisms working in conjunction with doped electrode materials. The doping profiles of different components can be optimized to respond differently to thermal events, creating a coordinated defense system that can contain and mitigate thermal incidents before they propagate throughout the battery.
02 Advanced electrode materials with thermal runaway resistance
Specialized electrode materials can be developed through controlled doping to resist thermal runaway conditions. These materials incorporate specific dopants that create thermal barriers within the electrode structure, preventing the propagation of thermal events. The modified electrode compositions demonstrate enhanced structural integrity under high-temperature conditions, effectively increasing the safety margin of electrochemical systems.Expand Specific Solutions03 Electrolyte additives for thermal stability enhancement
Incorporating specific additives into electrolyte formulations can significantly improve thermal runaway resistance in electrochemical systems. These additives work by forming protective films on electrode surfaces during cycling, which act as barriers against thermal degradation. Additionally, some additives can function as flame retardants or heat absorbers, providing multiple layers of protection against thermal events.Expand Specific Solutions04 Battery management systems for thermal runaway prevention
Advanced battery management systems can be designed to monitor and control electrochemical doping processes to prevent thermal runaway. These systems utilize sensors and algorithms to detect early signs of thermal instability and adjust operating parameters accordingly. By implementing real-time monitoring and adaptive control strategies, these management systems can effectively mitigate thermal risks in electrochemical energy storage devices.Expand Specific Solutions05 Structural design innovations for thermal runaway resistance
Novel structural designs can be implemented in electrochemical cells to enhance thermal runaway resistance. These designs include specialized cell architectures, thermal isolation layers, and heat dissipation pathways that work together to contain and manage thermal events. By incorporating physical barriers and thermal management features directly into the cell structure, the propagation of thermal runaway can be effectively limited even when initial thermal events occur.Expand Specific Solutions
Leading Companies in Battery Safety Innovation
The electrochemical doping technology for battery thermal runaway resistance is evolving rapidly in a market transitioning from early development to growth phase. The global battery safety market is expanding significantly, driven by electric vehicle adoption and energy storage demands. Technology maturity varies among key players, with Tesla and Samsung SDI leading commercial implementation, while CATL (Ningde Amperex) and LG Chem focus on advanced R&D. ProLogium and Murata Manufacturing are pioneering solid-state solutions incorporating electrochemical doping principles. Research institutions like Nanjing Tech University and CEA collaborate with industry partners to address fundamental challenges. The competitive landscape is characterized by strategic partnerships between automotive manufacturers (GM, Ford, Geely) and battery specialists to accelerate innovation in this critical safety technology.
Ningde Amperex Technology Ltd.
Technical Solution: CATL (Ningde Amperex Technology) has developed an innovative electrochemical doping approach focused on both cathode and anode materials to create comprehensive thermal runaway resistance. Their technology employs nano-scale doping of elements such as boron, fluorine, and phosphorus to modify the surface chemistry of cathode particles, creating a thermally stable interface layer. CATL's "cell-to-pack" technology integrates these doped materials into a structural design that enhances heat dissipation. Their proprietary "thermal gradient regulation" system works with the doped materials to prevent thermal propagation between cells. CATL has also pioneered the use of artificial intelligence to optimize dopant combinations and concentrations, resulting in materials that maintain high energy density while significantly improving thermal stability under extreme conditions.
Strengths: Holistic approach addressing both cathode and anode materials; integration with advanced thermal management systems; scalable manufacturing processes already implemented in gigafactories. Weaknesses: Some dopant combinations may affect cycle life at extreme temperatures; higher material costs compared to standard formulations; requires specialized equipment for precise dopant control.
Commissariat à l´énergie atomique et aux énergies Alternatives
Technical Solution: The French Alternative Energies and Atomic Energy Commission (CEA) has developed sophisticated electrochemical doping techniques focused on fundamental material science approaches. Their research has pioneered the use of lanthanide series elements as dopants in lithium-ion battery cathodes, creating unique electronic structures that suppress oxygen release during thermal events. CEA's approach includes atomic layer deposition techniques to create precisely controlled doped surface layers on cathode particles. Their technology also incorporates computational modeling to predict the thermal behavior of doped materials under various abuse conditions, allowing for targeted dopant selection. CEA has demonstrated that their doped materials can withstand temperatures up to 50°C higher than conventional cathodes before thermal runaway initiation, representing a significant safety improvement for applications in aerospace and defense sectors.
Strengths: Fundamental scientific approach with strong theoretical foundation; precise atomic-level control of doping processes; extensive characterization capabilities to validate thermal stability improvements. Weaknesses: Currently at lower technology readiness level compared to commercial implementations; higher production costs due to specialized deposition techniques; some approaches require rare earth elements with supply chain concerns.
Key Patents in Electrochemical Doping for Battery Safety
Passive vent system
PatentPendingUS20240204335A1
Innovation
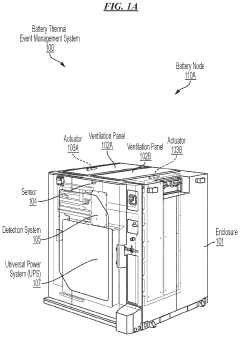
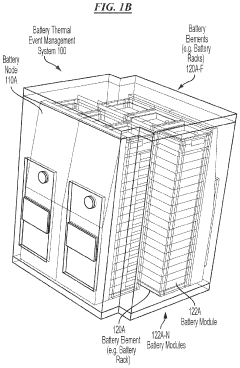
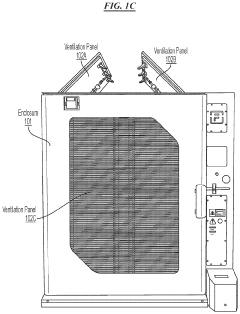
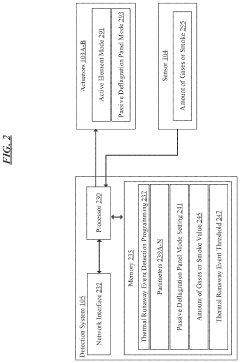
- A battery thermal event management system that includes sensors to detect indicative parameters of thermal runaway events and actuators to open ventilation panels, allowing gases or smoke to escape, thereby limiting combustible volume and pressure within the enclosure.
device for triggering thermal runaway of an electrochemical accumulator, in particular a metal-ion accumulator, associated method.
PatentActiveFR3106021A1
Innovation
- A device comprising two electrical current passage elements, such as conductive rings or foils, is used to heat the metal casing or flexible packaging of the accumulator by the Joule effect, allowing rapid thermal runaway initiation with high current application, typically in less than 5 minutes, using a power supply independent of the accumulator.
Safety Standards and Regulatory Framework
The regulatory landscape for battery safety has evolved significantly in response to thermal runaway incidents across various industries. International standards such as IEC 62133 and UL 1642 have established comprehensive testing protocols specifically addressing thermal stability in lithium-ion batteries. These standards now increasingly recognize electrochemical doping as a critical factor in thermal runaway resistance, requiring manufacturers to document doping agents and their concentration levels used in electrode materials.
The UN Transportation Testing requirements (UN 38.3) have been updated to include specific provisions for batteries with advanced doping technologies, acknowledging their potentially different behavior under thermal stress conditions. These regulations mandate more stringent testing for thermal propagation resistance, with particular attention to how doped materials respond to thermal triggers.
Regional frameworks show notable variations in their approach to electrochemical doping. The European Union, through its Battery Directive and upcoming Battery Regulation, has implemented specific requirements for documenting all chemical additives, including dopants that affect thermal stability. Meanwhile, China's GB/T standards have pioneered specific testing methodologies for evaluating the effectiveness of doped cathode materials in preventing thermal cascades.
Industry-specific regulations have emerged in sectors with heightened safety concerns. Aviation standards (RTCA DO-311A) now include specialized testing for batteries with novel doping configurations, while electric vehicle regulations increasingly mandate disclosure of thermal runaway mitigation strategies, including electrochemical doping approaches.
Regulatory gaps remain evident regarding the long-term stability of doping agents and their potential environmental impacts. Current frameworks primarily focus on immediate safety performance rather than the degradation of doping effectiveness over battery lifetime. This has prompted calls for lifecycle-oriented safety standards that account for how doping agents may change in effectiveness throughout a battery's operational life.
Compliance documentation requirements have expanded to include detailed information about doping agents, their concentration levels, and supporting test data demonstrating their effectiveness in thermal runaway prevention. Manufacturers must now maintain comprehensive technical files documenting how their doping strategies align with safety standards, creating significant barriers to market entry for companies without sophisticated materials science capabilities.
The UN Transportation Testing requirements (UN 38.3) have been updated to include specific provisions for batteries with advanced doping technologies, acknowledging their potentially different behavior under thermal stress conditions. These regulations mandate more stringent testing for thermal propagation resistance, with particular attention to how doped materials respond to thermal triggers.
Regional frameworks show notable variations in their approach to electrochemical doping. The European Union, through its Battery Directive and upcoming Battery Regulation, has implemented specific requirements for documenting all chemical additives, including dopants that affect thermal stability. Meanwhile, China's GB/T standards have pioneered specific testing methodologies for evaluating the effectiveness of doped cathode materials in preventing thermal cascades.
Industry-specific regulations have emerged in sectors with heightened safety concerns. Aviation standards (RTCA DO-311A) now include specialized testing for batteries with novel doping configurations, while electric vehicle regulations increasingly mandate disclosure of thermal runaway mitigation strategies, including electrochemical doping approaches.
Regulatory gaps remain evident regarding the long-term stability of doping agents and their potential environmental impacts. Current frameworks primarily focus on immediate safety performance rather than the degradation of doping effectiveness over battery lifetime. This has prompted calls for lifecycle-oriented safety standards that account for how doping agents may change in effectiveness throughout a battery's operational life.
Compliance documentation requirements have expanded to include detailed information about doping agents, their concentration levels, and supporting test data demonstrating their effectiveness in thermal runaway prevention. Manufacturers must now maintain comprehensive technical files documenting how their doping strategies align with safety standards, creating significant barriers to market entry for companies without sophisticated materials science capabilities.
Environmental Impact of Advanced Battery Materials
The environmental implications of advanced battery materials are increasingly significant as the global adoption of energy storage technologies accelerates. Electrochemical doping, a critical factor in enhancing thermal runaway resistance, presents both environmental challenges and opportunities that warrant careful consideration in the sustainable development of battery technologies.
The production processes for dopants used in electrochemical modification often involve energy-intensive manufacturing and rare earth element extraction. These processes generate substantial carbon emissions and can lead to habitat destruction, particularly in regions with less stringent environmental regulations. For instance, the mining of cobalt and nickel, commonly used as dopants, has been associated with significant environmental degradation in countries like the Democratic Republic of Congo and Indonesia.
Water consumption and contamination represent another environmental concern. The synthesis of advanced electrode materials with enhanced thermal stability often requires substantial water resources, while chemical leaching during extraction can introduce toxic compounds into local water systems. Recent studies indicate that the production of one ton of lithium, essential for many advanced battery formulations, can consume up to 2 million liters of water in arid regions.
However, electrochemical doping also offers environmental benefits through improved battery safety and longevity. By enhancing thermal runaway resistance, these technologies reduce the frequency of catastrophic battery failures that can release toxic chemicals and heavy metals into ecosystems. Additionally, the extended lifecycle of thermally stable batteries decreases the overall material demand and waste generation across the battery value chain.
End-of-life management presents both challenges and opportunities. While doped materials may introduce additional complexity to recycling processes, they also create potential for more efficient material recovery. Advanced separation techniques specifically designed for doped electrodes are emerging, potentially enabling higher recovery rates for critical materials and reducing the environmental burden of battery disposal.
The environmental footprint of electrochemically doped batteries must be evaluated through comprehensive life cycle assessment (LCA) methodologies. Recent LCAs suggest that while production impacts may be higher for advanced doped materials, these are often offset by efficiency gains and extended service life. The net environmental benefit depends significantly on the specific dopants used, their concentration, and the manufacturing processes employed.
The production processes for dopants used in electrochemical modification often involve energy-intensive manufacturing and rare earth element extraction. These processes generate substantial carbon emissions and can lead to habitat destruction, particularly in regions with less stringent environmental regulations. For instance, the mining of cobalt and nickel, commonly used as dopants, has been associated with significant environmental degradation in countries like the Democratic Republic of Congo and Indonesia.
Water consumption and contamination represent another environmental concern. The synthesis of advanced electrode materials with enhanced thermal stability often requires substantial water resources, while chemical leaching during extraction can introduce toxic compounds into local water systems. Recent studies indicate that the production of one ton of lithium, essential for many advanced battery formulations, can consume up to 2 million liters of water in arid regions.
However, electrochemical doping also offers environmental benefits through improved battery safety and longevity. By enhancing thermal runaway resistance, these technologies reduce the frequency of catastrophic battery failures that can release toxic chemicals and heavy metals into ecosystems. Additionally, the extended lifecycle of thermally stable batteries decreases the overall material demand and waste generation across the battery value chain.
End-of-life management presents both challenges and opportunities. While doped materials may introduce additional complexity to recycling processes, they also create potential for more efficient material recovery. Advanced separation techniques specifically designed for doped electrodes are emerging, potentially enabling higher recovery rates for critical materials and reducing the environmental burden of battery disposal.
The environmental footprint of electrochemically doped batteries must be evaluated through comprehensive life cycle assessment (LCA) methodologies. Recent LCAs suggest that while production impacts may be higher for advanced doped materials, these are often offset by efficiency gains and extended service life. The net environmental benefit depends significantly on the specific dopants used, their concentration, and the manufacturing processes employed.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!