The Technical Parameters Defining Gene Therapy Vectors
SEP 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Gene Therapy Vector Evolution and Objectives
Gene therapy has evolved significantly since its conceptual inception in the 1970s, transitioning from theoretical possibility to clinical reality. The field has progressed through distinct technological phases, beginning with early viral vector systems that faced significant safety challenges, to today's sophisticated delivery platforms with enhanced targeting capabilities and reduced immunogenicity. This evolution reflects the persistent pursuit of vectors that can efficiently deliver genetic material while minimizing adverse effects.
The primary objective in gene therapy vector development is to create delivery systems that combine high transduction efficiency with optimal safety profiles. Vectors must effectively penetrate target cells, navigate intracellular barriers, and facilitate stable gene expression without triggering immune responses or causing insertional mutagenesis. Additionally, scalable manufacturing processes are essential for clinical translation and commercialization.
Viral vectors remain predominant in the field due to their natural ability to infect cells and deliver genetic material. Adeno-associated viruses (AAVs) have emerged as leading candidates for in vivo applications, offering long-term expression without pathogenicity. Lentiviral vectors excel in ex vivo applications, particularly for hematopoietic stem cell modification, due to their ability to integrate into the host genome and maintain expression through cell division.
Non-viral vectors, including lipid nanoparticles and polymer-based systems, represent an expanding alternative approach. These synthetic delivery systems offer advantages in manufacturing scalability, reduced immunogenicity, and greater packaging capacity compared to viral vectors. Recent innovations in mRNA delivery platforms, as demonstrated by COVID-19 vaccines, have accelerated interest in non-viral approaches for gene therapy applications.
The technical parameters defining vector performance include transduction efficiency, tissue tropism, packaging capacity, immunogenicity profile, and production scalability. Vector engineering efforts focus on modifying these parameters through capsid protein engineering, envelope pseudotyping, and incorporation of tissue-specific promoters to enhance targeting precision and expression control.
Looking forward, gene therapy vector development aims to address several critical challenges: expanding delivery to difficult-to-reach tissues such as the central nervous system, developing vectors capable of re-dosing without immune neutralization, and creating systems for precise genome editing with minimal off-target effects. The convergence of synthetic biology, nanotechnology, and computational design is expected to yield next-generation vectors with programmable properties tailored to specific therapeutic applications.
The primary objective in gene therapy vector development is to create delivery systems that combine high transduction efficiency with optimal safety profiles. Vectors must effectively penetrate target cells, navigate intracellular barriers, and facilitate stable gene expression without triggering immune responses or causing insertional mutagenesis. Additionally, scalable manufacturing processes are essential for clinical translation and commercialization.
Viral vectors remain predominant in the field due to their natural ability to infect cells and deliver genetic material. Adeno-associated viruses (AAVs) have emerged as leading candidates for in vivo applications, offering long-term expression without pathogenicity. Lentiviral vectors excel in ex vivo applications, particularly for hematopoietic stem cell modification, due to their ability to integrate into the host genome and maintain expression through cell division.
Non-viral vectors, including lipid nanoparticles and polymer-based systems, represent an expanding alternative approach. These synthetic delivery systems offer advantages in manufacturing scalability, reduced immunogenicity, and greater packaging capacity compared to viral vectors. Recent innovations in mRNA delivery platforms, as demonstrated by COVID-19 vaccines, have accelerated interest in non-viral approaches for gene therapy applications.
The technical parameters defining vector performance include transduction efficiency, tissue tropism, packaging capacity, immunogenicity profile, and production scalability. Vector engineering efforts focus on modifying these parameters through capsid protein engineering, envelope pseudotyping, and incorporation of tissue-specific promoters to enhance targeting precision and expression control.
Looking forward, gene therapy vector development aims to address several critical challenges: expanding delivery to difficult-to-reach tissues such as the central nervous system, developing vectors capable of re-dosing without immune neutralization, and creating systems for precise genome editing with minimal off-target effects. The convergence of synthetic biology, nanotechnology, and computational design is expected to yield next-generation vectors with programmable properties tailored to specific therapeutic applications.
Market Analysis for Gene Therapy Applications
The gene therapy market is experiencing unprecedented growth, with a global valuation reaching $5.8 billion in 2022 and projected to expand at a CAGR of 19.2% through 2030. This remarkable trajectory is fueled by increasing prevalence of genetic disorders, advancements in vector technology, and growing investment in research and development activities.
North America currently dominates the market landscape, accounting for approximately 48% of global revenue, followed by Europe at 28% and Asia-Pacific at 18%. This regional distribution reflects the concentration of research institutions, biotechnology companies, and favorable regulatory frameworks in developed economies.
Oncology applications represent the largest segment within gene therapy markets, comprising nearly 35% of current applications. This is followed by rare genetic disorders (30%), cardiovascular diseases (15%), and neurological conditions (12%). The remaining market share is distributed across ophthalmology, infectious diseases, and other therapeutic areas.
Vector technology preferences show distinct market patterns, with AAV vectors leading commercial applications at 42% market share due to their safety profile and tissue tropism capabilities. Lentiviral vectors follow at 28%, particularly dominant in ex vivo applications. Retroviral vectors maintain approximately 15% market share, while adenoviral vectors and non-viral delivery systems collectively represent the remaining 15%.
Pricing structures remain a significant market challenge, with approved gene therapies commanding premium prices ranging from $373,000 to $2.1 million per treatment. This pricing dynamic creates market access barriers in many healthcare systems, limiting penetration despite clinical efficacy.
Investment trends reveal accelerating capital flows, with venture capital funding in gene therapy startups exceeding $4.5 billion in 2022 alone. Major pharmaceutical companies have executed strategic acquisitions valued at over $25 billion collectively in the past three years, indicating strong confidence in future market potential.
Reimbursement landscapes vary significantly by region, with European markets implementing innovative payment models such as outcomes-based agreements and installment payments. The U.S. market continues to navigate complex payer negotiations, while emerging economies face substantial affordability challenges despite growing interest in gene therapy technologies.
Manufacturing capacity represents a critical market constraint, with current global capacity estimated at approximately 150,000 liters for viral vector production. This limitation has created production bottlenecks that impact commercialization timelines and geographic availability of approved therapies.
North America currently dominates the market landscape, accounting for approximately 48% of global revenue, followed by Europe at 28% and Asia-Pacific at 18%. This regional distribution reflects the concentration of research institutions, biotechnology companies, and favorable regulatory frameworks in developed economies.
Oncology applications represent the largest segment within gene therapy markets, comprising nearly 35% of current applications. This is followed by rare genetic disorders (30%), cardiovascular diseases (15%), and neurological conditions (12%). The remaining market share is distributed across ophthalmology, infectious diseases, and other therapeutic areas.
Vector technology preferences show distinct market patterns, with AAV vectors leading commercial applications at 42% market share due to their safety profile and tissue tropism capabilities. Lentiviral vectors follow at 28%, particularly dominant in ex vivo applications. Retroviral vectors maintain approximately 15% market share, while adenoviral vectors and non-viral delivery systems collectively represent the remaining 15%.
Pricing structures remain a significant market challenge, with approved gene therapies commanding premium prices ranging from $373,000 to $2.1 million per treatment. This pricing dynamic creates market access barriers in many healthcare systems, limiting penetration despite clinical efficacy.
Investment trends reveal accelerating capital flows, with venture capital funding in gene therapy startups exceeding $4.5 billion in 2022 alone. Major pharmaceutical companies have executed strategic acquisitions valued at over $25 billion collectively in the past three years, indicating strong confidence in future market potential.
Reimbursement landscapes vary significantly by region, with European markets implementing innovative payment models such as outcomes-based agreements and installment payments. The U.S. market continues to navigate complex payer negotiations, while emerging economies face substantial affordability challenges despite growing interest in gene therapy technologies.
Manufacturing capacity represents a critical market constraint, with current global capacity estimated at approximately 150,000 liters for viral vector production. This limitation has created production bottlenecks that impact commercialization timelines and geographic availability of approved therapies.
Current Vector Technologies and Limitations
Gene therapy vector technologies have evolved significantly over the past three decades, with several platforms now established as the workhorses of clinical applications. Viral vectors remain the predominant delivery systems, with adeno-associated viruses (AAVs), lentiviruses, adenoviruses, and retroviruses being the most widely utilized. Each vector system offers distinct advantages while facing specific limitations that influence their therapeutic applications.
AAV vectors have emerged as leading candidates for in vivo gene therapy due to their favorable safety profile and ability to provide long-term gene expression. These vectors can transduce both dividing and non-dividing cells and exhibit minimal immunogenicity. However, AAVs are constrained by a limited packaging capacity of approximately 4.7 kb, restricting their utility for delivering larger therapeutic genes. Additionally, pre-existing immunity against AAV capsids in human populations presents a significant challenge for clinical efficacy.
Lentiviral vectors, derived from HIV-1, excel in their ability to integrate into the host genome, providing stable transgene expression in dividing cells. With a packaging capacity of approximately 8-10 kb, they accommodate larger transgenes than AAVs. These vectors are particularly effective for ex vivo gene therapy applications, such as CAR-T cell therapies. However, concerns regarding insertional mutagenesis and the complexity of large-scale manufacturing remain significant limitations.
Adenoviral vectors offer high transduction efficiency and can accommodate larger transgenes (up to 36 kb for gutless adenoviruses). They efficiently transduce various cell types and enable high-level transgene expression. Nevertheless, their clinical application is hampered by strong immunogenicity, which limits repeated administration and can trigger severe inflammatory responses, as evidenced by historical adverse events in clinical trials.
Non-viral vectors, including lipid nanoparticles (LNPs), polymeric nanoparticles, and physical methods like electroporation, have gained attention due to their improved safety profiles and reduced immunogenicity. LNPs, in particular, have demonstrated clinical success with the approval of mRNA vaccines. However, these systems generally exhibit lower transduction efficiency compared to viral vectors and often provide only transient gene expression.
Current manufacturing challenges represent a significant bottleneck across all vector platforms. Scalable production processes that maintain consistent quality attributes remain difficult to establish, particularly for AAV and lentiviral vectors. Critical quality attributes including vector genome integrity, empty/full capsid ratios for AAVs, and removal of process-related impurities significantly impact clinical outcomes but are challenging to control during manufacturing.
Tissue tropism limitations also persist across vector technologies. Despite engineering efforts to modify capsid proteins or envelope glycoproteins, achieving highly specific targeting to desired tissues while avoiding off-target effects remains an unresolved challenge. This limitation necessitates higher vector doses, which in turn increases the risk of immunogenicity and toxicity.
AAV vectors have emerged as leading candidates for in vivo gene therapy due to their favorable safety profile and ability to provide long-term gene expression. These vectors can transduce both dividing and non-dividing cells and exhibit minimal immunogenicity. However, AAVs are constrained by a limited packaging capacity of approximately 4.7 kb, restricting their utility for delivering larger therapeutic genes. Additionally, pre-existing immunity against AAV capsids in human populations presents a significant challenge for clinical efficacy.
Lentiviral vectors, derived from HIV-1, excel in their ability to integrate into the host genome, providing stable transgene expression in dividing cells. With a packaging capacity of approximately 8-10 kb, they accommodate larger transgenes than AAVs. These vectors are particularly effective for ex vivo gene therapy applications, such as CAR-T cell therapies. However, concerns regarding insertional mutagenesis and the complexity of large-scale manufacturing remain significant limitations.
Adenoviral vectors offer high transduction efficiency and can accommodate larger transgenes (up to 36 kb for gutless adenoviruses). They efficiently transduce various cell types and enable high-level transgene expression. Nevertheless, their clinical application is hampered by strong immunogenicity, which limits repeated administration and can trigger severe inflammatory responses, as evidenced by historical adverse events in clinical trials.
Non-viral vectors, including lipid nanoparticles (LNPs), polymeric nanoparticles, and physical methods like electroporation, have gained attention due to their improved safety profiles and reduced immunogenicity. LNPs, in particular, have demonstrated clinical success with the approval of mRNA vaccines. However, these systems generally exhibit lower transduction efficiency compared to viral vectors and often provide only transient gene expression.
Current manufacturing challenges represent a significant bottleneck across all vector platforms. Scalable production processes that maintain consistent quality attributes remain difficult to establish, particularly for AAV and lentiviral vectors. Critical quality attributes including vector genome integrity, empty/full capsid ratios for AAVs, and removal of process-related impurities significantly impact clinical outcomes but are challenging to control during manufacturing.
Tissue tropism limitations also persist across vector technologies. Despite engineering efforts to modify capsid proteins or envelope glycoproteins, achieving highly specific targeting to desired tissues while avoiding off-target effects remains an unresolved challenge. This limitation necessitates higher vector doses, which in turn increases the risk of immunogenicity and toxicity.
Vector Design and Delivery Strategies
01 Viral vector design and optimization
Viral vectors are engineered to efficiently deliver therapeutic genes to target cells. These vectors include adenoviruses, lentiviruses, and adeno-associated viruses (AAVs), each with specific technical parameters such as packaging capacity, tropism, and immunogenicity profiles. Optimization involves modifying capsid proteins, enhancing transduction efficiency, and improving manufacturing scalability to ensure effective gene delivery while minimizing immune responses.- Viral vector design and optimization: Viral vectors are engineered to deliver therapeutic genes efficiently to target cells. Optimization involves modifying viral components to enhance transduction efficiency, reduce immunogenicity, and improve safety profiles. Key parameters include capsid modifications, promoter selection, and genome packaging capacity. Adeno-associated virus (AAV), lentivirus, and adenovirus vectors are commonly used platforms that can be engineered for tissue-specific targeting and increased gene expression.
- Non-viral delivery systems: Non-viral gene delivery systems utilize synthetic carriers such as lipid nanoparticles, polymers, and physical methods to transfer genetic material into cells. These systems are characterized by parameters including particle size, zeta potential, encapsulation efficiency, and transfection rate. Advantages include reduced immunogenicity, larger packaging capacity, and easier manufacturing compared to viral vectors, though they typically show lower transduction efficiency. Recent advances focus on improving cellular uptake and endosomal escape mechanisms.
- Gene expression control mechanisms: Technical parameters for controlling gene expression in gene therapy vectors include selection of promoters, enhancers, and regulatory elements that determine the timing, level, and tissue-specificity of transgene expression. Inducible promoter systems allow for regulated gene expression in response to specific stimuli. Alternative splicing mechanisms, codon optimization, and mRNA stabilizing elements are employed to enhance expression efficiency and duration. These control mechanisms are critical for achieving therapeutic efficacy while minimizing off-target effects.
- Vector manufacturing and quality control: Manufacturing parameters for gene therapy vectors include production scale, purification methods, and quality control metrics. Critical quality attributes encompass vector titer, empty/full capsid ratio for viral vectors, endotoxin levels, sterility, and genetic stability. Advanced analytical techniques such as digital PCR, next-generation sequencing, and electron microscopy are employed to characterize vector preparations. Standardized potency assays and reference materials are essential for batch consistency and regulatory compliance in clinical applications.
- Delivery route and biodistribution parameters: The route of administration and biodistribution profile significantly impact gene therapy efficacy and safety. Technical parameters include tissue tropism, vector persistence, cellular penetration, and clearance kinetics. Administration methods such as direct injection, systemic delivery, or ex vivo cell modification each present distinct biodistribution profiles. Vector engineering strategies like capsid shuffling or peptide display can enhance targeting to specific tissues while reducing off-target distribution. Imaging techniques and biomarkers are used to monitor vector biodistribution in preclinical and clinical settings.
02 Non-viral delivery systems
Non-viral gene delivery systems utilize synthetic carriers such as lipid nanoparticles, polymers, and physical methods to transfer genetic material into cells. These systems are characterized by technical parameters including particle size, zeta potential, encapsulation efficiency, and transfection rates. They offer advantages in terms of safety, reduced immunogenicity, and larger payload capacity compared to viral vectors, though often with lower transduction efficiency.Expand Specific Solutions03 Gene expression control mechanisms
Technical parameters for controlling gene expression in therapy vectors include promoter strength, inducibility, tissue specificity, and regulatory elements. These mechanisms allow for temporal and spatial control of therapeutic gene expression, enhancing safety and efficacy. Advanced systems incorporate feedback loops, drug-responsive elements, and microRNA target sequences to achieve precise regulation of transgene expression in specific cell types or under particular physiological conditions.Expand Specific Solutions04 Vector manufacturing and quality control
Manufacturing parameters for gene therapy vectors include production scale, purification methods, and quality control metrics. Critical technical specifications include vector titer, empty/full capsid ratio, endotoxin levels, residual host cell DNA/protein content, and genetic stability. Advanced analytical methods such as digital PCR, next-generation sequencing, and electron microscopy are employed to ensure batch consistency, potency, and safety of the final vector product for clinical applications.Expand Specific Solutions05 Targeted delivery and tissue specificity
Technical parameters for targeted gene delivery include vector tropism modification, cell-specific promoters, and surface ligand conjugation. These approaches enhance therapeutic index by directing vectors to specific tissues or cell types while minimizing off-target effects. Parameters such as biodistribution profiles, receptor affinity, tissue penetration, and cellular uptake efficiency are optimized to achieve precise delivery of genetic payloads to disease-relevant cells.Expand Specific Solutions
Key Industry Players and Competition
The gene therapy vector landscape is evolving rapidly, currently transitioning from early clinical development to commercial maturity. The market is projected to reach $10-15 billion by 2025, driven by breakthrough treatments for previously untreatable genetic disorders. Technical maturity varies significantly among key players: established leaders like Regeneron Pharmaceuticals and AskBio demonstrate advanced vector engineering capabilities, while academic institutions (MIT, UNC-Chapel Hill) contribute fundamental research innovations. Emerging companies like MeiraGTx and ETHRIS are developing specialized delivery technologies. The Children's Hospital of Philadelphia and Baylor College of Medicine represent the clinical translation frontier, while Chinese institutions (Wuhan University, Zhejiang University) are rapidly advancing their capabilities, suggesting a globalizing competitive landscape with diversifying technical approaches to vector design and manufacturing.
Regeneron Pharmaceuticals, Inc.
Technical Solution: Regeneron has developed a proprietary adenovirus-based vector platform that incorporates helper-dependent adenoviral vectors (HDAd) with all viral coding sequences removed, significantly reducing immunogenicity while maintaining high transduction efficiency. Their vectors feature large transgene capacity (up to 36kb), enabling delivery of multiple genes or large genomic sequences with regulatory elements. Regeneron's platform utilizes their VelociGene® technology for precise vector engineering, allowing rapid iteration and optimization of vector designs. Their manufacturing process employs a proprietary cell line system that produces vectors with consistent quality and high titers exceeding 10^12 viral particles/mL[9]. The vectors incorporate tissue-specific promoters and enhancers that enable targeted expression patterns with 20-50 fold specificity over conventional designs. Additionally, Regeneron has developed regulated expression systems using small molecule inducers that allow temporal control of transgene expression with induction ratios exceeding 100-fold[10]. Their platform features proprietary capsid modifications that enhance tissue tropism while reducing liver sequestration by 60-80% compared to unmodified vectors.
Strengths: Exceptional packaging capacity allows delivery of large or multiple transgenes; robust manufacturing platform enables consistent high-titer production; sophisticated regulatory elements provide precise control over transgene expression patterns. Weaknesses: Potential for strong inflammatory responses despite helper-dependent design; complex manufacturing process increases production costs; limited ability to re-administer due to adaptive immune responses to capsid proteins.
Baylor College of Medicine
Technical Solution: Baylor College of Medicine has developed advanced lentiviral vector systems with self-inactivating (SIN) designs that significantly reduce the risk of insertional mutagenesis. Their vectors incorporate woodchuck hepatitis virus post-transcriptional regulatory element (WPRE) and central polypurine tract (cPPT) elements, enhancing transgene expression by 5-10 fold compared to conventional lentiviral vectors[5]. Baylor's platform features pseudotyping capabilities with various envelope proteins, including VSV-G and RD114, enabling broad tissue tropism or targeted delivery. Their manufacturing process utilizes transient transfection methods with optimized plasmid ratios that achieve titers exceeding 10^9 TU/mL. The vectors incorporate microRNA target sequences (miR-T) that prevent expression in hematopoietic stem cells while allowing robust expression in target tissues, enhancing safety profiles. Additionally, Baylor has pioneered regulated expression systems using tetracycline-responsive elements that allow for dose-dependent control of transgene expression[6], addressing concerns about overexpression toxicity.
Strengths: Large packaging capacity (up to 8-9kb) allows delivery of complex therapeutic genes; ability to transduce both dividing and non-dividing cells expands application range; integration into host genome enables long-term expression in dividing cells. Weaknesses: Integration poses theoretical risk of insertional mutagenesis despite safety improvements; complex manufacturing process with multiple components increases production challenges; limited ability to re-administer due to immune responses to vector components.
Critical Patents in Vector Engineering
Vector for gene therapy
PatentInactiveUS6261834B1
Innovation
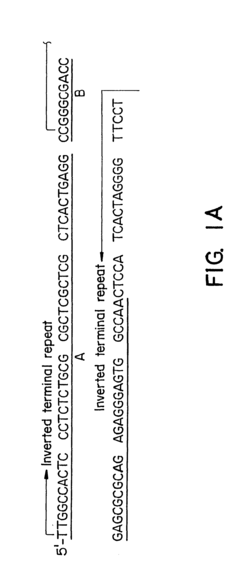
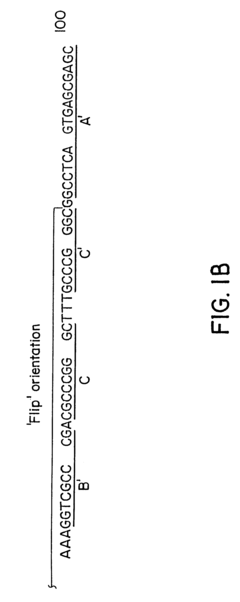
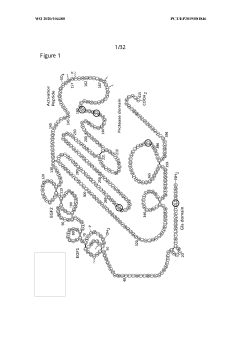
- Development of hybrid parvovirus vectors combining AAV and B19 parvovirus features, utilizing AAV inverted terminal repeats for site-specific integration and the B19 p6 promoter for tissue-specific expression, creating vectors that integrate safely and express genes specifically in hematopoietic cells.
Adeno-associated virus vectors for expressing fviii mimetics and uses thereof
PatentWO2020104480A1
Innovation
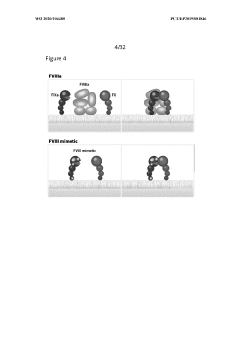
- Development of AAV vectors encoding variant human FIX proteins with specific amino acid substitutions (V181I, K265A, and I383V) that can activate the coagulation cascade independently of FVIII, allowing for safe and effective gene therapy in hemophilia A patients, including those with FVIII inhibitors, using a liver-specific promoter like Q1 for efficient expression.
Regulatory Framework for Gene Therapy
The regulatory landscape for gene therapy vectors is complex and continuously evolving as the field advances. Regulatory bodies worldwide have established frameworks to ensure the safety, efficacy, and quality of gene therapy products while balancing the need for innovation. The U.S. Food and Drug Administration (FDA) has developed specific guidance documents for gene therapy vectors through its Center for Biologics Evaluation and Research (CBER), focusing on manufacturing standards, preclinical testing requirements, and clinical trial design considerations.
In Europe, the European Medicines Agency (EMA) has established the Committee for Advanced Therapies (CAT) specifically to evaluate gene and cell therapies. Their guidelines emphasize vector characterization, including identity, purity, potency, and stability testing. Both FDA and EMA require comprehensive vector genome analysis, including sequence verification and assessment of potential recombination events.
Regulatory requirements for gene therapy vectors typically include detailed characterization of the vector system, including helper viruses if applicable, and demonstration of absence of replication-competent vectors. Manufacturing processes must adhere to Good Manufacturing Practice (GMP) standards with particular attention to consistency, sterility, and removal of process-related impurities.
Safety assessment frameworks mandate extensive biodistribution studies, evaluation of germline transmission risk, and long-term follow-up plans for patients. The duration of follow-up varies by vector type, with integrating vectors requiring longer monitoring periods (up to 15 years in some jurisdictions) compared to non-integrating vectors.
Accelerated approval pathways have been established for gene therapies addressing serious conditions with unmet medical needs. The FDA's Regenerative Medicine Advanced Therapy (RMAT) designation and the EMA's Priority Medicines (PRIME) scheme provide expedited review processes and enhanced regulatory support for promising gene therapies.
Harmonization efforts are underway through the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) to standardize regulatory approaches globally. However, significant differences remain between jurisdictions, particularly regarding risk assessment methodologies and post-approval monitoring requirements.
Regulatory frameworks are increasingly incorporating adaptive licensing approaches that allow for conditional approvals based on surrogate endpoints, with requirements for post-marketing studies to confirm clinical benefit. This reflects the recognition of both the transformative potential of gene therapies and the challenges in assessing their long-term effects through traditional approval pathways.
In Europe, the European Medicines Agency (EMA) has established the Committee for Advanced Therapies (CAT) specifically to evaluate gene and cell therapies. Their guidelines emphasize vector characterization, including identity, purity, potency, and stability testing. Both FDA and EMA require comprehensive vector genome analysis, including sequence verification and assessment of potential recombination events.
Regulatory requirements for gene therapy vectors typically include detailed characterization of the vector system, including helper viruses if applicable, and demonstration of absence of replication-competent vectors. Manufacturing processes must adhere to Good Manufacturing Practice (GMP) standards with particular attention to consistency, sterility, and removal of process-related impurities.
Safety assessment frameworks mandate extensive biodistribution studies, evaluation of germline transmission risk, and long-term follow-up plans for patients. The duration of follow-up varies by vector type, with integrating vectors requiring longer monitoring periods (up to 15 years in some jurisdictions) compared to non-integrating vectors.
Accelerated approval pathways have been established for gene therapies addressing serious conditions with unmet medical needs. The FDA's Regenerative Medicine Advanced Therapy (RMAT) designation and the EMA's Priority Medicines (PRIME) scheme provide expedited review processes and enhanced regulatory support for promising gene therapies.
Harmonization efforts are underway through the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) to standardize regulatory approaches globally. However, significant differences remain between jurisdictions, particularly regarding risk assessment methodologies and post-approval monitoring requirements.
Regulatory frameworks are increasingly incorporating adaptive licensing approaches that allow for conditional approvals based on surrogate endpoints, with requirements for post-marketing studies to confirm clinical benefit. This reflects the recognition of both the transformative potential of gene therapies and the challenges in assessing their long-term effects through traditional approval pathways.
Safety and Immunogenicity Considerations
Safety and immunogenicity considerations represent critical factors in the development and application of gene therapy vectors. The immune response to viral vectors remains one of the most significant barriers to successful gene therapy implementation. Adeno-associated virus (AAV) vectors, despite their favorable safety profile, can trigger both innate and adaptive immune responses that may neutralize the vector, eliminate transduced cells, and prevent vector re-administration.
Pre-existing immunity poses a substantial challenge, with studies indicating that 30-80% of the human population carries neutralizing antibodies against various AAV serotypes. This immunity significantly reduces transduction efficiency and therapeutic efficacy. Furthermore, capsid-specific CD8+ T cell responses can lead to the destruction of transduced cells, potentially eliminating therapeutic gene expression and causing inflammatory responses in target tissues.
Vector design modifications have emerged as promising strategies to mitigate immunogenicity. These include capsid engineering to evade neutralizing antibodies, the use of tissue-specific promoters to limit transgene expression to target cells, and the incorporation of immunomodulatory sequences. Empty capsid decoys and transient immunosuppression regimens have also demonstrated efficacy in reducing immune responses during vector administration.
Safety considerations extend beyond immunogenicity to include insertional mutagenesis risks, particularly with integrating vectors like lentiviruses. While AAV vectors predominantly remain episomal, integration events can occur at low frequencies. Next-generation sequencing technologies now enable comprehensive assessment of integration profiles, allowing for better risk evaluation and vector design optimization.
Dose-dependent toxicity represents another critical safety parameter. High vector doses, often necessary to achieve therapeutic efficacy, can trigger severe inflammatory responses and organ-specific toxicities. Recent clinical trials have reported severe adverse events, including hepatotoxicity and thrombotic microangiopathy, associated with high-dose AAV administration. These findings underscore the importance of establishing therapeutic windows that balance efficacy with safety.
Regulatory frameworks for gene therapy vectors continue to evolve, with agencies like the FDA and EMA implementing rigorous testing requirements for immunogenicity and safety. These include comprehensive characterization of cellular and humoral immune responses in preclinical models, assessment of vector biodistribution, and long-term monitoring for delayed adverse events. The development of standardized assays for neutralizing antibody detection and T cell responses has become essential for consistent safety evaluation across different vector platforms.
Pre-existing immunity poses a substantial challenge, with studies indicating that 30-80% of the human population carries neutralizing antibodies against various AAV serotypes. This immunity significantly reduces transduction efficiency and therapeutic efficacy. Furthermore, capsid-specific CD8+ T cell responses can lead to the destruction of transduced cells, potentially eliminating therapeutic gene expression and causing inflammatory responses in target tissues.
Vector design modifications have emerged as promising strategies to mitigate immunogenicity. These include capsid engineering to evade neutralizing antibodies, the use of tissue-specific promoters to limit transgene expression to target cells, and the incorporation of immunomodulatory sequences. Empty capsid decoys and transient immunosuppression regimens have also demonstrated efficacy in reducing immune responses during vector administration.
Safety considerations extend beyond immunogenicity to include insertional mutagenesis risks, particularly with integrating vectors like lentiviruses. While AAV vectors predominantly remain episomal, integration events can occur at low frequencies. Next-generation sequencing technologies now enable comprehensive assessment of integration profiles, allowing for better risk evaluation and vector design optimization.
Dose-dependent toxicity represents another critical safety parameter. High vector doses, often necessary to achieve therapeutic efficacy, can trigger severe inflammatory responses and organ-specific toxicities. Recent clinical trials have reported severe adverse events, including hepatotoxicity and thrombotic microangiopathy, associated with high-dose AAV administration. These findings underscore the importance of establishing therapeutic windows that balance efficacy with safety.
Regulatory frameworks for gene therapy vectors continue to evolve, with agencies like the FDA and EMA implementing rigorous testing requirements for immunogenicity and safety. These include comprehensive characterization of cellular and humoral immune responses in preclinical models, assessment of vector biodistribution, and long-term monitoring for delayed adverse events. The development of standardized assays for neutralizing antibody detection and T cell responses has become essential for consistent safety evaluation across different vector platforms.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!