TIM Cleanroom Handling SOPs: Contamination Control For High-Reliability Builds
AUG 27, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Cleanroom Technology Background and Objectives
Cleanroom technology has evolved significantly over the past several decades, transforming from basic controlled environments to sophisticated facilities with precise contamination control capabilities. The development of cleanroom standards began in the 1960s with the introduction of Federal Standard 209, which established classifications based on airborne particle concentrations. This standard has since evolved into ISO 14644, providing a comprehensive framework for cleanroom classification and monitoring globally.
The evolution of Thermal Interface Materials (TIM) has paralleled advancements in electronics, where increasing power densities and miniaturization have demanded more efficient heat dissipation solutions. TIM materials serve as critical components in electronic assemblies by filling microscopic air gaps between heat-generating components and heat sinks, thereby enhancing thermal conductivity and system reliability.
High-reliability builds, particularly in aerospace, defense, medical devices, and advanced computing applications, require exceptional contamination control during assembly processes. Even microscopic contaminants can compromise the performance and longevity of these critical systems, potentially leading to catastrophic failures in mission-critical applications.
The primary objective of TIM Cleanroom Handling Standard Operating Procedures (SOPs) is to establish comprehensive protocols that minimize contamination risks during the handling, application, and integration of thermal interface materials. These procedures aim to ensure consistent product quality, maximize thermal performance, and extend the operational lifespan of high-reliability electronic assemblies.
Current technological goals include developing standardized methodologies for contamination monitoring specific to TIM applications, implementing advanced training systems for cleanroom personnel, and creating adaptive SOPs that can accommodate emerging TIM formulations and application techniques. Additionally, there is a growing focus on establishing quantifiable metrics to evaluate the effectiveness of contamination control measures in TIM handling processes.
The industry is witnessing a trend toward more specialized cleanroom environments tailored specifically for TIM applications, incorporating features such as controlled humidity zones, electrostatic discharge protection, and real-time particle monitoring systems. These technological advancements aim to address the increasing complexity of TIM materials, which now include phase-change materials, liquid metal compounds, and nanocomposites with unique handling requirements.
Future technological objectives include the development of automated TIM application systems that minimize human interaction and associated contamination risks, as well as the integration of artificial intelligence for predictive contamination control and process optimization in cleanroom environments dedicated to high-reliability electronic assembly.
The evolution of Thermal Interface Materials (TIM) has paralleled advancements in electronics, where increasing power densities and miniaturization have demanded more efficient heat dissipation solutions. TIM materials serve as critical components in electronic assemblies by filling microscopic air gaps between heat-generating components and heat sinks, thereby enhancing thermal conductivity and system reliability.
High-reliability builds, particularly in aerospace, defense, medical devices, and advanced computing applications, require exceptional contamination control during assembly processes. Even microscopic contaminants can compromise the performance and longevity of these critical systems, potentially leading to catastrophic failures in mission-critical applications.
The primary objective of TIM Cleanroom Handling Standard Operating Procedures (SOPs) is to establish comprehensive protocols that minimize contamination risks during the handling, application, and integration of thermal interface materials. These procedures aim to ensure consistent product quality, maximize thermal performance, and extend the operational lifespan of high-reliability electronic assemblies.
Current technological goals include developing standardized methodologies for contamination monitoring specific to TIM applications, implementing advanced training systems for cleanroom personnel, and creating adaptive SOPs that can accommodate emerging TIM formulations and application techniques. Additionally, there is a growing focus on establishing quantifiable metrics to evaluate the effectiveness of contamination control measures in TIM handling processes.
The industry is witnessing a trend toward more specialized cleanroom environments tailored specifically for TIM applications, incorporating features such as controlled humidity zones, electrostatic discharge protection, and real-time particle monitoring systems. These technological advancements aim to address the increasing complexity of TIM materials, which now include phase-change materials, liquid metal compounds, and nanocomposites with unique handling requirements.
Future technological objectives include the development of automated TIM application systems that minimize human interaction and associated contamination risks, as well as the integration of artificial intelligence for predictive contamination control and process optimization in cleanroom environments dedicated to high-reliability electronic assembly.
Market Requirements for High-Reliability Manufacturing
The high-reliability manufacturing market is experiencing unprecedented growth driven by industries where failure is not an option. Aerospace, defense, medical devices, and critical infrastructure sectors demand manufacturing processes with near-zero defect rates. Market research indicates that the high-reliability electronics manufacturing sector alone is projected to reach $118 billion by 2027, growing at a CAGR of 5.8% from 2022.
Contamination control has emerged as a primary concern across these industries, with particulate contamination being responsible for approximately 70% of electronic component failures in high-reliability applications. A single microscopic contaminant can cause catastrophic failures in mission-critical systems, resulting in potential losses measured in millions of dollars or even human lives.
The market increasingly demands comprehensive cleanroom handling Standard Operating Procedures (SOPs) that can demonstrably reduce contamination-related failures. Surveys of manufacturing executives reveal that 83% consider contamination control protocols as "extremely important" to their quality assurance programs, with Thermal Interface Materials (TIMs) being particularly vulnerable to contamination issues.
Regulatory requirements are becoming more stringent, with standards like ISO 14644, NASA-STD-8739, and industry-specific requirements like AS9100 for aerospace driving the need for more sophisticated contamination control protocols. Companies must demonstrate compliance with these standards to remain competitive in high-reliability markets.
Customer expectations have evolved beyond basic quality metrics to include detailed documentation of cleanroom protocols, material handling procedures, and contamination control validation methods. OEMs are increasingly requiring suppliers to provide evidence of robust TIM handling procedures as part of their vendor qualification processes.
The economic impact of contamination-related failures extends beyond immediate repair costs. For medical device manufacturers, a contamination-related recall can cost an average of $8.5 million, while in aerospace applications, the cost of field failures can exceed $50 million per incident when accounting for investigation, replacement, and reputation damage.
Market analysis reveals a significant gap between current industry practices and optimal contamination control protocols specifically for TIM materials. This gap represents both a challenge and an opportunity for organizations that can develop and implement advanced cleanroom handling SOPs tailored to high-reliability manufacturing environments.
Contamination control has emerged as a primary concern across these industries, with particulate contamination being responsible for approximately 70% of electronic component failures in high-reliability applications. A single microscopic contaminant can cause catastrophic failures in mission-critical systems, resulting in potential losses measured in millions of dollars or even human lives.
The market increasingly demands comprehensive cleanroom handling Standard Operating Procedures (SOPs) that can demonstrably reduce contamination-related failures. Surveys of manufacturing executives reveal that 83% consider contamination control protocols as "extremely important" to their quality assurance programs, with Thermal Interface Materials (TIMs) being particularly vulnerable to contamination issues.
Regulatory requirements are becoming more stringent, with standards like ISO 14644, NASA-STD-8739, and industry-specific requirements like AS9100 for aerospace driving the need for more sophisticated contamination control protocols. Companies must demonstrate compliance with these standards to remain competitive in high-reliability markets.
Customer expectations have evolved beyond basic quality metrics to include detailed documentation of cleanroom protocols, material handling procedures, and contamination control validation methods. OEMs are increasingly requiring suppliers to provide evidence of robust TIM handling procedures as part of their vendor qualification processes.
The economic impact of contamination-related failures extends beyond immediate repair costs. For medical device manufacturers, a contamination-related recall can cost an average of $8.5 million, while in aerospace applications, the cost of field failures can exceed $50 million per incident when accounting for investigation, replacement, and reputation damage.
Market analysis reveals a significant gap between current industry practices and optimal contamination control protocols specifically for TIM materials. This gap represents both a challenge and an opportunity for organizations that can develop and implement advanced cleanroom handling SOPs tailored to high-reliability manufacturing environments.
Current Cleanroom Contamination Control Challenges
Despite significant advancements in cleanroom technology, contemporary contamination control in TIM (Thermal Interface Material) handling environments faces multifaceted challenges. Particle contamination remains the primary concern, with sub-micron particles capable of compromising thermal conductivity at critical interfaces. These particles, often invisible to standard inspection methods, can create air gaps that significantly reduce heat transfer efficiency in high-reliability electronic assemblies.
Molecular contamination presents another substantial challenge, particularly hydrocarbon compounds that can outgas from various sources including personnel, equipment, and even cleanroom construction materials. These molecular contaminants can form thin films on TIM surfaces, altering their chemical properties and degrading long-term performance characteristics.
Electrostatic discharge (ESD) control introduces a complex contradiction in cleanroom operations. While ESD protection is essential for sensitive electronic components, many ESD-preventive materials can generate particulate or molecular contamination. This creates a delicate balancing act between protecting components from electrical damage while maintaining the required cleanliness levels for optimal TIM performance.
Human factors continue to represent one of the most significant variables in contamination control. Despite rigorous training protocols, human movements generate particles, shed biological contaminants, and introduce variability in procedure execution. The increasing complexity of TIM application processes for advanced thermal management solutions demands unprecedented precision from operators, creating a growing gap between human capabilities and process requirements.
Environmental stability challenges have intensified as TIM formulations become more sophisticated. Modern high-performance TIMs often require precise temperature and humidity control during handling and application. Fluctuations outside narrow parameters can trigger premature curing, phase separation, or viscosity changes that compromise the material's ability to form proper interfaces.
Cross-contamination between different TIM materials presents an emerging challenge as manufacturing facilities handle multiple product types. Even trace amounts of incompatible materials can trigger chemical reactions that degrade thermal performance or mechanical properties of precision-engineered TIMs.
Monitoring and verification limitations constitute a significant technological gap. Current real-time monitoring systems lack the sensitivity to detect all relevant contaminants at the concentrations that affect TIM performance. This creates uncertainty in quality assurance processes and complicates root cause analysis when thermal performance issues arise in finished products.
Molecular contamination presents another substantial challenge, particularly hydrocarbon compounds that can outgas from various sources including personnel, equipment, and even cleanroom construction materials. These molecular contaminants can form thin films on TIM surfaces, altering their chemical properties and degrading long-term performance characteristics.
Electrostatic discharge (ESD) control introduces a complex contradiction in cleanroom operations. While ESD protection is essential for sensitive electronic components, many ESD-preventive materials can generate particulate or molecular contamination. This creates a delicate balancing act between protecting components from electrical damage while maintaining the required cleanliness levels for optimal TIM performance.
Human factors continue to represent one of the most significant variables in contamination control. Despite rigorous training protocols, human movements generate particles, shed biological contaminants, and introduce variability in procedure execution. The increasing complexity of TIM application processes for advanced thermal management solutions demands unprecedented precision from operators, creating a growing gap between human capabilities and process requirements.
Environmental stability challenges have intensified as TIM formulations become more sophisticated. Modern high-performance TIMs often require precise temperature and humidity control during handling and application. Fluctuations outside narrow parameters can trigger premature curing, phase separation, or viscosity changes that compromise the material's ability to form proper interfaces.
Cross-contamination between different TIM materials presents an emerging challenge as manufacturing facilities handle multiple product types. Even trace amounts of incompatible materials can trigger chemical reactions that degrade thermal performance or mechanical properties of precision-engineered TIMs.
Monitoring and verification limitations constitute a significant technological gap. Current real-time monitoring systems lack the sensitivity to detect all relevant contaminants at the concentrations that affect TIM performance. This creates uncertainty in quality assurance processes and complicates root cause analysis when thermal performance issues arise in finished products.
Current TIM Handling Solutions and Best Practices
01 Cleanroom design and air management systems
Cleanroom design incorporates specialized air management systems to control contamination. These systems include HEPA filtration, laminar airflow, and pressure differentials between rooms to prevent contaminant ingress. The design focuses on minimizing particle generation and maintaining appropriate air quality classifications according to industry standards. Proper ventilation systems ensure continuous removal of airborne contaminants while maintaining required temperature and humidity levels.- Cleanroom design and air management systems: Effective cleanroom design incorporates specialized air management systems to control contamination. These systems include HEPA filtration, laminar airflow, and pressure differentials between rooms to prevent contaminant migration. The design focuses on minimizing particle generation and optimizing air exchange rates to maintain cleanliness classifications according to industry standards. Proper ventilation systems are crucial for removing airborne contaminants and maintaining appropriate temperature and humidity levels.
- Personnel protocols and gowning procedures: Standard operating procedures for personnel entering and working in cleanrooms include specific gowning protocols to minimize contamination. These procedures detail the proper sequence for donning cleanroom garments, gloves, masks, and footwear. Training programs ensure staff understand contamination sources from human activities and how to minimize particle generation through controlled movements. Entry and exit protocols may include air showers, sticky mats, and hand sanitization to reduce contaminant introduction to the controlled environment.
- Material transfer and handling procedures: Protocols for transferring materials into and within cleanrooms are essential for contamination control. These procedures include proper decontamination methods such as wiping with appropriate cleaning agents, using transfer hatches or pass-through chambers, and implementing double-bagging techniques. Materials are often staged in transition areas before being introduced to the cleanroom environment. Handling procedures specify how to move, store, and process materials to minimize particle generation and cross-contamination risks.
- Cleaning and disinfection protocols: Comprehensive cleaning and disinfection protocols are fundamental to cleanroom contamination control. These procedures specify appropriate cleaning agents, application methods, contact times, and frequencies for different surfaces and equipment. The protocols typically follow a specific sequence from ceiling to floor and from cleanest to less clean areas. Validation methods such as surface sampling and particle counting are used to verify cleaning effectiveness. Special attention is given to high-touch surfaces and critical process areas to prevent microbial and particulate contamination.
- Monitoring and documentation systems: Effective contamination control relies on robust monitoring and documentation systems. These include continuous particle counting, microbial sampling, and environmental parameter tracking. Regular testing verifies that cleanroom conditions meet specified cleanliness classifications. Documentation systems record all activities within the cleanroom, including personnel entry/exit, cleaning procedures performed, and environmental monitoring results. Automated systems may provide real-time alerts when parameters exceed acceptable limits, allowing for immediate corrective actions to maintain cleanroom integrity.
02 Personnel protocols and gowning procedures
Standard operating procedures for personnel include specific gowning protocols to minimize contamination. These procedures detail the proper sequence for donning cleanroom garments, gloves, masks, and other protective equipment. Training programs ensure staff understand contamination sources from human activity and proper movement techniques within cleanroom environments. Protocols may include airlocks, gowning rooms, and specific entry/exit procedures to maintain cleanroom integrity.Expand Specific Solutions03 Material handling and transfer systems
Specialized systems for material handling and transfer help maintain cleanroom integrity while moving items between different cleanliness zones. These include pass-through chambers, airlocks, and automated transfer systems designed to minimize contamination during material movement. Procedures specify proper cleaning and packaging of materials before introduction to the cleanroom, along with protocols for handling containers and equipment to prevent particle generation and cross-contamination.Expand Specific Solutions04 Cleaning and maintenance procedures
Detailed cleaning and maintenance procedures are essential for contamination control in cleanrooms. These include specific protocols for surface cleaning, equipment maintenance, and waste removal that minimize particle generation. SOPs specify approved cleaning agents, techniques, equipment, and frequencies for different cleanroom classifications. Documentation requirements ensure consistent implementation and verification of cleaning effectiveness through environmental monitoring programs.Expand Specific Solutions05 Monitoring and documentation systems
Comprehensive monitoring and documentation systems track cleanroom performance and ensure compliance with contamination control standards. These include particle counting, microbial sampling, and environmental parameter monitoring. Real-time monitoring systems provide alerts when conditions exceed specified limits, while documentation procedures maintain records of all activities, test results, and corrective actions. Regular audits and certification processes verify continued compliance with cleanroom standards and identify areas for improvement.Expand Specific Solutions
Leading Cleanroom Technology Providers and Standards
The cleanroom handling and contamination control market for high-reliability builds is currently in a mature growth phase, with increasing demand driven by semiconductor, pharmaceutical, and advanced manufacturing sectors. The global market size is estimated to exceed $4 billion, growing at 5-7% annually as industries prioritize contamination-free environments. Leading players demonstrate varying levels of technical maturity: Taiwan Semiconductor Manufacturing and IBM represent advanced semiconductor cleanroom expertise; Veltek Associates and Ecolab USA specialize in contamination control solutions; while G-CON Manufacturing offers innovative prefabricated cleanroom units. Emerging players like Dycem Ltd. focus on specialized contamination control products, while established industrial giants such as DuPont and Festo provide comprehensive cleanroom infrastructure solutions. The ecosystem shows a balance between specialized niche providers and integrated solution companies across multiple geographic regions.
DuPont de Nemours, Inc.
Technical Solution: DuPont has developed the Tyvek® Clean Processing methodology specifically for high-reliability cleanroom applications. This comprehensive approach includes specialized single-use garments and material handling protocols designed to minimize particle generation and cross-contamination. Their system incorporates advanced material science principles with cleanroom-compatible polymers that resist particle shedding and chemical degradation. DuPont's contamination control methodology includes specialized cleaning validation protocols using fluorescent detection systems to verify surface cleanliness to sub-micron levels. They've also pioneered cleanroom-compatible packaging systems that maintain material integrity from production through final assembly. DuPont's approach integrates material science with procedural controls, creating a holistic contamination management system that addresses both particulate and molecular contamination vectors through specialized material selection and handling procedures.
Strengths: Their materials science expertise allows for specialized contamination control solutions tailored to specific manufacturing challenges. The single-use garment systems eliminate cross-contamination risks associated with reusable systems. Weaknesses: Disposable systems create higher ongoing operational costs and environmental waste management challenges compared to reusable alternatives.
Veltek Associates, Inc.
Technical Solution: Veltek Associates has developed the Core2Clean™ contamination control methodology specifically for high-reliability manufacturing environments. Their system integrates specialized cleaning chemistries with validated application protocols to achieve reproducible contamination control results. The Core2Clean™ system includes sterile, pre-saturated wipes and mops with documented endotoxin and particulate specifications, ensuring consistent cleaning performance. Veltek's approach incorporates environmental monitoring systems that integrate with their cleaning protocols, creating a closed-loop validation system. Their contamination control methodology includes specialized transfer systems for introducing materials into cleanroom environments while minimizing contamination risks. Additionally, Veltek has developed specialized documentation and training systems that ensure procedural compliance across manufacturing operations, with electronic record-keeping that facilitates contamination event investigation and resolution.
Strengths: Their specialized cleaning chemistries are formulated specifically for cleanroom applications with minimal residue and rapid action against microbial contaminants. The integrated monitoring and documentation systems provide comprehensive contamination control records for regulatory compliance. Weaknesses: The system requires significant staff training and ongoing validation to maintain effectiveness, creating potential implementation challenges in high-turnover environments.
Critical Contamination Control Methodologies
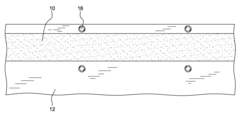
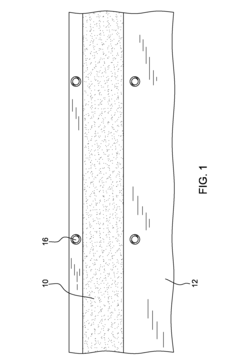
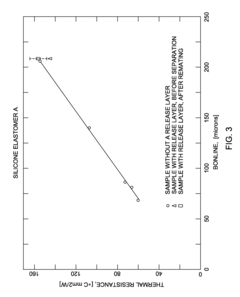
Precast thermal interface adhesive for easy and repeated, separation and remating
PatentActiveUS8268389B2
Innovation
- A precast curable TIM adhesive is applied to a heat spreader or heat sink surface, mated with a dummy substrate coated with a release layer, allowing for controlled bonding and easy separation and remating without solvent cleaning or mechanical wiping, using external spacers or particles to achieve target bondlines.
Thermal interface material
PatentWO2013140295A2
Innovation
- A TIM with an activable shrinkage material that increases in thickness upon activation, providing controllable physical dimensions and enhancing contact pressure between heat generating and conducting components, thereby eliminating the need for external pressure and improving robustness against surface curvature and roughness.
Risk Assessment and Mitigation Strategies
In the cleanroom environment for Thermal Interface Materials (TIM) handling, comprehensive risk assessment and mitigation strategies are essential to maintain contamination control standards. The primary risks include particulate contamination from personnel, equipment failures, improper material handling, and environmental fluctuations. Each risk factor must be systematically evaluated using Failure Mode and Effects Analysis (FMEA) methodology to determine severity, occurrence probability, and detection capability.
Personnel-related risks represent the most significant contamination source, with human operators introducing particles through movement, improper gowning, or inadequate training. Mitigation requires implementing strict gowning protocols, regular training programs, and minimizing unnecessary personnel movement within critical zones. Equipment-related risks include mechanical failures, inadequate maintenance, and improper calibration of critical systems such as HEPA filters and air handling units. These risks necessitate preventive maintenance schedules, redundant systems for critical functions, and real-time monitoring technologies.
Material handling risks encompass improper storage, transportation, and application of TIMs, potentially introducing contaminants that compromise thermal performance and reliability. Mitigation strategies include dedicated storage areas with controlled access, standardized transfer protocols, and specialized handling tools designed to minimize direct contact. Environmental control risks involve fluctuations in temperature, humidity, and pressure differentials that can compromise cleanroom integrity. These require continuous environmental monitoring systems with alert thresholds and automated response mechanisms.
Cross-contamination between different manufacturing processes represents another significant risk category, particularly when multiple TIM applications occur within the same facility. Implementing process segregation, dedicated equipment for specific materials, and unidirectional workflow patterns effectively mitigates these risks. Additionally, establishing contamination response protocols for incidents ensures rapid containment and remediation when breaches occur.
Risk prioritization matrices should be developed based on potential impact on product reliability, with highest priority given to risks affecting critical thermal interfaces in high-reliability applications. Each identified risk requires documented mitigation procedures, responsible parties, verification methods, and contingency plans. Regular risk reassessment cycles, typically quarterly, ensure that mitigation strategies evolve with changing production requirements and technological advancements.
Implementing a closed-loop feedback system connecting quality control data with risk management processes enables continuous improvement of mitigation strategies. This approach allows for data-driven refinement of SOPs based on actual contamination events and near-misses, creating an adaptive risk management framework that enhances long-term reliability of TIM applications in high-performance electronic assemblies.
Personnel-related risks represent the most significant contamination source, with human operators introducing particles through movement, improper gowning, or inadequate training. Mitigation requires implementing strict gowning protocols, regular training programs, and minimizing unnecessary personnel movement within critical zones. Equipment-related risks include mechanical failures, inadequate maintenance, and improper calibration of critical systems such as HEPA filters and air handling units. These risks necessitate preventive maintenance schedules, redundant systems for critical functions, and real-time monitoring technologies.
Material handling risks encompass improper storage, transportation, and application of TIMs, potentially introducing contaminants that compromise thermal performance and reliability. Mitigation strategies include dedicated storage areas with controlled access, standardized transfer protocols, and specialized handling tools designed to minimize direct contact. Environmental control risks involve fluctuations in temperature, humidity, and pressure differentials that can compromise cleanroom integrity. These require continuous environmental monitoring systems with alert thresholds and automated response mechanisms.
Cross-contamination between different manufacturing processes represents another significant risk category, particularly when multiple TIM applications occur within the same facility. Implementing process segregation, dedicated equipment for specific materials, and unidirectional workflow patterns effectively mitigates these risks. Additionally, establishing contamination response protocols for incidents ensures rapid containment and remediation when breaches occur.
Risk prioritization matrices should be developed based on potential impact on product reliability, with highest priority given to risks affecting critical thermal interfaces in high-reliability applications. Each identified risk requires documented mitigation procedures, responsible parties, verification methods, and contingency plans. Regular risk reassessment cycles, typically quarterly, ensure that mitigation strategies evolve with changing production requirements and technological advancements.
Implementing a closed-loop feedback system connecting quality control data with risk management processes enables continuous improvement of mitigation strategies. This approach allows for data-driven refinement of SOPs based on actual contamination events and near-misses, creating an adaptive risk management framework that enhances long-term reliability of TIM applications in high-performance electronic assemblies.
Cleanroom Certification and Compliance Requirements
Cleanroom certification and compliance requirements form the foundation of any effective contamination control strategy for Thermal Interface Materials (TIM) handling. ISO 14644 standards serve as the primary framework for cleanroom classification, with most high-reliability TIM applications requiring ISO Class 5 (formerly Class 100) to ISO Class 7 (formerly Class 10,000) environments. These classifications dictate the maximum allowable particle concentrations per cubic meter of air, with stringent limits on particles sized 0.5μm and larger.
Federal Standard 209E, though officially canceled in 2001, continues to influence industry terminology and practices, particularly in legacy facilities. For medical and aerospace applications involving TIMs, additional compliance with standards such as EU GMP Annex 1 or NASA-STD-8739.6 may be necessary, imposing supplementary requirements beyond basic ISO classifications.
Certification processes involve comprehensive testing protocols including particle counting, airflow velocity measurements, room pressurization verification, and microbial sampling. These tests must be conducted at regular intervals—typically semi-annually or annually—by qualified third-party certifiers. Documentation of these certifications must be maintained for regulatory inspections and quality assurance audits.
Environmental monitoring represents a critical ongoing requirement beyond initial certification. Continuous particle monitoring systems provide real-time data on cleanroom conditions, while settle plates and contact sampling detect microbial contamination that could compromise TIM performance. Temperature and humidity controls must maintain conditions within ±2°C and ±5% relative humidity of specified parameters to ensure TIM material stability.
Personnel qualification requirements mandate specialized training for all cleanroom staff handling TIMs. This includes gowning procedures, contamination awareness, material handling protocols, and emergency response procedures. Certification of personnel through standardized training programs with periodic requalification is essential for maintaining compliance.
Equipment validation protocols require all tools and machinery used in TIM handling to undergo Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ) processes. These validations must be documented and periodically reviewed to ensure continued compliance with cleanroom standards.
Regulatory oversight varies by industry, with FDA oversight for medical applications, NADCAP requirements for aerospace, and industry-specific standards for semiconductor manufacturing. Non-compliance can result in product recalls, regulatory penalties, and significant reputational damage, making rigorous adherence to certification requirements a business imperative rather than merely a technical consideration.
Federal Standard 209E, though officially canceled in 2001, continues to influence industry terminology and practices, particularly in legacy facilities. For medical and aerospace applications involving TIMs, additional compliance with standards such as EU GMP Annex 1 or NASA-STD-8739.6 may be necessary, imposing supplementary requirements beyond basic ISO classifications.
Certification processes involve comprehensive testing protocols including particle counting, airflow velocity measurements, room pressurization verification, and microbial sampling. These tests must be conducted at regular intervals—typically semi-annually or annually—by qualified third-party certifiers. Documentation of these certifications must be maintained for regulatory inspections and quality assurance audits.
Environmental monitoring represents a critical ongoing requirement beyond initial certification. Continuous particle monitoring systems provide real-time data on cleanroom conditions, while settle plates and contact sampling detect microbial contamination that could compromise TIM performance. Temperature and humidity controls must maintain conditions within ±2°C and ±5% relative humidity of specified parameters to ensure TIM material stability.
Personnel qualification requirements mandate specialized training for all cleanroom staff handling TIMs. This includes gowning procedures, contamination awareness, material handling protocols, and emergency response procedures. Certification of personnel through standardized training programs with periodic requalification is essential for maintaining compliance.
Equipment validation protocols require all tools and machinery used in TIM handling to undergo Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ) processes. These validations must be documented and periodically reviewed to ensure continued compliance with cleanroom standards.
Regulatory oversight varies by industry, with FDA oversight for medical applications, NADCAP requirements for aerospace, and industry-specific standards for semiconductor manufacturing. Non-compliance can result in product recalls, regulatory penalties, and significant reputational damage, making rigorous adherence to certification requirements a business imperative rather than merely a technical consideration.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!