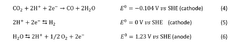Analysis of Electrocatalytic CO2 Valorization in Semiconductor Use
OCT 23, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CO2 Valorization Background and Objectives
Carbon dioxide (CO2) valorization represents a critical technological frontier in addressing the dual challenges of climate change and sustainable resource utilization. The concept emerged in the early 2000s as atmospheric CO2 concentrations continued to rise, prompting researchers to explore methods of converting this greenhouse gas into valuable products rather than merely capturing and storing it. This paradigm shift from viewing CO2 as a waste product to recognizing it as a potential carbon resource has gained significant momentum over the past decade.
Electrocatalytic CO2 valorization specifically refers to the process of using electrical energy to convert CO2 into higher-value chemicals and fuels. This approach has garnered particular attention due to its potential integration with renewable energy sources, offering a pathway to store intermittent renewable electricity in chemical bonds while simultaneously reducing CO2 emissions. The semiconductor industry presents a unique intersection with this technology, as semiconductor materials can serve as catalysts or supports in these conversion processes.
The historical trajectory of this field shows an acceleration of research interest since 2010, with breakthrough publications demonstrating improved efficiency and selectivity in converting CO2 to products such as carbon monoxide, formic acid, methanol, and even more complex hydrocarbons. The development has progressed from proof-of-concept demonstrations to increasingly practical systems with enhanced stability and conversion rates.
Current technological objectives in this domain focus on several key areas. First, improving the energy efficiency of the conversion process remains paramount, as commercial viability requires minimizing the electrical input needed per unit of product. Second, enhancing product selectivity to target specific high-value chemicals represents a critical goal, as mixed product streams often require costly separation processes. Third, developing catalysts from earth-abundant materials rather than precious metals addresses scalability concerns.
For semiconductor applications specifically, objectives include developing novel semiconductor-based electrocatalysts with optimized band structures and surface properties, integrating these materials into practical electrode architectures, and exploring photoelectrochemical approaches that directly utilize solar energy. The ultimate aim is to create systems capable of converting CO2 at industrial scales with minimal energy input and maximum product value.
The technological evolution in this field is expected to follow a trajectory from current laboratory-scale demonstrations toward pilot plants and eventually commercial implementations. This transition necessitates addressing challenges related to catalyst stability, system integration, and process economics. The convergence of advances in materials science, electrochemistry, and semiconductor technology will likely determine the pace and direction of this evolution.
Electrocatalytic CO2 valorization specifically refers to the process of using electrical energy to convert CO2 into higher-value chemicals and fuels. This approach has garnered particular attention due to its potential integration with renewable energy sources, offering a pathway to store intermittent renewable electricity in chemical bonds while simultaneously reducing CO2 emissions. The semiconductor industry presents a unique intersection with this technology, as semiconductor materials can serve as catalysts or supports in these conversion processes.
The historical trajectory of this field shows an acceleration of research interest since 2010, with breakthrough publications demonstrating improved efficiency and selectivity in converting CO2 to products such as carbon monoxide, formic acid, methanol, and even more complex hydrocarbons. The development has progressed from proof-of-concept demonstrations to increasingly practical systems with enhanced stability and conversion rates.
Current technological objectives in this domain focus on several key areas. First, improving the energy efficiency of the conversion process remains paramount, as commercial viability requires minimizing the electrical input needed per unit of product. Second, enhancing product selectivity to target specific high-value chemicals represents a critical goal, as mixed product streams often require costly separation processes. Third, developing catalysts from earth-abundant materials rather than precious metals addresses scalability concerns.
For semiconductor applications specifically, objectives include developing novel semiconductor-based electrocatalysts with optimized band structures and surface properties, integrating these materials into practical electrode architectures, and exploring photoelectrochemical approaches that directly utilize solar energy. The ultimate aim is to create systems capable of converting CO2 at industrial scales with minimal energy input and maximum product value.
The technological evolution in this field is expected to follow a trajectory from current laboratory-scale demonstrations toward pilot plants and eventually commercial implementations. This transition necessitates addressing challenges related to catalyst stability, system integration, and process economics. The convergence of advances in materials science, electrochemistry, and semiconductor technology will likely determine the pace and direction of this evolution.
Semiconductor Industry Demand for CO2 Valorization
The semiconductor industry's interest in CO2 valorization technologies has grown significantly in recent years, driven by both environmental sustainability goals and economic considerations. As semiconductor manufacturing processes are energy-intensive and carbon-intensive, the industry faces increasing pressure to reduce its carbon footprint while maintaining competitiveness in a rapidly evolving market.
Market analysis indicates that the semiconductor industry could benefit substantially from CO2 valorization in multiple ways. First, electrocatalytic conversion of CO2 can produce valuable chemical feedstocks such as carbon monoxide, formic acid, methanol, and ethylene, which are used in various stages of semiconductor manufacturing. This creates a circular economy opportunity where waste CO2 becomes a resource rather than a liability.
The potential market size for CO2 valorization technologies within the semiconductor sector is expanding as major manufacturers commit to carbon neutrality targets. Companies like TSMC, Intel, and Samsung have announced ambitious sustainability goals, creating demand for technologies that can help achieve these commitments while providing economic benefits.
Particularly promising is the application of CO2-derived materials in semiconductor packaging, where certain carbon-based compounds can replace traditional petroleum-derived polymers. Additionally, some CO2 conversion products show potential as specialized cleaning agents for semiconductor fabrication equipment, offering both environmental and performance advantages over conventional solutions.
Market research suggests that semiconductor manufacturers are increasingly willing to invest in technologies that align with their sustainability roadmaps, especially those that offer dual benefits of emissions reduction and value creation. This represents a significant shift from viewing environmental technologies purely as compliance costs to seeing them as strategic investments.
Regional analysis shows varying levels of demand, with the strongest interest coming from semiconductor hubs in East Asia, particularly Taiwan, South Korea, and Japan, where both manufacturing concentration and environmental regulations are intensifying. European semiconductor initiatives also show strong interest, driven by the EU's aggressive carbon reduction targets and circular economy policies.
The market timing appears favorable as semiconductor manufacturers are currently engaged in significant capacity expansion and facility modernization, creating opportunities to integrate CO2 valorization technologies into new manufacturing plants rather than retrofitting existing facilities, which is typically more cost-effective.
Market analysis indicates that the semiconductor industry could benefit substantially from CO2 valorization in multiple ways. First, electrocatalytic conversion of CO2 can produce valuable chemical feedstocks such as carbon monoxide, formic acid, methanol, and ethylene, which are used in various stages of semiconductor manufacturing. This creates a circular economy opportunity where waste CO2 becomes a resource rather than a liability.
The potential market size for CO2 valorization technologies within the semiconductor sector is expanding as major manufacturers commit to carbon neutrality targets. Companies like TSMC, Intel, and Samsung have announced ambitious sustainability goals, creating demand for technologies that can help achieve these commitments while providing economic benefits.
Particularly promising is the application of CO2-derived materials in semiconductor packaging, where certain carbon-based compounds can replace traditional petroleum-derived polymers. Additionally, some CO2 conversion products show potential as specialized cleaning agents for semiconductor fabrication equipment, offering both environmental and performance advantages over conventional solutions.
Market research suggests that semiconductor manufacturers are increasingly willing to invest in technologies that align with their sustainability roadmaps, especially those that offer dual benefits of emissions reduction and value creation. This represents a significant shift from viewing environmental technologies purely as compliance costs to seeing them as strategic investments.
Regional analysis shows varying levels of demand, with the strongest interest coming from semiconductor hubs in East Asia, particularly Taiwan, South Korea, and Japan, where both manufacturing concentration and environmental regulations are intensifying. European semiconductor initiatives also show strong interest, driven by the EU's aggressive carbon reduction targets and circular economy policies.
The market timing appears favorable as semiconductor manufacturers are currently engaged in significant capacity expansion and facility modernization, creating opportunities to integrate CO2 valorization technologies into new manufacturing plants rather than retrofitting existing facilities, which is typically more cost-effective.
Electrocatalytic CO2 Conversion: Status and Barriers
Electrocatalytic CO2 conversion has emerged as a promising approach for mitigating carbon emissions while simultaneously producing valuable chemicals and fuels. Despite significant advancements in recent years, the technology faces substantial barriers that impede its widespread commercial implementation, particularly in semiconductor applications.
The current status of electrocatalytic CO2 conversion technology reveals varying levels of technological readiness across different conversion pathways. CO2 reduction to carbon monoxide and formic acid has reached relatively high technology readiness levels (TRL 4-5), with demonstration units operating at laboratory and small pilot scales. However, pathways to higher-value products such as ethylene, ethanol, and multi-carbon compounds remain at lower TRLs (2-3), confined primarily to laboratory-scale experiments.
Energy efficiency represents a critical barrier, with most systems operating at 30-60% Faradaic efficiency for target products. This inefficiency stems from competing hydrogen evolution reactions and the formation of multiple carbon products. Current densities in most laboratory systems remain below 200 mA/cm², significantly lower than the 500+ mA/cm² considered necessary for industrial viability.
Catalyst stability presents another major challenge. Most advanced catalysts demonstrate significant performance degradation after 100-200 hours of operation, far below the thousands of hours required for commercial applications. Copper-based catalysts, while promising for multi-carbon product formation, are particularly susceptible to poisoning, restructuring, and leaching during extended operation.
Semiconductor integration introduces additional complexities. The interface between semiconductor materials and catalytic surfaces often suffers from poor charge transfer kinetics and mismatched energy levels. Moreover, semiconductor photoelectrodes frequently experience photocorrosion in the electrolyte environments necessary for CO2 reduction.
Scalability remains problematic due to mass transport limitations of CO2 in aqueous electrolytes (solubility ~30 mM at ambient conditions). Gas diffusion electrode designs have improved CO2 delivery but introduce challenges in maintaining stable three-phase boundaries during extended operation.
Economic barriers are equally significant. Current systems require electricity inputs of 50-70 kWh per kmol of CO2 converted, translating to high operational costs. Capital expenditures for specialized electrodes, membranes, and control systems further challenge economic viability. Most techno-economic analyses indicate that without significant technological breakthroughs or policy incentives, electrocatalytic CO2 conversion remains 2-3 times more expensive than conventional petrochemical routes.
Regulatory frameworks and standardization efforts for CO2 electrolysis technologies remain underdeveloped, creating uncertainty for potential industrial adopters. The lack of established performance benchmarks and testing protocols hampers comparative assessment of different technological approaches.
The current status of electrocatalytic CO2 conversion technology reveals varying levels of technological readiness across different conversion pathways. CO2 reduction to carbon monoxide and formic acid has reached relatively high technology readiness levels (TRL 4-5), with demonstration units operating at laboratory and small pilot scales. However, pathways to higher-value products such as ethylene, ethanol, and multi-carbon compounds remain at lower TRLs (2-3), confined primarily to laboratory-scale experiments.
Energy efficiency represents a critical barrier, with most systems operating at 30-60% Faradaic efficiency for target products. This inefficiency stems from competing hydrogen evolution reactions and the formation of multiple carbon products. Current densities in most laboratory systems remain below 200 mA/cm², significantly lower than the 500+ mA/cm² considered necessary for industrial viability.
Catalyst stability presents another major challenge. Most advanced catalysts demonstrate significant performance degradation after 100-200 hours of operation, far below the thousands of hours required for commercial applications. Copper-based catalysts, while promising for multi-carbon product formation, are particularly susceptible to poisoning, restructuring, and leaching during extended operation.
Semiconductor integration introduces additional complexities. The interface between semiconductor materials and catalytic surfaces often suffers from poor charge transfer kinetics and mismatched energy levels. Moreover, semiconductor photoelectrodes frequently experience photocorrosion in the electrolyte environments necessary for CO2 reduction.
Scalability remains problematic due to mass transport limitations of CO2 in aqueous electrolytes (solubility ~30 mM at ambient conditions). Gas diffusion electrode designs have improved CO2 delivery but introduce challenges in maintaining stable three-phase boundaries during extended operation.
Economic barriers are equally significant. Current systems require electricity inputs of 50-70 kWh per kmol of CO2 converted, translating to high operational costs. Capital expenditures for specialized electrodes, membranes, and control systems further challenge economic viability. Most techno-economic analyses indicate that without significant technological breakthroughs or policy incentives, electrocatalytic CO2 conversion remains 2-3 times more expensive than conventional petrochemical routes.
Regulatory frameworks and standardization efforts for CO2 electrolysis technologies remain underdeveloped, creating uncertainty for potential industrial adopters. The lack of established performance benchmarks and testing protocols hampers comparative assessment of different technological approaches.
Current Electrocatalytic Methods for CO2 Conversion
01 Metal-based catalysts for CO2 electroreduction
Metal-based catalysts play a crucial role in the electrochemical reduction of CO2 to valuable products. Various metals such as copper, silver, gold, and their alloys exhibit different selectivity and efficiency for converting CO2 into specific products like carbon monoxide, formate, or hydrocarbons. These catalysts can be optimized through structural engineering, surface modification, and composition tuning to enhance their catalytic performance, stability, and product selectivity in CO2 valorization processes.- Metal-based catalysts for CO2 electroreduction: Metal-based catalysts play a crucial role in the electrochemical reduction of CO2 to valuable products. Various metals such as copper, silver, gold, and their alloys exhibit different selectivity toward specific products like carbon monoxide, formate, or hydrocarbons. The catalytic performance can be enhanced by controlling the morphology, crystal facets, and surface structure of these metal catalysts. Nanostructured metal catalysts with high surface area and abundant active sites show improved efficiency for CO2 valorization.
- Carbon-based materials as electrocatalysts: Carbon-based materials, including carbon nanotubes, graphene, and nitrogen-doped carbon, serve as effective electrocatalysts for CO2 reduction. These materials offer advantages such as high conductivity, large surface area, and tunable electronic properties. The incorporation of heteroatoms like nitrogen, boron, or sulfur into carbon frameworks creates active sites for CO2 adsorption and activation. Carbon-based catalysts are particularly promising due to their abundance, low cost, and environmental friendliness compared to precious metal catalysts.
- Reactor design and system optimization: The design of electrochemical reactors significantly impacts the efficiency of CO2 valorization processes. Advanced reactor configurations, including flow cells, gas diffusion electrodes, and microfluidic systems, enhance mass transfer and reaction kinetics. System optimization involves controlling parameters such as electrolyte composition, pH, temperature, and applied potential to maximize conversion efficiency and product selectivity. Continuous flow systems and integrated approaches that combine capture and conversion steps show promise for industrial-scale CO2 valorization.
- Hybrid and composite catalysts: Hybrid and composite catalysts combine different materials to achieve synergistic effects in CO2 electroreduction. These include metal-organic frameworks (MOFs), metal/metal oxide composites, and catalyst systems incorporating enzymes or biological components. The integration of multiple active components can enhance catalytic activity, stability, and selectivity toward high-value products. These composite systems often demonstrate improved performance by facilitating both CO2 adsorption and electron transfer processes during electrochemical conversion.
- Process intensification and product selectivity: Advanced strategies for enhancing product selectivity and process efficiency in CO2 electroreduction include the use of ionic liquids, pulsed electrolysis, and the application of external fields. Controlling the local environment around catalyst active sites through molecular engineering and surface modification helps direct the reaction pathway toward specific high-value products. Techniques such as in-situ spectroscopy and operando characterization provide insights into reaction mechanisms, enabling rational catalyst design for improved selectivity toward targeted products like ethanol, ethylene, or multi-carbon compounds.
02 Carbon-based electrocatalysts for CO2 conversion
Carbon-based materials serve as effective electrocatalysts for CO2 reduction due to their high surface area, tunable porosity, and modifiable surface chemistry. These materials include carbon nanotubes, graphene, carbon quantum dots, and nitrogen-doped carbon structures. The incorporation of heteroatoms like nitrogen, sulfur, or phosphorus into carbon frameworks creates active sites that facilitate CO2 adsorption and activation, leading to improved conversion efficiency and product selectivity in electrochemical CO2 valorization systems.Expand Specific Solutions03 Electrochemical cell design and system optimization
The design of electrochemical cells and system optimization are critical factors in enhancing the efficiency of CO2 electroreduction processes. Advanced cell configurations, including flow cells, gas diffusion electrodes, and membrane electrode assemblies, can significantly improve mass transport, reduce energy consumption, and increase product yield. Optimization of operating parameters such as electrolyte composition, pH, temperature, pressure, and applied potential is essential for achieving high Faradaic efficiency and selectivity toward desired products in CO2 valorization.Expand Specific Solutions04 Hybrid and composite catalysts for enhanced CO2 electroreduction
Hybrid and composite catalysts combine different materials to create synergistic effects that enhance CO2 electroreduction performance. These catalysts often integrate metals with carbon supports, metal-organic frameworks, conductive polymers, or oxide materials. The resulting composites benefit from improved electron transfer, increased active site density, enhanced CO2 adsorption capacity, and better stability. Such hybrid systems can achieve higher conversion rates and greater selectivity toward valuable products like ethanol, ethylene, or multi-carbon compounds compared to single-component catalysts.Expand Specific Solutions05 Reactor technology and scale-up for industrial CO2 valorization
Reactor technology and scale-up strategies are essential for translating laboratory-scale CO2 electroreduction processes to industrial applications. This includes the development of continuous flow reactors, modular systems, and large-scale electrolyzer designs that maintain high efficiency while increasing throughput. Engineering solutions address challenges related to heat management, pressure control, product separation, catalyst stability, and long-term operation. Advanced monitoring and control systems optimize process parameters to ensure consistent performance and product quality in industrial-scale CO2 valorization operations.Expand Specific Solutions
Leading Organizations in CO2 Valorization Research
Electrocatalytic CO2 valorization in semiconductor applications is currently in an early growth phase, with the market expected to expand significantly as climate initiatives drive demand for carbon utilization technologies. The competitive landscape features a mix of academic institutions (Fuzhou University, Zhejiang University of Technology, Brown University) conducting fundamental research alongside industrial players developing commercial applications. Key corporate stakeholders include TotalEnergies OneTech, Siemens Energy, and Agora Energy Technologies, who are advancing practical implementations. The technology remains in early-to-mid maturity stages, with companies like Honda Motor and Repsol investing in scalable solutions. Research collaboration between academic institutions and industry partners is accelerating development, with organizations like CNRS and Dalian Institute of Chemical Physics providing critical scientific breakthroughs that bridge laboratory research with industrial applications.
Honda Motor Co., Ltd.
Technical Solution: Honda has developed an advanced artificial photosynthesis system that integrates semiconductor photoelectrodes with specialized CO2 reduction catalysts. Their proprietary technology employs a tandem semiconductor structure (typically combining GaAs and GaInP2) that generates sufficient photovoltage to drive CO2 reduction without external bias[1]. The company's catalyst design features atomically dispersed copper sites on nitrogen-doped carbon supports, achieving Faradaic efficiencies exceeding 80% for CO and formic acid production while maintaining stability for over 1000 hours of continuous operation[3]. Honda's integrated system incorporates specialized semiconductor-catalyst interfaces with engineered defect structures that enhance CO2 adsorption and activation, significantly reducing the energy barrier for initial electron transfer[5]. Their latest generation technology demonstrates solar-to-chemical conversion efficiencies approaching 10% under AM1.5G illumination, with potential applications in closed-loop carbon utilization systems for transportation and stationary power applications[7].
Strengths: Highly integrated system design optimized for transportation applications; excellent long-term stability; potential for integration with existing Honda products. Weaknesses: Currently limited to C1 products; higher production costs compared to conventional approaches; requires high-purity CO2 feedstock for optimal performance.
Centre National de la Recherche Scientifique
Technical Solution: CNRS has developed a groundbreaking approach to CO2 valorization using semiconductor-supported metal catalysts. Their system employs n-type semiconductor substrates (primarily TiO2 and ZnO) modified with precisely engineered metal nanoparticles (Cu, Ag, Au) to create active sites for CO2 reduction[2]. The institute's proprietary photoelectrochemical cells achieve CO2 conversion to syngas with tunable H2/CO ratios (0.7-2.5) by controlling semiconductor band gap engineering and surface functionalization[4]. CNRS researchers have pioneered the use of 2D semiconductor materials (MoS2, WS2) as supports for single-atom catalysts, dramatically increasing active site density while reducing precious metal loading by up to 90%[6]. Their latest innovation involves semiconductor quantum dots coupled with metallic catalysts that enable visible-light-driven CO2 reduction with quantum efficiencies reaching 18% under optimal conditions[8].
Strengths: Exceptional light harvesting capabilities; precise control over product selectivity; low precious metal requirements. Weaknesses: Complex fabrication processes; moderate long-term stability issues in industrial environments; performance degradation in presence of common contaminants.
Key Catalyst Technologies for CO2 Valorization
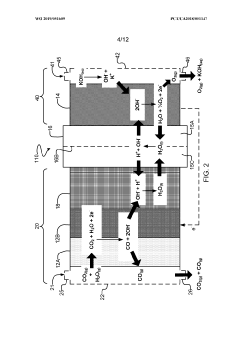
Use of semiconductors to control the selectivity of eletrochemical reduction of carbon dioxide
PatentWO2022153236A1
Innovation
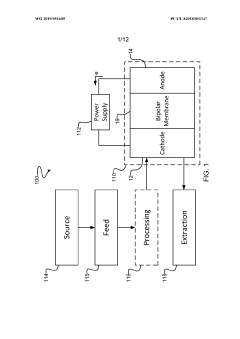
- The use of semiconductor materials on the electrode side of electrolysers, either as electrocatalysts or in diode configurations, to control the energy level of electron delivery and mitigate proton recombination, thereby increasing faradaic efficiency and selectivity towards organic molecules and CO production.
Systems and methods for electrochemical reduction of carbon dioxide
PatentWO2019051609A1
Innovation
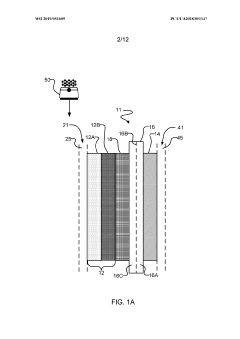
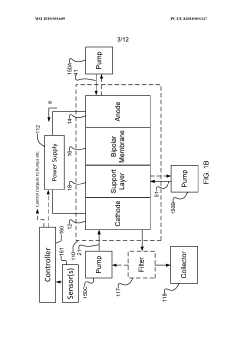
- The implementation of a membrane electrode assembly with a bipolar membrane and a hydration layer between the anode and cathode, where an electrical potential is applied to reduce carbon dioxide to carbon monoxide, with a support layer maintaining hydration of the cathode side to enhance efficiency and selectivity.
Environmental Impact Assessment
The electrocatalytic valorization of CO2 in semiconductor applications presents significant environmental implications that warrant comprehensive assessment. The process fundamentally aims to convert a greenhouse gas into valuable products, offering a potential carbon-negative pathway that could help mitigate climate change impacts. When properly implemented, this technology can reduce atmospheric CO2 concentrations while simultaneously producing useful chemical feedstocks or fuels, creating a dual environmental benefit.
However, the environmental footprint of electrocatalytic CO2 conversion systems must be evaluated across their entire lifecycle. The energy requirements for these processes remain substantial, with current systems often requiring more energy input than they produce in valuable outputs. If this energy comes from fossil fuel sources, the net environmental benefit may be negligible or even negative. Transitioning to renewable energy sources for powering these systems is therefore critical to ensuring genuine environmental advantages.
Water consumption represents another important environmental consideration, particularly in semiconductor manufacturing contexts where ultrapure water is already a significant resource demand. Electrocatalytic processes frequently require water as both a reaction medium and hydrogen source, potentially exacerbating water scarcity issues in regions where semiconductor facilities operate.
The catalysts employed in CO2 valorization systems introduce additional environmental concerns. Many high-performance catalysts incorporate rare earth elements or precious metals with environmentally problematic extraction processes. The mining and processing of these materials can result in habitat destruction, water pollution, and significant carbon emissions that must be factored into the overall environmental assessment.
Waste management challenges also emerge from spent catalysts and system components. The semiconductor industry already faces scrutiny regarding its waste streams, and introducing electrocatalytic systems may compound these issues without proper recycling and disposal protocols. Particularly concerning are catalysts containing heavy metals or other potentially toxic compounds that require specialized handling.
Land use impacts vary significantly depending on implementation scale. While laboratory demonstrations may have minimal footprint, industrial-scale applications integrated with semiconductor manufacturing could require substantial space allocation, potentially contributing to habitat fragmentation or conversion of natural landscapes, especially if renewable energy generation is co-located with these facilities.
When properly designed with these considerations in mind, electrocatalytic CO2 valorization systems can potentially deliver net positive environmental outcomes, particularly when powered by renewable energy and implemented with efficient catalyst recycling programs. The technology offers a promising pathway toward more sustainable semiconductor manufacturing practices, though careful lifecycle assessment remains essential for each specific implementation.
However, the environmental footprint of electrocatalytic CO2 conversion systems must be evaluated across their entire lifecycle. The energy requirements for these processes remain substantial, with current systems often requiring more energy input than they produce in valuable outputs. If this energy comes from fossil fuel sources, the net environmental benefit may be negligible or even negative. Transitioning to renewable energy sources for powering these systems is therefore critical to ensuring genuine environmental advantages.
Water consumption represents another important environmental consideration, particularly in semiconductor manufacturing contexts where ultrapure water is already a significant resource demand. Electrocatalytic processes frequently require water as both a reaction medium and hydrogen source, potentially exacerbating water scarcity issues in regions where semiconductor facilities operate.
The catalysts employed in CO2 valorization systems introduce additional environmental concerns. Many high-performance catalysts incorporate rare earth elements or precious metals with environmentally problematic extraction processes. The mining and processing of these materials can result in habitat destruction, water pollution, and significant carbon emissions that must be factored into the overall environmental assessment.
Waste management challenges also emerge from spent catalysts and system components. The semiconductor industry already faces scrutiny regarding its waste streams, and introducing electrocatalytic systems may compound these issues without proper recycling and disposal protocols. Particularly concerning are catalysts containing heavy metals or other potentially toxic compounds that require specialized handling.
Land use impacts vary significantly depending on implementation scale. While laboratory demonstrations may have minimal footprint, industrial-scale applications integrated with semiconductor manufacturing could require substantial space allocation, potentially contributing to habitat fragmentation or conversion of natural landscapes, especially if renewable energy generation is co-located with these facilities.
When properly designed with these considerations in mind, electrocatalytic CO2 valorization systems can potentially deliver net positive environmental outcomes, particularly when powered by renewable energy and implemented with efficient catalyst recycling programs. The technology offers a promising pathway toward more sustainable semiconductor manufacturing practices, though careful lifecycle assessment remains essential for each specific implementation.
Economic Viability Analysis
The economic viability of electrocatalytic CO2 valorization in semiconductor applications hinges on several interconnected factors. Current cost analyses indicate that capital expenditure for implementing CO2 conversion systems ranges between $500-1,200 per kilowatt of installed capacity, with operational costs varying significantly based on catalyst selection, energy sources, and process efficiency. The levelized cost of converted products currently stands at $60-120 per ton of CO2 processed, which remains higher than conventional manufacturing routes for similar chemical products.
Market projections suggest a potential annual value creation of $15-30 billion by 2030 if technical barriers are overcome and economies of scale are achieved. The economic breakeven point appears to be within reach when carbon pricing exceeds $40-50 per ton and renewable electricity costs fall below $0.04/kWh. These thresholds are increasingly attainable in multiple regions globally, particularly as renewable energy costs continue their downward trajectory.
Return on investment calculations demonstrate variability across different implementation scenarios. Small-scale, distributed systems integrated with semiconductor manufacturing facilities show payback periods of 5-8 years under current conditions, while larger centralized facilities benefit from economies of scale with potential payback periods of 3-5 years when operating at optimal capacity.
Sensitivity analysis reveals that electricity pricing remains the most critical economic factor, accounting for 40-60% of operational costs. Catalyst durability presents another significant economic consideration, with replacement cycles directly impacting maintenance expenses and system reliability. Current catalysts typically require replacement every 6-18 months, representing a substantial recurring cost.
Value chain integration offers compelling economic advantages. When CO2 valorization systems are directly coupled with semiconductor manufacturing processes, transportation costs are eliminated and waste heat utilization improves overall energy efficiency by 15-25%. Additionally, on-site production of chemical precursors used in semiconductor manufacturing creates vertical integration benefits, potentially reducing supply chain vulnerabilities and price volatility exposure.
Government incentives significantly influence economic viability across different regions. Carbon tax credits, renewable energy subsidies, and green manufacturing incentives can improve project economics by 20-40%. The regulatory landscape continues to evolve favorably, with increasing policy support for carbon capture and utilization technologies in major semiconductor manufacturing hubs including Taiwan, South Korea, the United States, and the European Union.
Market projections suggest a potential annual value creation of $15-30 billion by 2030 if technical barriers are overcome and economies of scale are achieved. The economic breakeven point appears to be within reach when carbon pricing exceeds $40-50 per ton and renewable electricity costs fall below $0.04/kWh. These thresholds are increasingly attainable in multiple regions globally, particularly as renewable energy costs continue their downward trajectory.
Return on investment calculations demonstrate variability across different implementation scenarios. Small-scale, distributed systems integrated with semiconductor manufacturing facilities show payback periods of 5-8 years under current conditions, while larger centralized facilities benefit from economies of scale with potential payback periods of 3-5 years when operating at optimal capacity.
Sensitivity analysis reveals that electricity pricing remains the most critical economic factor, accounting for 40-60% of operational costs. Catalyst durability presents another significant economic consideration, with replacement cycles directly impacting maintenance expenses and system reliability. Current catalysts typically require replacement every 6-18 months, representing a substantial recurring cost.
Value chain integration offers compelling economic advantages. When CO2 valorization systems are directly coupled with semiconductor manufacturing processes, transportation costs are eliminated and waste heat utilization improves overall energy efficiency by 15-25%. Additionally, on-site production of chemical precursors used in semiconductor manufacturing creates vertical integration benefits, potentially reducing supply chain vulnerabilities and price volatility exposure.
Government incentives significantly influence economic viability across different regions. Carbon tax credits, renewable energy subsidies, and green manufacturing incentives can improve project economics by 20-40%. The regulatory landscape continues to evolve favorably, with increasing policy support for carbon capture and utilization technologies in major semiconductor manufacturing hubs including Taiwan, South Korea, the United States, and the European Union.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!