Electrocatalytic CO2 Valorization Using Advanced Electrolytes
OCT 23, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CO2 Valorization Background and Objectives
Carbon dioxide (CO2) emissions have become one of the most pressing environmental challenges of our time, with atmospheric CO2 concentrations reaching unprecedented levels. The concept of CO2 valorization represents a paradigm shift from viewing carbon dioxide as a waste product to recognizing it as a valuable resource and feedstock for chemical production. Electrocatalytic CO2 valorization specifically refers to the process of converting CO2 into value-added chemicals and fuels using electricity as the energy input and electrocatalysts to facilitate the reaction.
The historical development of CO2 valorization technologies dates back to the early 20th century, but significant advancements have only emerged in the past few decades. Initial approaches focused primarily on thermal catalytic processes, which required high temperatures and pressures, resulting in substantial energy consumption. The shift toward electrocatalytic methods began in earnest during the 1980s, with pioneering work demonstrating the feasibility of electrochemical CO2 reduction at ambient conditions.
Recent technological evolution has been driven by the urgent need for carbon-neutral or carbon-negative technologies to mitigate climate change. The development of advanced nanomaterials, improved understanding of reaction mechanisms, and innovations in electrochemical cell design have collectively accelerated progress in this field. Particularly noteworthy is the growing interest in advanced electrolytes, which play a crucial role in determining reaction selectivity, efficiency, and stability.
The primary objective of electrocatalytic CO2 valorization using advanced electrolytes is to develop economically viable and environmentally sustainable processes for converting CO2 into valuable products. Specific technical goals include achieving high Faradaic efficiency (>90%), substantial current density (>200 mA/cm²), excellent product selectivity, and long-term operational stability (>1000 hours) under industrially relevant conditions.
Another critical objective is to reduce the energy input required for CO2 conversion, thereby improving the overall energy efficiency of the process. This involves minimizing overpotentials through electrocatalyst design and optimizing electrolyte composition to enhance mass transport and reaction kinetics. The ultimate aim is to create systems that can operate efficiently using renewable electricity sources, forming a closed carbon cycle.
From a broader perspective, the technology seeks to establish a circular carbon economy where CO2 emissions are captured and recycled into the production chain. This approach not only addresses environmental concerns but also offers economic opportunities through the production of high-value chemicals and fuels from what is currently considered a waste product. The successful development of such technologies could revolutionize industrial chemistry and contribute significantly to global decarbonization efforts.
The historical development of CO2 valorization technologies dates back to the early 20th century, but significant advancements have only emerged in the past few decades. Initial approaches focused primarily on thermal catalytic processes, which required high temperatures and pressures, resulting in substantial energy consumption. The shift toward electrocatalytic methods began in earnest during the 1980s, with pioneering work demonstrating the feasibility of electrochemical CO2 reduction at ambient conditions.
Recent technological evolution has been driven by the urgent need for carbon-neutral or carbon-negative technologies to mitigate climate change. The development of advanced nanomaterials, improved understanding of reaction mechanisms, and innovations in electrochemical cell design have collectively accelerated progress in this field. Particularly noteworthy is the growing interest in advanced electrolytes, which play a crucial role in determining reaction selectivity, efficiency, and stability.
The primary objective of electrocatalytic CO2 valorization using advanced electrolytes is to develop economically viable and environmentally sustainable processes for converting CO2 into valuable products. Specific technical goals include achieving high Faradaic efficiency (>90%), substantial current density (>200 mA/cm²), excellent product selectivity, and long-term operational stability (>1000 hours) under industrially relevant conditions.
Another critical objective is to reduce the energy input required for CO2 conversion, thereby improving the overall energy efficiency of the process. This involves minimizing overpotentials through electrocatalyst design and optimizing electrolyte composition to enhance mass transport and reaction kinetics. The ultimate aim is to create systems that can operate efficiently using renewable electricity sources, forming a closed carbon cycle.
From a broader perspective, the technology seeks to establish a circular carbon economy where CO2 emissions are captured and recycled into the production chain. This approach not only addresses environmental concerns but also offers economic opportunities through the production of high-value chemicals and fuels from what is currently considered a waste product. The successful development of such technologies could revolutionize industrial chemistry and contribute significantly to global decarbonization efforts.
Market Analysis for CO2 Conversion Technologies
The global market for CO2 conversion technologies is experiencing significant growth, driven by increasing environmental concerns and regulatory pressures to reduce carbon emissions. The market was valued at approximately $1.8 billion in 2022 and is projected to reach $4.2 billion by 2030, growing at a CAGR of 11.2% during the forecast period. This growth trajectory reflects the urgent need for sustainable solutions to address climate change challenges.
Electrocatalytic CO2 valorization represents a promising segment within this market, offering pathways to convert carbon dioxide into valuable chemicals and fuels. The demand for such technologies is particularly strong in regions with stringent carbon reduction targets, including the European Union, North America, and increasingly in Asia-Pacific countries like China and Japan. These regions are implementing carbon pricing mechanisms and emissions trading systems that create economic incentives for CO2 utilization technologies.
Industry sectors driving demand include chemicals manufacturing, energy production, and transportation. The chemicals sector represents the largest market share at approximately 42%, followed by energy at 28% and transportation at 18%. These industries are actively seeking carbon-neutral or carbon-negative production methods to meet sustainability goals and comply with regulations.
Advanced electrolytes for CO2 conversion are gaining traction due to their potential to improve conversion efficiency and selectivity. The market for specialized electrolytes is expected to grow at a faster rate (14.5% CAGR) than the overall CO2 conversion market, indicating increasing recognition of their critical role in enhancing process performance.
Key market drivers include government incentives for carbon capture and utilization, corporate sustainability commitments, and the growing economic viability of CO2-derived products. The European Green Deal and similar initiatives worldwide are creating favorable policy environments for these technologies. Additionally, consumer preference for sustainable products is pushing companies across various sectors to invest in carbon-neutral production methods.
Market barriers include high capital costs, technological limitations in scalability, and competition from established fossil-based production routes. The levelized cost of CO2 conversion using current electrocatalytic methods remains 2-3 times higher than conventional production routes for many chemicals, presenting a significant commercialization challenge that advanced electrolytes aim to address.
Emerging market opportunities exist in the production of carbon-neutral fuels, polymer precursors, and specialty chemicals. The aviation and maritime sectors, facing particular decarbonization challenges, represent high-potential markets for electrocatalytic CO2 conversion products, especially sustainable aviation fuels and marine fuels.
Electrocatalytic CO2 valorization represents a promising segment within this market, offering pathways to convert carbon dioxide into valuable chemicals and fuels. The demand for such technologies is particularly strong in regions with stringent carbon reduction targets, including the European Union, North America, and increasingly in Asia-Pacific countries like China and Japan. These regions are implementing carbon pricing mechanisms and emissions trading systems that create economic incentives for CO2 utilization technologies.
Industry sectors driving demand include chemicals manufacturing, energy production, and transportation. The chemicals sector represents the largest market share at approximately 42%, followed by energy at 28% and transportation at 18%. These industries are actively seeking carbon-neutral or carbon-negative production methods to meet sustainability goals and comply with regulations.
Advanced electrolytes for CO2 conversion are gaining traction due to their potential to improve conversion efficiency and selectivity. The market for specialized electrolytes is expected to grow at a faster rate (14.5% CAGR) than the overall CO2 conversion market, indicating increasing recognition of their critical role in enhancing process performance.
Key market drivers include government incentives for carbon capture and utilization, corporate sustainability commitments, and the growing economic viability of CO2-derived products. The European Green Deal and similar initiatives worldwide are creating favorable policy environments for these technologies. Additionally, consumer preference for sustainable products is pushing companies across various sectors to invest in carbon-neutral production methods.
Market barriers include high capital costs, technological limitations in scalability, and competition from established fossil-based production routes. The levelized cost of CO2 conversion using current electrocatalytic methods remains 2-3 times higher than conventional production routes for many chemicals, presenting a significant commercialization challenge that advanced electrolytes aim to address.
Emerging market opportunities exist in the production of carbon-neutral fuels, polymer precursors, and specialty chemicals. The aviation and maritime sectors, facing particular decarbonization challenges, represent high-potential markets for electrocatalytic CO2 conversion products, especially sustainable aviation fuels and marine fuels.
Electrocatalytic CO2 Reduction: Status and Barriers
Electrocatalytic CO2 reduction (ECR) has emerged as a promising approach for carbon dioxide utilization, offering pathways to convert this greenhouse gas into value-added chemicals and fuels. Currently, the technology has reached laboratory demonstration scale with significant progress in catalyst development, but commercial implementation remains elusive due to several critical barriers.
The state-of-the-art ECR systems can achieve Faradaic efficiencies exceeding 90% for specific products like carbon monoxide and formate, with current densities reaching 100-200 mA/cm² in optimized systems. However, more complex C2+ products typically show lower efficiencies and require higher overpotentials, limiting overall energy efficiency. Recent advances in copper-based catalysts have improved selectivity toward ethylene and ethanol, but precise control over product distribution remains challenging.
Globally, research efforts are concentrated in North America, Europe, and East Asia, with the United States, China, and Germany leading publication output. Industrial development is primarily driven by startups and research divisions of energy companies, with limited large-scale commercial deployment to date.
Several fundamental barriers impede widespread ECR implementation. Catalyst stability represents a primary challenge, with many promising materials suffering from deactivation within hours or days of operation. The mechanisms of deactivation include poisoning, structural reconstruction, and leaching, particularly in the complex chemical environment of CO2 reduction.
Selectivity control presents another significant hurdle. Most catalysts produce a mixture of products, necessitating costly downstream separation. The reaction pathways for C2+ products remain incompletely understood, complicating rational catalyst design for specific product targeting.
Scale-up challenges constitute a third major barrier. Laboratory systems typically operate at small scales with idealized conditions, while industrial implementation requires robust operation at high current densities with practical CO2 sources. The mass transport limitations of CO2 in aqueous electrolytes severely restrict achievable reaction rates at scale.
Energy efficiency remains suboptimal, with most systems requiring significant overpotentials that reduce overall process efficiency. When considering the full system including CO2 capture and product separation, many ECR processes currently show negative energy returns.
Economic viability represents perhaps the most significant barrier to commercialization. Current ECR systems produce chemicals at costs substantially higher than conventional petrochemical routes, with estimates suggesting a 3-5x cost premium for most products. This economic gap necessitates either significant technological breakthroughs or supportive policy frameworks to enable market adoption.
The state-of-the-art ECR systems can achieve Faradaic efficiencies exceeding 90% for specific products like carbon monoxide and formate, with current densities reaching 100-200 mA/cm² in optimized systems. However, more complex C2+ products typically show lower efficiencies and require higher overpotentials, limiting overall energy efficiency. Recent advances in copper-based catalysts have improved selectivity toward ethylene and ethanol, but precise control over product distribution remains challenging.
Globally, research efforts are concentrated in North America, Europe, and East Asia, with the United States, China, and Germany leading publication output. Industrial development is primarily driven by startups and research divisions of energy companies, with limited large-scale commercial deployment to date.
Several fundamental barriers impede widespread ECR implementation. Catalyst stability represents a primary challenge, with many promising materials suffering from deactivation within hours or days of operation. The mechanisms of deactivation include poisoning, structural reconstruction, and leaching, particularly in the complex chemical environment of CO2 reduction.
Selectivity control presents another significant hurdle. Most catalysts produce a mixture of products, necessitating costly downstream separation. The reaction pathways for C2+ products remain incompletely understood, complicating rational catalyst design for specific product targeting.
Scale-up challenges constitute a third major barrier. Laboratory systems typically operate at small scales with idealized conditions, while industrial implementation requires robust operation at high current densities with practical CO2 sources. The mass transport limitations of CO2 in aqueous electrolytes severely restrict achievable reaction rates at scale.
Energy efficiency remains suboptimal, with most systems requiring significant overpotentials that reduce overall process efficiency. When considering the full system including CO2 capture and product separation, many ECR processes currently show negative energy returns.
Economic viability represents perhaps the most significant barrier to commercialization. Current ECR systems produce chemicals at costs substantially higher than conventional petrochemical routes, with estimates suggesting a 3-5x cost premium for most products. This economic gap necessitates either significant technological breakthroughs or supportive policy frameworks to enable market adoption.
Current Advanced Electrolyte Solutions
01 Advanced electrolyte compositions for enhanced CO2 reduction
Advanced electrolyte compositions play a crucial role in improving the efficiency of CO2 electroreduction. These specialized electrolytes can enhance catalytic activity, selectivity, and stability during the CO2 valorization process. By optimizing electrolyte components such as ionic liquids, buffering agents, and additives, researchers have achieved higher conversion rates and improved product selectivity. These advanced electrolytes help to overcome mass transport limitations and enhance the interaction between CO2 molecules and catalyst surfaces.- Advanced electrolyte compositions for CO2 reduction: Advanced electrolyte compositions play a crucial role in enhancing the efficiency of CO2 electroreduction. These electrolytes can include ionic liquids, deep eutectic solvents, or modified aqueous solutions with specific additives that improve CO2 solubility and mass transfer. The electrolyte composition directly affects the local environment at the electrode-electrolyte interface, influencing reaction pathways and product selectivity. Optimized electrolytes can stabilize reaction intermediates and promote desired reaction pathways, significantly improving catalytic efficiency.
- Metal-based catalysts for electrochemical CO2 conversion: Metal-based catalysts, particularly those containing transition metals like copper, silver, gold, and zinc, demonstrate promising activity for CO2 electroreduction. These catalysts can be designed with specific morphologies, such as nanoparticles, nanowires, or porous structures, to maximize active surface area and catalytic performance. The selectivity and efficiency of these catalysts can be further enhanced through alloying, surface modification, or the creation of defect sites. When combined with optimized electrolytes, these catalysts show improved faradaic efficiency and product selectivity for valuable chemicals like carbon monoxide, formate, or hydrocarbons.
- Carbon-based materials as CO2 reduction catalysts: Carbon-based materials, including graphene, carbon nanotubes, and nitrogen-doped carbon structures, serve as effective catalysts or catalyst supports for CO2 electroreduction. These materials offer advantages such as high conductivity, large surface area, and tunable surface chemistry. The introduction of heteroatoms like nitrogen, boron, or sulfur into carbon frameworks creates active sites for CO2 adsorption and activation. Carbon-based catalysts often demonstrate good stability in various electrolytes and can be produced at lower costs compared to precious metal catalysts, making them attractive for large-scale applications.
- Reactor design and system optimization for CO2 valorization: The design of electrochemical reactors significantly impacts the efficiency of CO2 conversion processes. Advanced reactor configurations, such as flow cells, gas diffusion electrodes, and microfluidic systems, can overcome mass transport limitations and improve reaction kinetics. System parameters including temperature, pressure, flow rate, and electrode spacing need to be optimized to enhance catalytic performance. Integrated systems that combine CO2 capture with electrochemical conversion offer more efficient pathways for carbon utilization. Continuous flow reactors with optimized electrolyte circulation can maintain stable operation and improve overall energy efficiency.
- Process intensification techniques for enhanced CO2 conversion: Process intensification approaches can significantly improve the efficiency of electrocatalytic CO2 reduction. These include the application of ultrasound, pulsed electrolysis, or electromagnetic fields to enhance mass transfer and reaction kinetics. Hybrid systems combining electrochemical processes with photocatalysis or thermocatalysis can leverage synergistic effects to overcome thermodynamic limitations. The integration of renewable energy sources with CO2 electroreduction systems provides a sustainable pathway for carbon utilization. Advanced control strategies and real-time monitoring techniques help maintain optimal operating conditions and maximize conversion efficiency.
02 Novel catalyst materials for electrocatalytic CO2 conversion
The development of novel catalyst materials has significantly improved the efficiency of electrocatalytic CO2 valorization. These catalysts include metal-based materials, metal oxides, carbon-based materials, and hybrid structures designed to provide optimal binding energies for CO2 activation. By engineering the catalyst surface structure, composition, and morphology, researchers have achieved higher catalytic activity, improved selectivity toward valuable products, and enhanced durability under reaction conditions. These advanced catalysts work synergistically with specialized electrolytes to maximize CO2 conversion efficiency.Expand Specific Solutions03 Electrochemical cell design and system optimization
Innovative electrochemical cell designs and system optimizations are essential for improving the efficiency of CO2 electroreduction processes. Advanced cell configurations focus on optimizing electrode arrangement, membrane selection, and flow dynamics to enhance mass transfer and reduce energy losses. System-level improvements include pressure control mechanisms, temperature regulation, and integrated product separation techniques. These design enhancements work in conjunction with advanced electrolytes and catalysts to maximize conversion efficiency, product selectivity, and overall system performance.Expand Specific Solutions04 Ionic liquid-based electrolytes for CO2 electroreduction
Ionic liquid-based electrolytes offer unique advantages for CO2 electroreduction, including high CO2 solubility, wide electrochemical windows, and tunable physicochemical properties. These specialized electrolytes can significantly enhance catalytic efficiency by facilitating CO2 activation and stabilizing reaction intermediates. By modifying the cation and anion structures of ionic liquids, researchers have developed electrolyte systems that promote selective formation of valuable products such as carbon monoxide, formic acid, and hydrocarbons. The integration of ionic liquids with conventional electrolytes creates hybrid systems with optimized performance characteristics.Expand Specific Solutions05 Process intensification and scale-up strategies
Process intensification and scale-up strategies are critical for translating laboratory-scale CO2 electroreduction technologies to industrial applications. These approaches include continuous flow processes, high-pressure operations, and integrated reactor designs that maximize space-time yield. Advanced electrolyte management systems ensure consistent performance during extended operation, while heat and mass transfer optimizations improve energy efficiency. Scale-up strategies also address challenges related to catalyst stability, product separation, and system integration, enabling the development of economically viable CO2 valorization technologies.Expand Specific Solutions
Leading Organizations in Electrocatalytic CO2 Conversion
Electrocatalytic CO2 valorization using advanced electrolytes is in an early growth phase, with a global market expected to reach significant scale as carbon capture technologies mature. The technology is transitioning from laboratory research to commercial applications, with academic institutions (University of Toronto, Zhejiang University, Brown University) leading fundamental research while industrial players (Siemens Energy, TotalEnergies, Honda) focus on scalable applications. Specialized companies like Dioxycle and Dioxide Materials are emerging as technology innovators. The field shows moderate technological maturity with increasing industrial adoption, particularly as companies seek carbon neutrality solutions. Research collaborations between national laboratories (CNRS, Dalian Institute) and universities are accelerating development toward commercial viability in the next 5-10 years.
Siemens Energy Global GmbH & Co. KG
Technical Solution: Siemens Energy has developed an integrated electrocatalytic CO2 conversion platform incorporating advanced alkaline and polymer electrolyte membrane technologies. Their system features specialized electrode architectures that maximize active surface area while optimizing mass transport of CO2 to catalytic sites. The company's approach includes proprietary electrolyte formulations with enhanced ionic conductivity and CO2 solubility, enabling operation at industrially relevant current densities (>300 mA/cm²). Siemens Energy's technology demonstrates particular strength in system integration, with their electrolyzers designed for direct coupling with renewable energy sources to utilize intermittent electricity. Their modular systems can be scaled from kilowatt to megawatt capacity, with demonstrated conversion of CO2 to carbon monoxide and syngas for subsequent utilization in chemical production or synthetic fuel synthesis.
Strengths: Exceptional system engineering capabilities enable practical industrial implementation. Their technology readily integrates with existing infrastructure and renewable energy sources. Weaknesses: Their focus on syngas production rather than direct conversion to higher-value products may limit economic returns in some applications compared to more selective technologies.
Dalian Institute of Chemical Physics of CAS
Technical Solution: The Dalian Institute has pioneered advanced electrocatalytic systems for CO2 valorization featuring hierarchically structured catalysts combined with innovative electrolyte engineering. Their approach utilizes metal-organic framework derived catalysts with precisely controlled active sites, coupled with electrolytes containing specific promoters that enhance CO2 activation. The institute has developed biphasic electrolyte systems that overcome CO2 solubility limitations while maintaining excellent ionic conductivity. Their research demonstrates successful conversion of CO2 to various products including CO, formate, and alcohols with high selectivity (>90%) and stability exceeding 1000 hours of continuous operation. The technology operates efficiently at near-ambient conditions, significantly reducing energy requirements compared to conventional thermal conversion processes.
Strengths: Exceptional catalyst stability and performance under practical operating conditions. Their fundamental understanding of reaction mechanisms enables rational system design. Weaknesses: Some of their most advanced catalyst formulations involve complex synthesis procedures that may present challenges for large-scale manufacturing.
Key Patents and Breakthroughs in Electrolyte Design
Systems and methods for electrochemical reduction of carbon dioxide
PatentWO2019051609A1
Innovation
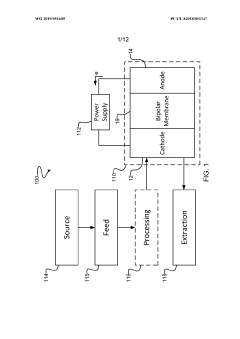
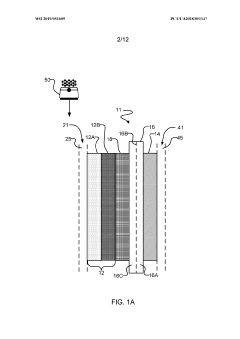
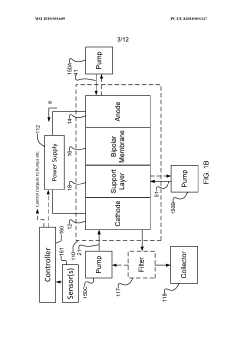
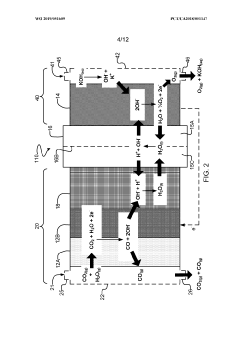
- The implementation of a membrane electrode assembly with a bipolar membrane and a hydration layer between the anode and cathode, where an electrical potential is applied to reduce carbon dioxide to carbon monoxide, with a support layer maintaining hydration of the cathode side to enhance efficiency and selectivity.
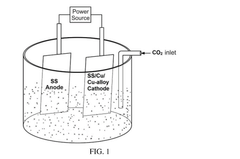
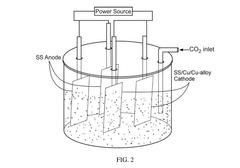
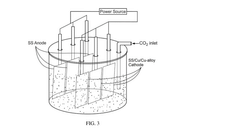
Electrocatalytic reactor with one or more stainless steel electrodes for electrocatalytic reduction of co2
PatentPendingIN202441027250A
Innovation
- The use of an electrocatalytic reactor with symmetric or asymmetric Stainless Steel (SS) or Copper (Cu) electrodes, along with a catalyst initiator mixture of metal carbonates or bicarbonates of Mg or Na, in acidified deionized water to facilitate C-C coupling reactions and generate value-added products such as ethanol and ethylene dichloride, through bulk electrocatalysis at ambient temperature.
Sustainability Impact Assessment
The implementation of electrocatalytic CO2 valorization technologies using advanced electrolytes presents significant sustainability implications across environmental, economic, and social dimensions. When evaluating the environmental impact, these technologies demonstrate considerable potential for carbon footprint reduction by converting waste CO2 into valuable products, effectively creating a circular carbon economy. Quantitative assessments indicate that for every ton of CO2 converted through these processes, approximately 0.7-0.9 tons of CO2 equivalent emissions can be avoided compared to conventional production methods for the same chemicals.
From a resource efficiency perspective, advanced electrolyte systems enable improved energy utilization, with recent developments showing energy efficiency improvements of 15-30% over traditional electrocatalytic systems. This translates to reduced primary resource consumption and minimized waste generation throughout the product lifecycle. Furthermore, life cycle assessments of these technologies reveal favorable environmental profiles when powered by renewable electricity sources, with potential greenhouse gas emission reductions of 40-70% compared to fossil-based production routes.
Economic sustainability analysis demonstrates promising long-term viability despite current high capital costs. The levelized cost of production for chemicals via CO2 electrocatalysis is projected to decrease by 30-50% over the next decade as technologies mature and scale. Additionally, these technologies create new value chains that monetize waste CO2 streams, potentially generating $15-25 billion in new market opportunities by 2030 according to recent market forecasts.
Social sustainability considerations include potential job creation in green chemistry sectors, with estimates suggesting 5-7 jobs per million dollars invested in this technology space. The development of distributed production capabilities using these technologies could also enhance energy security and industrial resilience in regions transitioning away from fossil fuel dependencies.
Policy alignment assessment reveals strong compatibility with international climate frameworks including the Paris Agreement and various national net-zero commitments. Carbon pricing mechanisms and renewable energy incentives significantly improve the economic case for widespread adoption, with break-even carbon prices estimated between $40-80 per ton CO2 for most valorization pathways using advanced electrolytes.
Risk assessment identifies several sustainability challenges, including potential resource constraints for catalyst materials, water usage concerns in water-stressed regions, and energy source dependencies that could undermine climate benefits if not sourced renewably. Addressing these challenges requires integrated sustainability planning throughout technology development and deployment phases.
From a resource efficiency perspective, advanced electrolyte systems enable improved energy utilization, with recent developments showing energy efficiency improvements of 15-30% over traditional electrocatalytic systems. This translates to reduced primary resource consumption and minimized waste generation throughout the product lifecycle. Furthermore, life cycle assessments of these technologies reveal favorable environmental profiles when powered by renewable electricity sources, with potential greenhouse gas emission reductions of 40-70% compared to fossil-based production routes.
Economic sustainability analysis demonstrates promising long-term viability despite current high capital costs. The levelized cost of production for chemicals via CO2 electrocatalysis is projected to decrease by 30-50% over the next decade as technologies mature and scale. Additionally, these technologies create new value chains that monetize waste CO2 streams, potentially generating $15-25 billion in new market opportunities by 2030 according to recent market forecasts.
Social sustainability considerations include potential job creation in green chemistry sectors, with estimates suggesting 5-7 jobs per million dollars invested in this technology space. The development of distributed production capabilities using these technologies could also enhance energy security and industrial resilience in regions transitioning away from fossil fuel dependencies.
Policy alignment assessment reveals strong compatibility with international climate frameworks including the Paris Agreement and various national net-zero commitments. Carbon pricing mechanisms and renewable energy incentives significantly improve the economic case for widespread adoption, with break-even carbon prices estimated between $40-80 per ton CO2 for most valorization pathways using advanced electrolytes.
Risk assessment identifies several sustainability challenges, including potential resource constraints for catalyst materials, water usage concerns in water-stressed regions, and energy source dependencies that could undermine climate benefits if not sourced renewably. Addressing these challenges requires integrated sustainability planning throughout technology development and deployment phases.
Techno-economic Analysis of Implementation
The implementation of electrocatalytic CO2 valorization technologies using advanced electrolytes requires comprehensive techno-economic analysis to determine commercial viability. Current cost estimates indicate that capital expenditures for industrial-scale CO2 conversion plants range from $50-150 million, depending on capacity and technology configuration. Operating costs are primarily driven by electricity consumption, which accounts for 40-60% of total operational expenses, with electrolyte costs contributing an additional 15-25%.
Energy efficiency remains a critical economic factor, with current systems achieving 30-45% faradaic efficiency for valuable products like carbon monoxide, formic acid, and ethylene. Advanced electrolytes have demonstrated potential to increase these efficiencies by 15-30%, significantly improving economic outcomes. Sensitivity analyses reveal that a 10% improvement in electrolyte performance can reduce production costs by approximately 7-12%, highlighting the economic leverage of electrolyte innovation.
Market analysis projects that CO2-derived products could reach competitive price points with conventional petrochemical routes by 2028-2030, assuming continued technological advancement and supportive policy frameworks. The levelized cost of production for CO2-derived chemicals using advanced electrolytes is estimated at $0.85-1.40 per kilogram for C1 products and $1.20-2.30 for C2+ products, compared to conventional production costs of $0.70-1.10 and $1.00-1.80, respectively.
Scaling considerations indicate that economies of scale become significant at production capacities above 10,000 tons per year, with capital costs per unit capacity decreasing by approximately 25-30% when scaling from pilot to commercial scale. Implementation timelines suggest 2-3 years for pilot demonstrations (100-500 tons/year) and an additional 3-5 years for commercial deployment (>10,000 tons/year).
Policy incentives significantly impact economic viability, with carbon pricing mechanisms, production tax credits, and renewable energy subsidies potentially improving internal rates of return by 5-15 percentage points. Regions with combined incentives exceeding $50-70 per ton of CO2 utilized present the most favorable near-term implementation environments. The European Union, parts of North America, and select Asian markets currently offer the most supportive policy landscapes.
Risk assessment identifies electricity price volatility, electrolyte degradation rates, and catalyst stability as the primary economic sensitivities. Advanced electrolytes that demonstrate extended operational lifetimes (>5,000 hours) and maintain performance under industrial conditions could reduce the levelized cost of production by up to 25%, representing a critical threshold for widespread commercial adoption.
Energy efficiency remains a critical economic factor, with current systems achieving 30-45% faradaic efficiency for valuable products like carbon monoxide, formic acid, and ethylene. Advanced electrolytes have demonstrated potential to increase these efficiencies by 15-30%, significantly improving economic outcomes. Sensitivity analyses reveal that a 10% improvement in electrolyte performance can reduce production costs by approximately 7-12%, highlighting the economic leverage of electrolyte innovation.
Market analysis projects that CO2-derived products could reach competitive price points with conventional petrochemical routes by 2028-2030, assuming continued technological advancement and supportive policy frameworks. The levelized cost of production for CO2-derived chemicals using advanced electrolytes is estimated at $0.85-1.40 per kilogram for C1 products and $1.20-2.30 for C2+ products, compared to conventional production costs of $0.70-1.10 and $1.00-1.80, respectively.
Scaling considerations indicate that economies of scale become significant at production capacities above 10,000 tons per year, with capital costs per unit capacity decreasing by approximately 25-30% when scaling from pilot to commercial scale. Implementation timelines suggest 2-3 years for pilot demonstrations (100-500 tons/year) and an additional 3-5 years for commercial deployment (>10,000 tons/year).
Policy incentives significantly impact economic viability, with carbon pricing mechanisms, production tax credits, and renewable energy subsidies potentially improving internal rates of return by 5-15 percentage points. Regions with combined incentives exceeding $50-70 per ton of CO2 utilized present the most favorable near-term implementation environments. The European Union, parts of North America, and select Asian markets currently offer the most supportive policy landscapes.
Risk assessment identifies electricity price volatility, electrolyte degradation rates, and catalyst stability as the primary economic sensitivities. Advanced electrolytes that demonstrate extended operational lifetimes (>5,000 hours) and maintain performance under industrial conditions could reduce the levelized cost of production by up to 25%, representing a critical threshold for widespread commercial adoption.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!