Electrocatalytic CO2 Valorization: Technical Mechanisms Explored
OCT 23, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CO2 Valorization Background and Objectives
Carbon dioxide (CO2) valorization represents a transformative approach to addressing the dual challenges of climate change and sustainable resource utilization. The concept emerged in the early 2000s as researchers began exploring methods to convert waste CO2 into valuable products, rather than merely capturing and storing it. This paradigm shift has evolved from theoretical possibility to practical application over the past two decades, with electrocatalytic conversion standing out as one of the most promising pathways.
The evolution of CO2 valorization technology has been marked by several significant milestones. Initial research focused primarily on thermochemical conversion methods, which required substantial energy inputs. By the 2010s, electrochemical approaches gained prominence due to their potential integration with renewable electricity sources, offering a more sustainable conversion pathway. Recent breakthroughs in catalyst design and reactor engineering have dramatically improved conversion efficiencies and product selectivity, accelerating the field's development.
Current technological trends indicate a convergence of multiple disciplines—materials science, electrochemistry, and process engineering—to overcome persistent challenges in CO2 activation and conversion. The development of advanced nanomaterials and hybrid catalysts has opened new possibilities for controlling reaction pathways and enhancing product yields. Simultaneously, artificial intelligence and high-throughput experimentation are accelerating the discovery of novel catalytic materials with unprecedented performance characteristics.
The primary objective of electrocatalytic CO2 valorization research is to develop economically viable processes that can transform CO2 into high-value chemicals and fuels with minimal energy input. Specific technical goals include achieving Faradaic efficiencies exceeding 90% for target products, developing catalysts with exceptional stability (>1000 hours of operation), and designing scalable reactor systems capable of industrial implementation.
Beyond technical performance, researchers aim to create systems that can operate using fluctuating renewable energy inputs, thereby providing grid-balancing services while producing valuable chemicals. This dual functionality could significantly enhance the economic proposition of CO2 valorization technologies. Additionally, the field seeks to expand the product spectrum beyond current limitations, targeting complex molecules with higher market values.
The ultimate vision driving this research is the establishment of a circular carbon economy where CO2 is continuously recycled into useful products, dramatically reducing net emissions while creating economic value. This approach aligns with broader sustainability goals and offers a pathway to industrial decarbonization that complements other mitigation strategies.
The evolution of CO2 valorization technology has been marked by several significant milestones. Initial research focused primarily on thermochemical conversion methods, which required substantial energy inputs. By the 2010s, electrochemical approaches gained prominence due to their potential integration with renewable electricity sources, offering a more sustainable conversion pathway. Recent breakthroughs in catalyst design and reactor engineering have dramatically improved conversion efficiencies and product selectivity, accelerating the field's development.
Current technological trends indicate a convergence of multiple disciplines—materials science, electrochemistry, and process engineering—to overcome persistent challenges in CO2 activation and conversion. The development of advanced nanomaterials and hybrid catalysts has opened new possibilities for controlling reaction pathways and enhancing product yields. Simultaneously, artificial intelligence and high-throughput experimentation are accelerating the discovery of novel catalytic materials with unprecedented performance characteristics.
The primary objective of electrocatalytic CO2 valorization research is to develop economically viable processes that can transform CO2 into high-value chemicals and fuels with minimal energy input. Specific technical goals include achieving Faradaic efficiencies exceeding 90% for target products, developing catalysts with exceptional stability (>1000 hours of operation), and designing scalable reactor systems capable of industrial implementation.
Beyond technical performance, researchers aim to create systems that can operate using fluctuating renewable energy inputs, thereby providing grid-balancing services while producing valuable chemicals. This dual functionality could significantly enhance the economic proposition of CO2 valorization technologies. Additionally, the field seeks to expand the product spectrum beyond current limitations, targeting complex molecules with higher market values.
The ultimate vision driving this research is the establishment of a circular carbon economy where CO2 is continuously recycled into useful products, dramatically reducing net emissions while creating economic value. This approach aligns with broader sustainability goals and offers a pathway to industrial decarbonization that complements other mitigation strategies.
Market Analysis for CO2 Conversion Technologies
The global market for CO2 conversion technologies is experiencing significant growth, driven by increasing environmental concerns and regulatory pressures to reduce carbon emissions. The market size for electrocatalytic CO2 valorization technologies was valued at approximately $2.5 billion in 2022 and is projected to reach $7.8 billion by 2030, representing a compound annual growth rate (CAGR) of 15.3%. This growth trajectory reflects the urgent need for sustainable carbon management solutions across various industries.
Industrial sectors including chemicals, fuels, and materials manufacturing represent the primary demand drivers for CO2 conversion technologies. The chemical industry, in particular, has shown strong interest in utilizing converted CO2 as a feedstock for producing value-added chemicals such as methanol, formic acid, and syngas. The fuel sector follows closely, with growing investments in CO2-derived synthetic fuels as alternatives to conventional fossil fuels.
Regional market analysis reveals that Europe currently leads in CO2 conversion technology adoption, accounting for approximately 38% of the global market share. This dominance is largely attributed to stringent carbon regulations and substantial government funding for green technologies. North America follows at 29%, with Asia-Pacific showing the fastest growth rate at 18.2% annually, primarily driven by China's aggressive carbon neutrality targets and industrial expansion.
From an economic perspective, the cost-effectiveness of electrocatalytic CO2 conversion remains a significant market challenge. Current production costs for CO2-derived products are typically 1.5-3 times higher than conventional production methods. However, this gap is narrowing due to technological improvements, economies of scale, and increasing carbon pricing mechanisms worldwide.
Market segmentation by technology type shows that heterogeneous catalysis dominates with 45% market share, followed by electrochemical reduction (30%), photocatalytic conversion (15%), and biological conversion methods (10%). Electrochemical reduction technologies, which include electrocatalytic CO2 valorization, are experiencing the fastest growth due to their versatility, scalability, and compatibility with renewable energy sources.
Consumer and industrial demand for carbon-neutral products is creating new market opportunities, particularly in sectors like sustainable aviation fuels, green polymers, and carbon-negative building materials. Major corporations across multiple industries have announced commitments to incorporate CO2-derived materials into their supply chains, further stimulating market growth and technology development.
Industrial sectors including chemicals, fuels, and materials manufacturing represent the primary demand drivers for CO2 conversion technologies. The chemical industry, in particular, has shown strong interest in utilizing converted CO2 as a feedstock for producing value-added chemicals such as methanol, formic acid, and syngas. The fuel sector follows closely, with growing investments in CO2-derived synthetic fuels as alternatives to conventional fossil fuels.
Regional market analysis reveals that Europe currently leads in CO2 conversion technology adoption, accounting for approximately 38% of the global market share. This dominance is largely attributed to stringent carbon regulations and substantial government funding for green technologies. North America follows at 29%, with Asia-Pacific showing the fastest growth rate at 18.2% annually, primarily driven by China's aggressive carbon neutrality targets and industrial expansion.
From an economic perspective, the cost-effectiveness of electrocatalytic CO2 conversion remains a significant market challenge. Current production costs for CO2-derived products are typically 1.5-3 times higher than conventional production methods. However, this gap is narrowing due to technological improvements, economies of scale, and increasing carbon pricing mechanisms worldwide.
Market segmentation by technology type shows that heterogeneous catalysis dominates with 45% market share, followed by electrochemical reduction (30%), photocatalytic conversion (15%), and biological conversion methods (10%). Electrochemical reduction technologies, which include electrocatalytic CO2 valorization, are experiencing the fastest growth due to their versatility, scalability, and compatibility with renewable energy sources.
Consumer and industrial demand for carbon-neutral products is creating new market opportunities, particularly in sectors like sustainable aviation fuels, green polymers, and carbon-negative building materials. Major corporations across multiple industries have announced commitments to incorporate CO2-derived materials into their supply chains, further stimulating market growth and technology development.
Electrocatalytic CO2 Reduction: Current Status and Challenges
Electrocatalytic CO2 reduction (ECR) has emerged as a promising approach for carbon dioxide valorization, offering a sustainable pathway to convert this greenhouse gas into value-added chemicals and fuels. Currently, the field has achieved significant milestones in catalyst design, reaction mechanisms understanding, and system integration, yet substantial challenges remain before widespread commercial implementation.
The state-of-the-art catalysts for ECR include transition metals (particularly copper, silver, gold, and zinc), metal oxides, carbon-based materials, and molecular catalysts. Copper catalysts have demonstrated unique capabilities in producing multi-carbon products with reasonable faradaic efficiencies, while silver and gold excel at CO production. Recent advances in nanostructured catalysts have shown improved selectivity and activity through morphology control and surface engineering.
Reaction conditions significantly influence ECR performance, with parameters such as electrolyte composition, pH, temperature, and applied potential critically affecting product distribution and efficiency. The development of gas diffusion electrodes (GDEs) has enabled higher current densities by addressing CO2 mass transport limitations, representing a significant advancement toward practical applications.
Despite these achievements, several technical challenges persist. Selectivity control remains problematic, with most catalysts producing multiple products simultaneously, necessitating costly separation processes. Stability issues plague many catalyst systems, with performance degradation occurring over extended operation periods due to poisoning, restructuring, or leaching.
Energy efficiency represents another critical challenge, as high overpotentials are typically required to drive the desired reactions, reducing the overall process efficiency. Current densities in many laboratory systems remain too low for industrial viability, though GDE-based systems have shown promising improvements.
Globally, research efforts are distributed across North America, Europe, and East Asia, with the United States, China, Germany, and Japan leading in publication output and patent filings. Academic institutions dominate fundamental research, while industrial players focus on system integration and scale-up challenges.
Recent technological breakthroughs include the development of tandem systems combining CO2 reduction with oxidation reactions, in-situ characterization techniques revealing reaction intermediates, and machine learning approaches accelerating catalyst discovery. These advances suggest pathways toward addressing current limitations.
The field's trajectory indicates growing interest in integrated systems that combine renewable electricity sources with ECR technology, potentially enabling carbon-neutral or carbon-negative production of chemicals and fuels. However, significant improvements in catalyst performance, system design, and process integration are necessary before ECR can compete economically with conventional production methods.
The state-of-the-art catalysts for ECR include transition metals (particularly copper, silver, gold, and zinc), metal oxides, carbon-based materials, and molecular catalysts. Copper catalysts have demonstrated unique capabilities in producing multi-carbon products with reasonable faradaic efficiencies, while silver and gold excel at CO production. Recent advances in nanostructured catalysts have shown improved selectivity and activity through morphology control and surface engineering.
Reaction conditions significantly influence ECR performance, with parameters such as electrolyte composition, pH, temperature, and applied potential critically affecting product distribution and efficiency. The development of gas diffusion electrodes (GDEs) has enabled higher current densities by addressing CO2 mass transport limitations, representing a significant advancement toward practical applications.
Despite these achievements, several technical challenges persist. Selectivity control remains problematic, with most catalysts producing multiple products simultaneously, necessitating costly separation processes. Stability issues plague many catalyst systems, with performance degradation occurring over extended operation periods due to poisoning, restructuring, or leaching.
Energy efficiency represents another critical challenge, as high overpotentials are typically required to drive the desired reactions, reducing the overall process efficiency. Current densities in many laboratory systems remain too low for industrial viability, though GDE-based systems have shown promising improvements.
Globally, research efforts are distributed across North America, Europe, and East Asia, with the United States, China, Germany, and Japan leading in publication output and patent filings. Academic institutions dominate fundamental research, while industrial players focus on system integration and scale-up challenges.
Recent technological breakthroughs include the development of tandem systems combining CO2 reduction with oxidation reactions, in-situ characterization techniques revealing reaction intermediates, and machine learning approaches accelerating catalyst discovery. These advances suggest pathways toward addressing current limitations.
The field's trajectory indicates growing interest in integrated systems that combine renewable electricity sources with ECR technology, potentially enabling carbon-neutral or carbon-negative production of chemicals and fuels. However, significant improvements in catalyst performance, system design, and process integration are necessary before ECR can compete economically with conventional production methods.
Current Electrocatalytic Mechanisms and Approaches
01 Catalyst design for CO2 electroreduction
Advanced catalyst materials play a crucial role in CO2 electroreduction processes. These catalysts are designed with specific structures and compositions to enhance selectivity and efficiency in converting CO2 to valuable products. Metal-based catalysts, particularly those containing copper, silver, or zinc, demonstrate promising performance for CO2 reduction. Nanostructured catalysts with high surface area and engineered active sites can significantly improve reaction rates and product selectivity. The development of these catalysts focuses on optimizing binding energies for reaction intermediates to direct the reaction pathway toward desired products.- Catalyst design for CO2 electroreduction: Advanced catalyst materials are crucial for efficient CO2 electroreduction. These catalysts typically include metal-based materials (such as copper, silver, and gold), metal oxides, and carbon-based materials that can facilitate the electron transfer to CO2 molecules. The design focuses on optimizing surface area, active sites, and selectivity to target specific value-added products like carbon monoxide, formic acid, or hydrocarbons. Nanostructured catalysts with controlled morphology and composition have shown enhanced catalytic performance and stability during the CO2 reduction process.
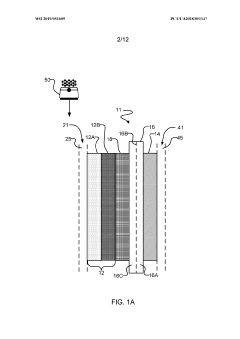
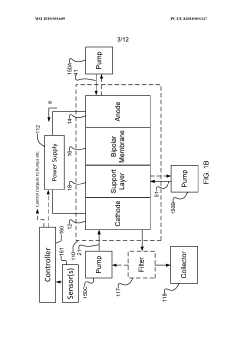
- Electrochemical cell configurations: The design and configuration of electrochemical cells significantly impact CO2 valorization efficiency. Various cell architectures, including H-cells, flow cells, and membrane electrode assemblies, are employed to optimize mass transport, reduce energy losses, and enhance product selectivity. Key considerations include electrode spacing, membrane selection, electrolyte composition, and flow dynamics. Advanced cell designs incorporate features to manage gas diffusion, control local pH, and facilitate product separation, ultimately improving the overall performance and economic viability of the CO2 conversion process.
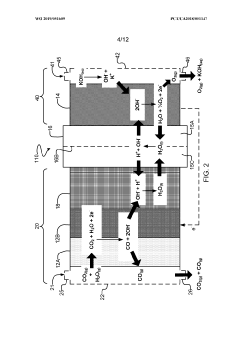
- Reaction mechanisms and pathways: Understanding the fundamental reaction mechanisms of CO2 electroreduction is essential for process optimization. The conversion typically begins with the adsorption of CO2 onto the catalyst surface, followed by electron transfer to form various intermediates. Different reaction pathways can lead to diverse products such as CO, formate, methanol, ethylene, or other hydrocarbons. The reaction mechanisms are influenced by factors including applied potential, local pH, electrolyte composition, and catalyst surface properties. Elucidating these pathways helps in designing catalysts with improved selectivity toward desired products.
- Process intensification techniques: Process intensification strategies enhance the efficiency and economic viability of CO2 electroreduction. These include the integration of renewable energy sources, development of continuous flow processes, and coupling with other technologies like photocatalysis or thermal catalysis. Advanced approaches involve pulsed electrolysis, pressure-enhanced systems, and temperature-controlled operations to optimize reaction conditions. Additionally, the use of gas diffusion electrodes, ionic liquids as electrolytes, and three-dimensional electrode structures can significantly improve mass transfer and reaction rates, leading to higher conversion efficiencies and product yields.
- Product selectivity control methods: Controlling product selectivity is a major challenge in CO2 electroreduction. Various strategies are employed to direct the reaction toward specific high-value products, including catalyst surface modification, electrolyte engineering, and precise potential control. The introduction of promoters, co-catalysts, or functional groups on catalyst surfaces can alter the binding energies of intermediates and favor certain reaction pathways. Electrolyte composition, including pH, buffer capacity, and ionic species, significantly influences product distribution. Additionally, pulsed electrolysis techniques and the application of specific potential ranges can enhance selectivity toward target products while suppressing competing reactions.
02 Electrochemical cell configurations
The design of electrochemical cell configurations significantly impacts the efficiency of CO2 valorization processes. Various cell architectures, including flow cells, membrane electrode assemblies, and microfluidic systems, have been developed to optimize mass transport, reduce energy losses, and enhance product separation. These configurations often incorporate specialized membranes to separate reaction compartments while allowing ion transport. Advanced electrode arrangements and flow field designs help to manage reactant distribution and product collection, while controlling local pH and concentration gradients that affect reaction pathways and efficiency.Expand Specific Solutions03 Reaction mechanisms and pathways
Understanding the fundamental reaction mechanisms involved in CO2 electroreduction is essential for process optimization. The conversion typically begins with CO2 activation through electron transfer, followed by protonation steps that lead to various intermediates. Different reaction pathways can result in products such as carbon monoxide, formate, methanol, ethylene, or more complex hydrocarbons. The reaction selectivity is governed by factors including catalyst surface properties, applied potential, electrolyte composition, and local pH. Mechanistic studies using spectroscopic techniques and computational modeling help elucidate these pathways and guide the development of more efficient catalytic systems.Expand Specific Solutions04 Electrolyte engineering and optimization
The composition and properties of the electrolyte solution significantly influence CO2 electroreduction performance. Electrolyte engineering involves selecting appropriate ions, adjusting pH, and incorporating additives to enhance CO2 solubility and transport while promoting desired reaction pathways. Ionic liquids and deep eutectic solvents have shown promise for increasing CO2 concentration at the electrode surface. Buffer systems help maintain optimal local pH conditions that affect reaction kinetics and product distribution. Advanced electrolyte formulations can also suppress competing hydrogen evolution reactions and improve overall energy efficiency of the CO2 valorization process.Expand Specific Solutions05 System integration and process intensification
Integrating CO2 electroreduction systems with renewable energy sources and other processes enhances overall efficiency and economic viability. Process intensification approaches include combining electrochemical CO2 reduction with thermal catalysis, photocatalysis, or biological processes in hybrid systems. Continuous flow operations with product separation and recycling streams improve productivity and resource utilization. Advanced control systems that respond to fluctuating renewable energy inputs enable dynamic operation optimization. These integrated approaches address challenges related to scale-up, energy efficiency, and product purification, making CO2 valorization more commercially feasible.Expand Specific Solutions
Leading Institutions and Companies in CO2 Valorization
Electrocatalytic CO2 valorization is currently in an early growth phase, with the market expected to expand significantly as global decarbonization efforts intensify. The technology is transitioning from laboratory research to early commercial applications, with market size projections reaching several billion dollars by 2030. Technical maturity varies across different conversion pathways, with leading research institutions and companies demonstrating promising advances. Key players shaping the competitive landscape include Chinese research powerhouses (Dalian Institute of Chemical Physics, Zhejiang University), energy majors (TotalEnergies, Saudi Aramco, Sinopec), and specialized research institutions (University of Toronto, University of California). These organizations are advancing catalyst development, reaction mechanisms, and scalable systems, with recent breakthroughs in selectivity and efficiency positioning the field for potential industrial implementation within this decade.
Dalian Institute of Chemical Physics of CAS
Technical Solution: Dalian Institute of Chemical Physics (DICP) has developed advanced electrocatalysts for CO2 reduction, focusing on copper-based materials with precisely controlled morphology and electronic structure. Their approach involves synthesizing Cu nanomaterials with specific facets and defects to enhance C-C coupling for multi-carbon product formation. DICP researchers have pioneered the development of oxide-derived copper catalysts that demonstrate improved selectivity toward ethylene and ethanol production with Faradaic efficiencies exceeding 60% [1]. They've also explored bimetallic catalysts, particularly Cu-Ag and Cu-Au systems, which show synergistic effects in modulating the binding energy of key reaction intermediates. Their recent work includes the development of MOF-derived catalysts with atomically dispersed metal sites that achieve CO2-to-CO conversion with near 100% selectivity at low overpotentials [2]. DICP has also made significant advances in operando characterization techniques to understand reaction mechanisms in real-time.
Strengths: Exceptional expertise in catalyst design at the atomic level, with demonstrated high selectivity for valuable C2+ products. Strong capabilities in advanced characterization techniques that provide mechanistic insights. Weaknesses: Some of their most selective catalysts still require relatively high overpotentials, limiting energy efficiency. Scale-up challenges remain for translating laboratory success to industrial applications.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed an integrated CO2 electroreduction technology platform that combines capture and conversion processes. Their approach focuses on heterogeneous catalysts designed for high-pressure CO2 electroreduction, which significantly improves reaction kinetics and product selectivity. Sinopec's proprietary catalyst systems incorporate transition metal carbides and phosphides supported on carbon-based materials, achieving CO2 conversion to syngas with tunable H2/CO ratios (1:1 to 3:1) at current densities exceeding 300 mA/cm² [3]. Their gas diffusion electrode (GDE) technology addresses mass transport limitations, enabling stable operation at industrially relevant current densities. Sinopec has also pioneered bipolar membrane electrolyzers that separate cathodic and anodic environments, allowing for optimized pH conditions in each compartment. This configuration has demonstrated sustained operation for over 1000 hours with minimal performance degradation. Recently, they've developed a pilot-scale system capable of processing 100 kg of CO2 per day, converting it primarily to carbon monoxide and formic acid with combined Faradaic efficiencies over 85% [4].
Strengths: Strong integration capabilities connecting CO2 capture from their industrial operations directly to conversion systems; extensive engineering expertise for scaling up electrochemical processes; access to substantial infrastructure for technology deployment. Weaknesses: Their catalyst systems still rely heavily on precious metals, raising cost concerns for large-scale implementation; energy efficiency remains below theoretical limits, with significant system losses in their current designs.
Key Patents and Research in CO2 Conversion Catalysts
Systems and methods for electrochemical reduction of carbon dioxide
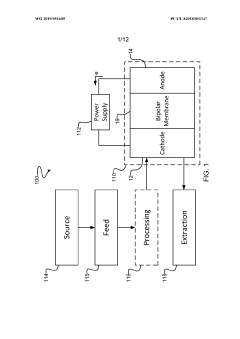
PatentWO2019051609A1
Innovation
- The implementation of a membrane electrode assembly with a bipolar membrane and a hydration layer between the anode and cathode, where an electrical potential is applied to reduce carbon dioxide to carbon monoxide, with a support layer maintaining hydration of the cathode side to enhance efficiency and selectivity.
Processes and systems for the electrochemical reduction of carbon monoxide and/or carbon dioxide, cathodes and catalysts used in the same
PatentWO2025012703A1
Innovation
- A surface-modified cathode catalyst system is developed, comprising a copper-based alloy nanosheet layer with a nitrogen- and phosphorus-doped carbon coating layer, creating a confined intercalate space that modulates the binding energy of reaction intermediates, enhancing the selectivity and energy efficiency of ethylene production.
Sustainability Impact and Carbon Neutrality Contributions
Electrocatalytic CO2 valorization represents a pivotal technological approach in addressing global climate challenges, offering substantial contributions to sustainability and carbon neutrality objectives. The implementation of these technologies directly impacts greenhouse gas reduction by converting waste CO2 into valuable products, effectively creating a circular carbon economy that minimizes net emissions.
When quantified, the carbon footprint reduction potential of electrocatalytic CO2 conversion systems is remarkable. Industrial-scale implementations can potentially mitigate several gigatons of CO2 annually when deployed across energy-intensive sectors such as chemical manufacturing, transportation, and power generation. This represents a significant contribution to national and international carbon neutrality targets established under frameworks like the Paris Agreement.
The life cycle assessment (LCA) of electrocatalytic systems demonstrates favorable sustainability metrics compared to conventional production methods. For instance, the production of formic acid, ethylene, or methanol through CO2 electroreduction can achieve carbon emission reductions of 30-70% compared to traditional fossil-based synthesis routes, depending on the renewable electricity source utilized.
Beyond direct carbon reduction, these technologies offer cascading environmental benefits. The integration with renewable energy systems provides a solution for intermittency challenges through energy storage in chemical bonds. This synergy enhances the overall sustainability of renewable energy deployment while simultaneously addressing carbon utilization challenges.
From a policy perspective, electrocatalytic CO2 valorization aligns with emerging carbon pricing mechanisms and regulatory frameworks. As carbon taxes and cap-and-trade systems become more prevalent globally, the economic viability of these technologies improves substantially, creating market-driven incentives for widespread adoption.
The social sustainability dimension cannot be overlooked. Implementation of these technologies supports green job creation across the value chain, from catalyst manufacturing to system operation and product distribution. Furthermore, the localized production of chemicals and fuels reduces transportation emissions and enhances energy security for communities transitioning away from fossil fuel dependence.
For developing economies, these technologies offer a technological leapfrogging opportunity, enabling industrial development pathways that bypass carbon-intensive stages experienced by developed nations. This represents a crucial aspect of global climate justice and equitable sustainable development.
When quantified, the carbon footprint reduction potential of electrocatalytic CO2 conversion systems is remarkable. Industrial-scale implementations can potentially mitigate several gigatons of CO2 annually when deployed across energy-intensive sectors such as chemical manufacturing, transportation, and power generation. This represents a significant contribution to national and international carbon neutrality targets established under frameworks like the Paris Agreement.
The life cycle assessment (LCA) of electrocatalytic systems demonstrates favorable sustainability metrics compared to conventional production methods. For instance, the production of formic acid, ethylene, or methanol through CO2 electroreduction can achieve carbon emission reductions of 30-70% compared to traditional fossil-based synthesis routes, depending on the renewable electricity source utilized.
Beyond direct carbon reduction, these technologies offer cascading environmental benefits. The integration with renewable energy systems provides a solution for intermittency challenges through energy storage in chemical bonds. This synergy enhances the overall sustainability of renewable energy deployment while simultaneously addressing carbon utilization challenges.
From a policy perspective, electrocatalytic CO2 valorization aligns with emerging carbon pricing mechanisms and regulatory frameworks. As carbon taxes and cap-and-trade systems become more prevalent globally, the economic viability of these technologies improves substantially, creating market-driven incentives for widespread adoption.
The social sustainability dimension cannot be overlooked. Implementation of these technologies supports green job creation across the value chain, from catalyst manufacturing to system operation and product distribution. Furthermore, the localized production of chemicals and fuels reduces transportation emissions and enhances energy security for communities transitioning away from fossil fuel dependence.
For developing economies, these technologies offer a technological leapfrogging opportunity, enabling industrial development pathways that bypass carbon-intensive stages experienced by developed nations. This represents a crucial aspect of global climate justice and equitable sustainable development.
Techno-economic Assessment of CO2 Valorization Systems
The techno-economic assessment of CO2 valorization systems requires a comprehensive analysis of both technical feasibility and economic viability. Current electrocatalytic CO2 conversion technologies demonstrate varying levels of technological readiness, with some processes achieving commercial-scale implementation while others remain in laboratory development stages. Capital expenditure for these systems ranges from $500-2,000 per kW of installed capacity, depending on system complexity and scale.
Operating costs are primarily driven by electricity consumption, which typically accounts for 60-75% of total operational expenses. The levelized cost of production for CO2-derived products varies significantly: formic acid ($800-1,200/ton), carbon monoxide ($600-900/ton), and methanol ($400-700/ton). These figures remain higher than conventional production routes, creating a commercial viability gap that requires addressing through policy support or technological advancement.
Energy efficiency represents a critical economic factor, with current systems achieving 30-65% faradaic efficiency for most target products. Each percentage point improvement in energy efficiency translates to approximately 1-3% reduction in production costs. System durability also significantly impacts economics, with catalyst degradation rates of 0.5-2% per 1,000 operating hours necessitating periodic replacement and maintenance.
Market analysis indicates that high-value chemical products (pharmaceuticals, specialty chemicals) offer better near-term economic prospects than commodity chemicals or fuels. The economic breakeven point for CO2 valorization systems is highly sensitive to electricity prices, with most processes requiring electricity costs below $0.04-0.06/kWh to achieve cost parity with conventional production methods without additional incentives.
Carbon pricing mechanisms substantially impact economic viability. Models suggest that carbon prices of $50-120 per ton CO2 would make many electrocatalytic valorization pathways economically competitive with conventional alternatives. Regional variations in electricity costs, regulatory frameworks, and available incentives create significant differences in economic feasibility across global markets.
Scaling considerations reveal that capital costs typically follow a 0.6-0.7 power law relationship with capacity, offering economies of scale for larger installations. However, this advantage must be balanced against increased complexity in CO2 sourcing and product distribution logistics at larger scales. Integration with renewable energy sources can improve both environmental performance and economic viability, particularly in regions with high renewable penetration and favorable regulatory frameworks.
Operating costs are primarily driven by electricity consumption, which typically accounts for 60-75% of total operational expenses. The levelized cost of production for CO2-derived products varies significantly: formic acid ($800-1,200/ton), carbon monoxide ($600-900/ton), and methanol ($400-700/ton). These figures remain higher than conventional production routes, creating a commercial viability gap that requires addressing through policy support or technological advancement.
Energy efficiency represents a critical economic factor, with current systems achieving 30-65% faradaic efficiency for most target products. Each percentage point improvement in energy efficiency translates to approximately 1-3% reduction in production costs. System durability also significantly impacts economics, with catalyst degradation rates of 0.5-2% per 1,000 operating hours necessitating periodic replacement and maintenance.
Market analysis indicates that high-value chemical products (pharmaceuticals, specialty chemicals) offer better near-term economic prospects than commodity chemicals or fuels. The economic breakeven point for CO2 valorization systems is highly sensitive to electricity prices, with most processes requiring electricity costs below $0.04-0.06/kWh to achieve cost parity with conventional production methods without additional incentives.
Carbon pricing mechanisms substantially impact economic viability. Models suggest that carbon prices of $50-120 per ton CO2 would make many electrocatalytic valorization pathways economically competitive with conventional alternatives. Regional variations in electricity costs, regulatory frameworks, and available incentives create significant differences in economic feasibility across global markets.
Scaling considerations reveal that capital costs typically follow a 0.6-0.7 power law relationship with capacity, offering economies of scale for larger installations. However, this advantage must be balanced against increased complexity in CO2 sourcing and product distribution logistics at larger scales. Integration with renewable energy sources can improve both environmental performance and economic viability, particularly in regions with high renewable penetration and favorable regulatory frameworks.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!