Electrocatalytic CO2 Valorization: Coating Materials and Techniques
OCT 23, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CO2 Valorization Background and Objectives
Carbon dioxide (CO2) valorization represents a critical technological frontier in addressing the dual challenges of climate change and sustainable resource utilization. The atmospheric concentration of CO2 has increased dramatically since the industrial revolution, reaching levels unprecedented in human history and contributing significantly to global warming. Rather than viewing CO2 merely as a waste product and environmental liability, valorization approaches reframe it as a potential carbon resource that can be transformed into valuable products through various conversion pathways.
Electrocatalytic CO2 valorization specifically refers to the process of converting CO2 into value-added chemicals and fuels using electricity as the energy input and specialized catalysts to facilitate the reaction. This approach has gained substantial attention over the past decade due to its potential integration with renewable electricity sources, offering a pathway to store intermittent renewable energy in chemical bonds while simultaneously reducing CO2 emissions.
The historical development of CO2 valorization technologies dates back to the early 20th century, but significant advancements in electrocatalytic approaches have primarily occurred within the last 30 years. Early research focused on fundamental electrochemical reduction mechanisms, while recent efforts have shifted toward developing high-performance catalysts and practical electrochemical systems capable of industrial-scale implementation.
Coating materials and techniques represent a crucial aspect of electrocatalytic CO2 valorization, as they directly influence catalyst performance, stability, and selectivity. The evolution of coating technologies has progressed from simple deposition methods to sophisticated techniques enabling precise control over catalyst structure at the nanoscale.
The primary objectives of current research in electrocatalytic CO2 valorization coatings include: enhancing catalytic activity to achieve commercially viable conversion rates; improving selectivity toward specific high-value products; developing durable coating materials capable of withstanding industrial operating conditions; reducing precious metal content to decrease costs; and designing scalable coating techniques compatible with mass manufacturing processes.
Future technological goals include the development of coating materials that can achieve near-100% faradaic efficiency for target products, operate at industrially relevant current densities (>200 mA/cm²), maintain stability for thousands of hours, and utilize earth-abundant elements rather than scarce precious metals. Additionally, there is growing interest in multifunctional coatings that can simultaneously address multiple performance parameters and self-healing coating systems that can extend catalyst lifetimes in harsh operating environments.
Electrocatalytic CO2 valorization specifically refers to the process of converting CO2 into value-added chemicals and fuels using electricity as the energy input and specialized catalysts to facilitate the reaction. This approach has gained substantial attention over the past decade due to its potential integration with renewable electricity sources, offering a pathway to store intermittent renewable energy in chemical bonds while simultaneously reducing CO2 emissions.
The historical development of CO2 valorization technologies dates back to the early 20th century, but significant advancements in electrocatalytic approaches have primarily occurred within the last 30 years. Early research focused on fundamental electrochemical reduction mechanisms, while recent efforts have shifted toward developing high-performance catalysts and practical electrochemical systems capable of industrial-scale implementation.
Coating materials and techniques represent a crucial aspect of electrocatalytic CO2 valorization, as they directly influence catalyst performance, stability, and selectivity. The evolution of coating technologies has progressed from simple deposition methods to sophisticated techniques enabling precise control over catalyst structure at the nanoscale.
The primary objectives of current research in electrocatalytic CO2 valorization coatings include: enhancing catalytic activity to achieve commercially viable conversion rates; improving selectivity toward specific high-value products; developing durable coating materials capable of withstanding industrial operating conditions; reducing precious metal content to decrease costs; and designing scalable coating techniques compatible with mass manufacturing processes.
Future technological goals include the development of coating materials that can achieve near-100% faradaic efficiency for target products, operate at industrially relevant current densities (>200 mA/cm²), maintain stability for thousands of hours, and utilize earth-abundant elements rather than scarce precious metals. Additionally, there is growing interest in multifunctional coatings that can simultaneously address multiple performance parameters and self-healing coating systems that can extend catalyst lifetimes in harsh operating environments.
Market Analysis for CO2 Conversion Technologies
The global market for CO2 conversion technologies is experiencing significant growth, driven by increasing environmental concerns and regulatory pressures to reduce carbon emissions. The market was valued at approximately $1.8 billion in 2022 and is projected to reach $4.2 billion by 2030, representing a compound annual growth rate (CAGR) of 11.2% during the forecast period. This growth trajectory reflects the urgent need for sustainable solutions to address climate change challenges.
Electrocatalytic CO2 valorization technologies, particularly those involving advanced coating materials and techniques, represent a rapidly expanding segment within this market. These technologies offer promising pathways for converting CO2 into value-added products such as carbon monoxide, formic acid, methanol, and other hydrocarbons, which can be utilized as feedstock for various industrial applications.
The market demand for these technologies is being fueled by several factors. First, stringent carbon emission regulations and carbon pricing mechanisms implemented by governments worldwide are incentivizing industries to adopt carbon capture and utilization technologies. The European Union's Carbon Border Adjustment Mechanism and similar policies in other regions are creating economic drivers for CO2 conversion solutions.
Second, there is growing interest from chemical and energy companies seeking to develop carbon-neutral or carbon-negative production processes. Major corporations including BASF, Shell, and Siemens Energy have made substantial investments in electrocatalytic CO2 conversion technologies, recognizing their potential to transform industrial processes while addressing sustainability goals.
The market for coating materials used in electrocatalytic CO2 conversion is particularly dynamic. Metal-based catalysts, especially those containing copper, silver, and gold nanoparticles, dominate the current market landscape due to their relatively high efficiency and selectivity. However, emerging materials such as metal-organic frameworks (MOFs), carbon-based materials, and bimetallic catalysts are gaining traction due to their enhanced performance characteristics and potential cost advantages.
Regional analysis indicates that North America and Europe currently lead the market for CO2 conversion technologies, accounting for approximately 65% of the global market share. However, the Asia-Pacific region, particularly China, Japan, and South Korea, is expected to witness the highest growth rate over the next decade, driven by aggressive carbon neutrality targets and substantial government investments in green technologies.
The market is also seeing increased collaboration between academic institutions, technology startups, and established industrial players, creating a robust innovation ecosystem that is accelerating technology development and commercialization pathways for electrocatalytic CO2 valorization solutions.
Electrocatalytic CO2 valorization technologies, particularly those involving advanced coating materials and techniques, represent a rapidly expanding segment within this market. These technologies offer promising pathways for converting CO2 into value-added products such as carbon monoxide, formic acid, methanol, and other hydrocarbons, which can be utilized as feedstock for various industrial applications.
The market demand for these technologies is being fueled by several factors. First, stringent carbon emission regulations and carbon pricing mechanisms implemented by governments worldwide are incentivizing industries to adopt carbon capture and utilization technologies. The European Union's Carbon Border Adjustment Mechanism and similar policies in other regions are creating economic drivers for CO2 conversion solutions.
Second, there is growing interest from chemical and energy companies seeking to develop carbon-neutral or carbon-negative production processes. Major corporations including BASF, Shell, and Siemens Energy have made substantial investments in electrocatalytic CO2 conversion technologies, recognizing their potential to transform industrial processes while addressing sustainability goals.
The market for coating materials used in electrocatalytic CO2 conversion is particularly dynamic. Metal-based catalysts, especially those containing copper, silver, and gold nanoparticles, dominate the current market landscape due to their relatively high efficiency and selectivity. However, emerging materials such as metal-organic frameworks (MOFs), carbon-based materials, and bimetallic catalysts are gaining traction due to their enhanced performance characteristics and potential cost advantages.
Regional analysis indicates that North America and Europe currently lead the market for CO2 conversion technologies, accounting for approximately 65% of the global market share. However, the Asia-Pacific region, particularly China, Japan, and South Korea, is expected to witness the highest growth rate over the next decade, driven by aggressive carbon neutrality targets and substantial government investments in green technologies.
The market is also seeing increased collaboration between academic institutions, technology startups, and established industrial players, creating a robust innovation ecosystem that is accelerating technology development and commercialization pathways for electrocatalytic CO2 valorization solutions.
Electrocatalytic CO2 Reduction: Current Status and Barriers
Electrocatalytic CO2 reduction (ECR) has emerged as a promising approach for carbon dioxide valorization, offering a sustainable pathway to convert this greenhouse gas into value-added chemicals and fuels. Currently, the technology has advanced from laboratory-scale demonstrations to pilot projects, with significant improvements in catalyst design, electrode architectures, and reactor configurations. However, widespread commercial implementation remains limited due to several critical barriers.
The current state of ECR technology is characterized by moderate energy efficiency, typically ranging from 30-60% depending on the target product. Faradaic efficiencies for C1 products like carbon monoxide and formate have reached commercial viability thresholds (>90%), while multi-carbon product selectivity has improved substantially but remains challenging to control precisely. Catalyst stability has extended from hours to weeks in laboratory settings, though industrial applications would require months or years of consistent performance.
A primary barrier to ECR advancement is the limited understanding of reaction mechanisms at the catalyst-electrolyte interface. The complex multi-electron, multi-proton transfer processes involved in CO2 reduction create numerous possible reaction pathways, making selective product formation difficult to achieve. This fundamental challenge manifests in practical limitations such as product selectivity control and catalyst deactivation.
Material constraints represent another significant barrier. Current noble metal catalysts (gold, silver, palladium) deliver high performance but at prohibitive costs for large-scale deployment. Alternative earth-abundant materials often suffer from lower activity, selectivity, or durability. Coating techniques for these catalysts frequently struggle to create uniform, defect-free layers with optimal thickness and porosity for efficient CO2 activation.
Engineering challenges further impede progress, particularly in reactor design and system integration. Mass transport limitations restrict reaction rates, as CO2 has limited solubility in aqueous electrolytes. The competition between hydrogen evolution and CO2 reduction reactions reduces overall efficiency, while product separation from complex mixtures remains energy-intensive and costly.
Economic barriers are equally significant. Current ECR systems face high capital costs and operational expenses that make them uncompetitive with conventional fossil-based production routes. The technology requires inexpensive renewable electricity to be economically viable and environmentally beneficial, creating dependency on broader energy transition timelines.
Regulatory frameworks and standardization efforts for ECR technologies remain underdeveloped, creating uncertainty for potential industrial adopters. The lack of comprehensive life cycle assessments and techno-economic analyses specific to different ECR pathways further complicates investment decisions and policy support mechanisms.
The current state of ECR technology is characterized by moderate energy efficiency, typically ranging from 30-60% depending on the target product. Faradaic efficiencies for C1 products like carbon monoxide and formate have reached commercial viability thresholds (>90%), while multi-carbon product selectivity has improved substantially but remains challenging to control precisely. Catalyst stability has extended from hours to weeks in laboratory settings, though industrial applications would require months or years of consistent performance.
A primary barrier to ECR advancement is the limited understanding of reaction mechanisms at the catalyst-electrolyte interface. The complex multi-electron, multi-proton transfer processes involved in CO2 reduction create numerous possible reaction pathways, making selective product formation difficult to achieve. This fundamental challenge manifests in practical limitations such as product selectivity control and catalyst deactivation.
Material constraints represent another significant barrier. Current noble metal catalysts (gold, silver, palladium) deliver high performance but at prohibitive costs for large-scale deployment. Alternative earth-abundant materials often suffer from lower activity, selectivity, or durability. Coating techniques for these catalysts frequently struggle to create uniform, defect-free layers with optimal thickness and porosity for efficient CO2 activation.
Engineering challenges further impede progress, particularly in reactor design and system integration. Mass transport limitations restrict reaction rates, as CO2 has limited solubility in aqueous electrolytes. The competition between hydrogen evolution and CO2 reduction reactions reduces overall efficiency, while product separation from complex mixtures remains energy-intensive and costly.
Economic barriers are equally significant. Current ECR systems face high capital costs and operational expenses that make them uncompetitive with conventional fossil-based production routes. The technology requires inexpensive renewable electricity to be economically viable and environmentally beneficial, creating dependency on broader energy transition timelines.
Regulatory frameworks and standardization efforts for ECR technologies remain underdeveloped, creating uncertainty for potential industrial adopters. The lack of comprehensive life cycle assessments and techno-economic analyses specific to different ECR pathways further complicates investment decisions and policy support mechanisms.
State-of-the-Art Electrocatalytic Coating Solutions
01 Metal-based catalytic coatings for CO2 reduction
Metal-based catalytic coatings, particularly those using copper, silver, gold, and transition metals, are applied to electrodes to enhance CO2 reduction efficiency. These coatings can be deposited through various techniques including electrodeposition, sputtering, and vapor deposition methods. The metal catalysts facilitate electron transfer to CO2 molecules, enabling their conversion to valuable products such as carbon monoxide, formic acid, and hydrocarbons. The selectivity and activity of these catalysts can be tuned by controlling the coating thickness, morphology, and composition.- Metal-based catalytic coatings for CO2 reduction: Metal-based catalytic coatings, particularly those using copper, silver, gold, and their alloys, are effective for electrocatalytic CO2 reduction. These metals can be applied as thin films, nanoparticles, or structured surfaces to enhance catalytic activity and selectivity. The coating techniques include electrodeposition, sputtering, and vapor deposition methods that control the morphology and surface properties of the catalyst, leading to improved CO2 conversion efficiency and product selectivity.
- Carbon-based coating materials for electrocatalysts: Carbon-based materials such as graphene, carbon nanotubes, and doped carbon structures serve as effective supports or active components in electrocatalytic CO2 valorization. These materials provide high surface area, excellent electrical conductivity, and can be functionalized to enhance catalytic performance. Coating techniques include chemical vapor deposition, solution-based methods, and spray coating that enable precise control over the carbon structure and its interaction with catalytic metal sites.
- Oxide and hydroxide coating techniques for CO2 electrocatalysis: Metal oxides and hydroxides, particularly those of transition metals, serve as efficient catalysts or catalyst supports for CO2 electroreduction. These materials can be applied as thin films or nanostructured coatings using techniques such as sol-gel processing, atomic layer deposition, and hydrothermal synthesis. The resulting oxide layers provide unique active sites for CO2 adsorption and activation, while also improving catalyst stability and selectivity toward valuable products like carbon monoxide, formate, or hydrocarbons.
- Polymer and composite coating materials for electrocatalysts: Polymer and composite coatings combine organic materials with inorganic catalysts to create enhanced electrocatalytic systems for CO2 reduction. These coatings include conductive polymers, ion-exchange membranes, and polymer-metal composites that can be applied through techniques such as electropolymerization, dip-coating, and layer-by-layer assembly. The polymer components can improve catalyst stability, provide selective transport of reactants and products, and create favorable microenvironments that enhance CO2 conversion efficiency.
- Advanced deposition techniques for nanostructured catalytic coatings: Advanced deposition methods enable precise control over the nanostructure and composition of electrocatalytic coatings for CO2 valorization. These techniques include atomic layer deposition, pulsed laser deposition, magnetron sputtering, and electrospray deposition. By controlling parameters such as deposition rate, substrate temperature, and precursor chemistry, these methods create catalytic surfaces with optimized morphology, exposed active sites, and tailored electronic properties that significantly enhance CO2 conversion performance and product selectivity.
02 Carbon-based coating materials for electrocatalysts
Carbon-based materials such as graphene, carbon nanotubes, and doped carbon structures serve as effective coating materials for CO2 electroreduction catalysts. These materials provide high surface area, excellent electrical conductivity, and can be functionalized to enhance catalyst binding and activity. The carbon-based coatings can be applied through techniques including dip-coating, spray coating, and chemical vapor deposition. These coatings not only improve the catalytic performance but also enhance the stability and durability of the electrocatalysts during long-term operation in CO2 valorization processes.Expand Specific Solutions03 Nanostructured coating techniques for enhanced catalytic performance
Nanostructured coatings with controlled morphology, such as nanowires, nanoparticles, and hierarchical structures, significantly enhance the performance of electrocatalysts for CO2 reduction. These nanostructured coatings can be fabricated using techniques like atomic layer deposition, sol-gel processing, and template-assisted growth. The nanoscale architecture increases the active surface area, provides more catalytic sites, and facilitates mass transport of reactants and products. Additionally, the nanostructured coatings can create unique local environments that favor specific reaction pathways, improving selectivity toward desired CO2 conversion products.Expand Specific Solutions04 Polymer and composite coating materials for electrocatalysts
Polymer and composite coatings combine organic polymers with inorganic catalytic materials to create synergistic effects in CO2 electroreduction. These coatings can be applied through techniques such as layer-by-layer assembly, electropolymerization, and solution casting. The polymer components can provide selective permeability to CO2, protect catalysts from deactivation, and create favorable microenvironments for the reaction. Composite coatings that integrate multiple functional materials can address multiple aspects of the catalytic process simultaneously, including CO2 adsorption, electron transfer, and product selectivity, resulting in improved overall performance for CO2 valorization.Expand Specific Solutions05 Surface modification techniques for catalyst stability and selectivity
Surface modification techniques are employed to enhance the stability and selectivity of electrocatalysts for CO2 reduction. These include atomic doping, ligand functionalization, and the creation of defect sites on catalyst surfaces. The modifications can be achieved through methods such as plasma treatment, chemical etching, and thermal annealing. By carefully engineering the surface properties, catalysts can be tailored to bind CO2 with optimal strength, direct the reaction pathway toward specific products, and resist deactivation mechanisms such as poisoning and dissolution. These surface modifications are crucial for developing practical electrocatalytic systems for long-term CO2 valorization applications.Expand Specific Solutions
Leading Companies and Research Institutions in CO2 Valorization
Electrocatalytic CO2 valorization is currently in a transitional phase from early research to commercial application, with a global market expected to reach $8-10 billion by 2030. The technology maturity varies significantly across different coating materials and techniques. Academic institutions like MIT, Zhejiang University, and University of Toronto lead fundamental research, while industrial players are advancing toward commercialization. TotalEnergies and DuPont focus on scaling up promising technologies, while specialized companies like Faraday Technology and Agora Energy Technologies develop proprietary coating methods. Chinese institutions demonstrate strength in catalyst development, while European and North American organizations excel in system integration. Cross-sector collaboration between academia and industry is accelerating technology transfer and commercial deployment.
Dalian Institute of Chemical Physics of CAS
Technical Solution: Dalian Institute of Chemical Physics (DICP) has developed advanced electrocatalytic CO2 valorization systems utilizing novel coating materials and techniques. Their approach centers on metal-organic framework (MOF) derived catalysts with atomically dispersed metal sites that significantly enhance CO2 reduction selectivity. DICP researchers have pioneered a controlled pyrolysis method for synthesizing M-N-C (metal-nitrogen-carbon) catalysts with precisely engineered pore structures and active site distributions. Their coating techniques include atomic layer deposition (ALD) for creating ultrathin protective layers that enhance catalyst stability while maintaining electron transfer efficiency. DICP has also developed innovative electrodeposition methods for creating gradient-structured catalysts that optimize both mass transport and reaction kinetics. Their recent breakthrough involves a dual-function coating that simultaneously facilitates CO2 adsorption and suppresses the competing hydrogen evolution reaction, achieving Faradaic efficiencies exceeding 90% for CO production at industrially relevant current densities.
Strengths: Exceptional control over atomic-level catalyst structure, leading to superior selectivity and efficiency. Their coating techniques demonstrate excellent scalability potential for industrial applications. Weaknesses: Some of their advanced coating methods require specialized equipment and precise control conditions that may limit widespread adoption. The long-term stability of some catalyst systems under industrial conditions remains to be fully validated.
TotalEnergies SE
Technical Solution: TotalEnergies SE has developed a comprehensive approach to electrocatalytic CO2 valorization focusing on innovative coating materials and techniques for industrial-scale implementation. Their technology centers on nanostructured copper-based catalysts with precisely engineered surface modifications to enhance selectivity toward higher-value C2+ products. TotalEnergies employs a proprietary plasma-enhanced chemical vapor deposition (PECVD) technique to create uniform catalyst coatings with controlled thickness and composition across large electrode surfaces. Their system incorporates a multi-layer coating architecture: a conductive support layer, an active catalyst layer with optimized porosity, and a protective ionomer overlay that enhances CO2 transport while minimizing degradation. The company has pioneered the use of ionic liquid-modified interfaces that significantly reduce the overpotential required for CO2 reduction. Their recent innovations include the development of bimetallic catalyst formulations with synergistic effects that achieve Faradaic efficiencies exceeding 85% for ethylene and ethanol production at industrially viable current densities. TotalEnergies has successfully demonstrated the scalability of these coating techniques on electrode areas exceeding 500 cm².
Strengths: Exceptional focus on industrial scalability and process integration with existing infrastructure. Their multi-layer coating approach effectively addresses both performance and durability challenges. Weaknesses: Higher implementation costs compared to simpler catalyst systems. Some of their advanced coating formulations rely on rare or expensive elements that may limit widespread adoption in cost-sensitive applications.
Key Patents and Breakthroughs in Catalyst Coating Technology
Doped carbon material for the electrocatalytic conversion of co 2 into hydrocarbons, uses of the material and conversion method using said material
PatentWO2013004882A9
Innovation
- Development of doped carbon materials, specifically carbon gels with transition metals like Ni, Cu, or Fe, which provide a high surface area and porosity, allowing for efficient electro-catalytic transformation of CO2 into hydrocarbons at atmospheric pressure with minimized metal leaching.
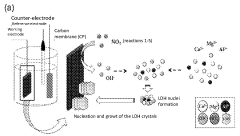
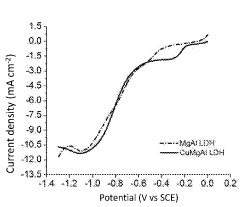
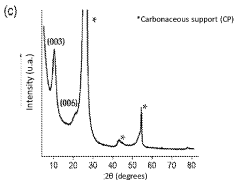
Layered double-hydroxide catalytic coatings containing: CU, mg e al, achievable electro¬ chemically, for uses such as electrochemical reduction of carbon dioxide
PatentWO2024127296A1
Innovation
- A nanostructured composite material with a layered hydrotalcite-type ternary structure of CuMgAl LDH, containing metal copper and cuprous ion as a redox couple, is developed, which is electrochemically deposited on a carbon gas diffusion membrane to enhance catalytic activity and selectivity for C2 products.
Sustainability Impact and Carbon Footprint Assessment
The implementation of electrocatalytic CO2 valorization technologies presents significant implications for environmental sustainability and carbon footprint reduction. When evaluating these systems, a comprehensive life cycle assessment (LCA) approach is essential to quantify the true environmental impact beyond direct CO2 conversion efficiency.
Electrocatalytic CO2 conversion processes offer a promising pathway for carbon neutrality by transforming waste CO2 into valuable chemicals and fuels. The net carbon impact depends on multiple factors including energy source, catalyst production methods, and system longevity. Studies indicate that when powered by renewable energy sources, these systems can achieve carbon negativity, potentially removing 0.5-2.0 tons of CO2 equivalent per ton of product depending on the specific valorization pathway.
The sustainability profile of coating materials used in electrocatalysts deserves particular attention. Traditional noble metal catalysts (Pt, Pd, Ru) carry substantial environmental burdens due to energy-intensive mining and refining processes. Recent advancements in earth-abundant transition metal catalysts and carbon-based materials have significantly improved the sustainability profile, reducing embodied carbon by approximately 60-85% compared to noble metal alternatives.
Coating techniques also contribute substantially to the overall environmental footprint. Physical vapor deposition (PVD) and chemical vapor deposition (CVD) methods typically consume 30-50% more energy than solution-based approaches like electrodeposition or sol-gel methods. However, the durability advantages of vapor deposition techniques may offset initial carbon costs through extended catalyst lifetimes, highlighting the importance of considering temporal factors in sustainability assessments.
Water consumption represents another critical sustainability metric, particularly for aqueous electrolysis systems. Advanced membrane coating technologies have demonstrated potential to reduce water requirements by 25-40% while simultaneously improving system efficiency. This dual benefit amplifies the positive environmental impact, especially in water-stressed regions where electrocatalytic facilities might be deployed.
The end-of-life management of coating materials presents both challenges and opportunities. While some catalyst materials contain potentially toxic components requiring specialized disposal, recent developments in catalyst recovery and regeneration techniques have shown promise for creating closed-loop systems. Effective recovery protocols can reclaim up to 85-95% of precious metals and 60-75% of transition metals from spent catalysts, substantially reducing the life-cycle environmental impact.
Electrocatalytic CO2 conversion processes offer a promising pathway for carbon neutrality by transforming waste CO2 into valuable chemicals and fuels. The net carbon impact depends on multiple factors including energy source, catalyst production methods, and system longevity. Studies indicate that when powered by renewable energy sources, these systems can achieve carbon negativity, potentially removing 0.5-2.0 tons of CO2 equivalent per ton of product depending on the specific valorization pathway.
The sustainability profile of coating materials used in electrocatalysts deserves particular attention. Traditional noble metal catalysts (Pt, Pd, Ru) carry substantial environmental burdens due to energy-intensive mining and refining processes. Recent advancements in earth-abundant transition metal catalysts and carbon-based materials have significantly improved the sustainability profile, reducing embodied carbon by approximately 60-85% compared to noble metal alternatives.
Coating techniques also contribute substantially to the overall environmental footprint. Physical vapor deposition (PVD) and chemical vapor deposition (CVD) methods typically consume 30-50% more energy than solution-based approaches like electrodeposition or sol-gel methods. However, the durability advantages of vapor deposition techniques may offset initial carbon costs through extended catalyst lifetimes, highlighting the importance of considering temporal factors in sustainability assessments.
Water consumption represents another critical sustainability metric, particularly for aqueous electrolysis systems. Advanced membrane coating technologies have demonstrated potential to reduce water requirements by 25-40% while simultaneously improving system efficiency. This dual benefit amplifies the positive environmental impact, especially in water-stressed regions where electrocatalytic facilities might be deployed.
The end-of-life management of coating materials presents both challenges and opportunities. While some catalyst materials contain potentially toxic components requiring specialized disposal, recent developments in catalyst recovery and regeneration techniques have shown promise for creating closed-loop systems. Effective recovery protocols can reclaim up to 85-95% of precious metals and 60-75% of transition metals from spent catalysts, substantially reducing the life-cycle environmental impact.
Techno-economic Analysis of CO2 Valorization Processes
The techno-economic analysis of CO2 valorization processes reveals significant economic challenges despite technological advancements. Current capital expenditure (CAPEX) for electrochemical CO2 reduction plants ranges from $1,500-3,000 per kW of installed capacity, substantially higher than conventional chemical production facilities. Operating expenses (OPEX) are dominated by electricity costs, accounting for 60-75% of total operational expenditures, with catalyst coating materials representing 5-15% depending on precious metal content.
Energy efficiency remains a critical economic factor, with most systems operating at 30-50% faradaic efficiency for valuable products like CO, ethylene, and formic acid. This translates to energy consumption of 6-12 kWh per kg of CO2 converted, significantly impacting economic viability in regions with high electricity prices. Sensitivity analysis indicates that a reduction in electricity costs by $0.02/kWh could improve profitability by 15-20%.
Coating material selection presents clear economic trade-offs. Noble metal catalysts (Au, Ag, Pt) demonstrate superior performance but at prohibitive costs ($40-60/g), while transition metal alternatives (Cu, Ni, Fe) offer more economical options ($0.1-5/g) with reduced efficiency. Recent advances in metal-organic frameworks and carbon-supported catalysts show promise for balancing cost and performance, potentially reducing catalyst costs by 30-40% while maintaining acceptable conversion rates.
Scale-up economics reveal interesting inflection points. Laboratory-scale systems (<1 kg CO2/day) show production costs of $800-1,200 per ton of converted CO2, while pilot plants (100-500 kg CO2/day) reduce this to $300-500 per ton. Industrial-scale facilities (>10 tons CO2/day) project costs of $150-250 per ton, approaching economic viability for high-value products like formic acid and ethanol, which command market prices of $800-1,200 and $600-900 per ton respectively.
Lifecycle assessment indicates that CO2 valorization processes must convert at least 70% of input CO2 to valuable products to achieve net carbon reduction benefits when accounting for electricity-related emissions. This threshold decreases to 40-50% when using renewable electricity sources, significantly improving the environmental and economic proposition simultaneously.
Market analysis projects that electrochemical CO2 valorization could become economically competitive with conventional production methods for select chemicals by 2030, assuming continued catalyst improvements and declining renewable electricity costs. The most promising near-term applications appear in regions combining low-cost renewable electricity, carbon pricing mechanisms, and proximity to industrial CO2 sources.
Energy efficiency remains a critical economic factor, with most systems operating at 30-50% faradaic efficiency for valuable products like CO, ethylene, and formic acid. This translates to energy consumption of 6-12 kWh per kg of CO2 converted, significantly impacting economic viability in regions with high electricity prices. Sensitivity analysis indicates that a reduction in electricity costs by $0.02/kWh could improve profitability by 15-20%.
Coating material selection presents clear economic trade-offs. Noble metal catalysts (Au, Ag, Pt) demonstrate superior performance but at prohibitive costs ($40-60/g), while transition metal alternatives (Cu, Ni, Fe) offer more economical options ($0.1-5/g) with reduced efficiency. Recent advances in metal-organic frameworks and carbon-supported catalysts show promise for balancing cost and performance, potentially reducing catalyst costs by 30-40% while maintaining acceptable conversion rates.
Scale-up economics reveal interesting inflection points. Laboratory-scale systems (<1 kg CO2/day) show production costs of $800-1,200 per ton of converted CO2, while pilot plants (100-500 kg CO2/day) reduce this to $300-500 per ton. Industrial-scale facilities (>10 tons CO2/day) project costs of $150-250 per ton, approaching economic viability for high-value products like formic acid and ethanol, which command market prices of $800-1,200 and $600-900 per ton respectively.
Lifecycle assessment indicates that CO2 valorization processes must convert at least 70% of input CO2 to valuable products to achieve net carbon reduction benefits when accounting for electricity-related emissions. This threshold decreases to 40-50% when using renewable electricity sources, significantly improving the environmental and economic proposition simultaneously.
Market analysis projects that electrochemical CO2 valorization could become economically competitive with conventional production methods for select chemicals by 2030, assuming continued catalyst improvements and declining renewable electricity costs. The most promising near-term applications appear in regions combining low-cost renewable electricity, carbon pricing mechanisms, and proximity to industrial CO2 sources.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!