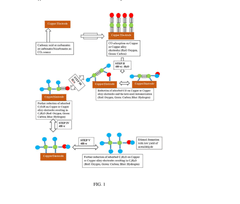
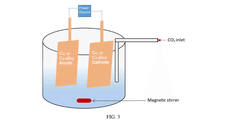
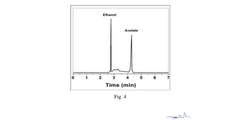
Comparison of Electrocatalytic CO2 Valorization vs Traditional Methods
OCT 23, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CO2 Valorization Background and Objectives
Carbon dioxide (CO2) valorization represents a critical technological frontier in addressing global climate change challenges while simultaneously creating economic value. The concept has evolved significantly over the past decades, transitioning from theoretical research to practical applications across various industries. Initially viewed primarily as a waste product and environmental pollutant, CO2 is increasingly recognized as a potential carbon resource that can be transformed into valuable products through appropriate technological interventions.
The historical trajectory of CO2 valorization technologies began with traditional chemical conversion methods in the early 20th century, such as the Bosch-Meiser process for urea production. These conventional approaches typically require high temperatures, pressures, and substantial energy inputs, limiting their environmental benefits. The 1970s energy crisis sparked renewed interest in carbon utilization, leading to exploration of catalytic conversion pathways with improved efficiency.
Recent technological breakthroughs, particularly in electrocatalysis, have revolutionized the field by offering potentially more sustainable pathways for CO2 conversion. Electrocatalytic methods leverage renewable electricity to drive chemical transformations under milder conditions, potentially reducing the overall carbon footprint of the conversion process. This represents a significant paradigm shift from traditional thermochemical approaches.
The primary objective of modern CO2 valorization research is to develop economically viable and environmentally beneficial pathways to transform carbon dioxide into value-added products. This includes fuels (methanol, syngas), chemicals (formic acid, carbon monoxide), materials (polymers, carbonates), and agricultural products. The ideal technology would operate with minimal energy requirements, utilize renewable energy sources, achieve high conversion rates and selectivity, and demonstrate economic competitiveness with conventional production methods.
Current technological trends indicate growing interest in integrated systems that combine capture and conversion processes, as well as hybrid approaches that leverage biological and chemical pathways. The emergence of advanced nanomaterials and catalyst designs has significantly improved reaction efficiencies and selectivity, addressing previous limitations in conversion rates and product specificity.
The global imperative to reduce greenhouse gas emissions has accelerated research and development in this field, with significant investments from both public and private sectors. Policy frameworks increasingly support carbon capture and utilization technologies as complementary to carbon capture and storage approaches, recognizing their potential to create circular carbon economies.
As we examine the comparative advantages of electrocatalytic methods versus traditional approaches, it becomes essential to understand the fundamental principles, technological readiness levels, and potential market applications of each pathway to effectively evaluate their respective merits and limitations.
The historical trajectory of CO2 valorization technologies began with traditional chemical conversion methods in the early 20th century, such as the Bosch-Meiser process for urea production. These conventional approaches typically require high temperatures, pressures, and substantial energy inputs, limiting their environmental benefits. The 1970s energy crisis sparked renewed interest in carbon utilization, leading to exploration of catalytic conversion pathways with improved efficiency.
Recent technological breakthroughs, particularly in electrocatalysis, have revolutionized the field by offering potentially more sustainable pathways for CO2 conversion. Electrocatalytic methods leverage renewable electricity to drive chemical transformations under milder conditions, potentially reducing the overall carbon footprint of the conversion process. This represents a significant paradigm shift from traditional thermochemical approaches.
The primary objective of modern CO2 valorization research is to develop economically viable and environmentally beneficial pathways to transform carbon dioxide into value-added products. This includes fuels (methanol, syngas), chemicals (formic acid, carbon monoxide), materials (polymers, carbonates), and agricultural products. The ideal technology would operate with minimal energy requirements, utilize renewable energy sources, achieve high conversion rates and selectivity, and demonstrate economic competitiveness with conventional production methods.
Current technological trends indicate growing interest in integrated systems that combine capture and conversion processes, as well as hybrid approaches that leverage biological and chemical pathways. The emergence of advanced nanomaterials and catalyst designs has significantly improved reaction efficiencies and selectivity, addressing previous limitations in conversion rates and product specificity.
The global imperative to reduce greenhouse gas emissions has accelerated research and development in this field, with significant investments from both public and private sectors. Policy frameworks increasingly support carbon capture and utilization technologies as complementary to carbon capture and storage approaches, recognizing their potential to create circular carbon economies.
As we examine the comparative advantages of electrocatalytic methods versus traditional approaches, it becomes essential to understand the fundamental principles, technological readiness levels, and potential market applications of each pathway to effectively evaluate their respective merits and limitations.
Market Analysis for CO2 Conversion Technologies
The global market for CO2 conversion technologies is experiencing significant growth, driven by increasing environmental concerns and regulatory pressures to reduce carbon emissions. The market size for carbon capture, utilization, and storage (CCUS) technologies was valued at approximately $2.5 billion in 2022 and is projected to reach $7.0 billion by 2030, growing at a CAGR of 13.8%. This growth trajectory reflects the urgent need for sustainable solutions to address climate change challenges.
Electrocatalytic CO2 valorization represents an emerging segment within this market, with particularly strong growth potential. While traditional CO2 conversion methods currently dominate with over 85% market share, electrocatalytic approaches are expected to grow at a faster rate of 17.2% annually through 2030, gradually increasing their market penetration.
Regional analysis reveals distinct market characteristics across different geographies. North America leads the market with approximately 35% share, driven by substantial investments in research and development, favorable government policies, and the presence of major industry players. Europe follows closely at 30%, with the European Union's ambitious climate targets and carbon pricing mechanisms accelerating adoption. The Asia-Pacific region, particularly China and Japan, is witnessing the fastest growth rate at 19.5% annually, fueled by rapid industrialization and government initiatives to reduce carbon intensity.
From an end-user perspective, the chemical manufacturing sector represents the largest market segment (40%), followed by energy production (25%), and fuel synthesis (20%). The remaining 15% is distributed across various applications including pharmaceuticals, agriculture, and food processing. This distribution highlights the versatility of CO2 conversion technologies across multiple industries.
Investment trends indicate growing confidence in the commercial viability of CO2 conversion technologies. Venture capital funding in this sector has increased by 145% over the past five years, reaching $1.2 billion in 2022. Corporate investments from oil and gas majors, chemical companies, and utilities have similarly expanded, with many establishing dedicated carbon management divisions and strategic partnerships with technology developers.
Market barriers include high capital costs, energy intensity of conversion processes, and competition from established carbon-intensive processes. However, these barriers are gradually diminishing as technological advancements improve efficiency and reduce costs. The levelized cost of CO2 conversion has decreased by approximately 30% over the past decade, enhancing commercial competitiveness against traditional methods.
Electrocatalytic CO2 valorization represents an emerging segment within this market, with particularly strong growth potential. While traditional CO2 conversion methods currently dominate with over 85% market share, electrocatalytic approaches are expected to grow at a faster rate of 17.2% annually through 2030, gradually increasing their market penetration.
Regional analysis reveals distinct market characteristics across different geographies. North America leads the market with approximately 35% share, driven by substantial investments in research and development, favorable government policies, and the presence of major industry players. Europe follows closely at 30%, with the European Union's ambitious climate targets and carbon pricing mechanisms accelerating adoption. The Asia-Pacific region, particularly China and Japan, is witnessing the fastest growth rate at 19.5% annually, fueled by rapid industrialization and government initiatives to reduce carbon intensity.
From an end-user perspective, the chemical manufacturing sector represents the largest market segment (40%), followed by energy production (25%), and fuel synthesis (20%). The remaining 15% is distributed across various applications including pharmaceuticals, agriculture, and food processing. This distribution highlights the versatility of CO2 conversion technologies across multiple industries.
Investment trends indicate growing confidence in the commercial viability of CO2 conversion technologies. Venture capital funding in this sector has increased by 145% over the past five years, reaching $1.2 billion in 2022. Corporate investments from oil and gas majors, chemical companies, and utilities have similarly expanded, with many establishing dedicated carbon management divisions and strategic partnerships with technology developers.
Market barriers include high capital costs, energy intensity of conversion processes, and competition from established carbon-intensive processes. However, these barriers are gradually diminishing as technological advancements improve efficiency and reduce costs. The levelized cost of CO2 conversion has decreased by approximately 30% over the past decade, enhancing commercial competitiveness against traditional methods.
Electrocatalytic vs Traditional CO2 Conversion: Status and Challenges
The global landscape of CO2 conversion technologies presents a complex interplay between established traditional methods and emerging electrocatalytic approaches. Traditional CO2 conversion has historically relied on thermochemical processes such as Fischer-Tropsch synthesis, hydrogenation reactions, and thermal catalysis, which operate at high temperatures and pressures. These methods have been industrially implemented for decades but face significant challenges regarding energy efficiency and carbon footprint.
Electrocatalytic CO2 valorization represents a paradigm shift in carbon utilization technology, operating at ambient conditions through electrochemical reduction reactions. This approach offers the potential for direct coupling with renewable electricity sources, enabling carbon-neutral or even carbon-negative production pathways. However, the technology remains predominantly at laboratory and pilot scales, with limited commercial deployment.
Current technical challenges for electrocatalytic methods include low faradaic efficiency, product selectivity issues, catalyst stability concerns, and scaling limitations. Most electrocatalysts struggle to maintain performance beyond several hundred hours of operation, presenting a significant barrier to industrial adoption. Additionally, current density achievements typically remain below the 300 mA/cm² threshold required for commercial viability.
Traditional methods benefit from technological maturity and established infrastructure but suffer from high energy demands and reliance on fossil-derived hydrogen sources. The thermodynamic limitations of these processes result in conversion efficiencies typically below 60%, with substantial heat losses and greenhouse gas emissions throughout the production chain.
Geographic distribution of technology development shows concentration of electrocatalytic innovation in North America, Western Europe, and East Asia, particularly in academic and research institutions. Traditional conversion technologies are more widely distributed but with significant industrial capacity centered in regions with established petrochemical infrastructure.
The economic gap between approaches remains substantial, with traditional methods benefiting from decades of process optimization and economies of scale. Current production costs for electrocatalytic pathways exceed traditional methods by factors of 2-5× depending on the target product, though this gap has narrowed significantly over the past five years as renewable electricity costs have declined.
Regulatory frameworks increasingly favor low-carbon production methods, creating potential advantages for electrocatalytic approaches despite their current cost premium. Carbon pricing mechanisms, when applied comprehensively, can significantly alter the competitive landscape between these technological approaches.
Electrocatalytic CO2 valorization represents a paradigm shift in carbon utilization technology, operating at ambient conditions through electrochemical reduction reactions. This approach offers the potential for direct coupling with renewable electricity sources, enabling carbon-neutral or even carbon-negative production pathways. However, the technology remains predominantly at laboratory and pilot scales, with limited commercial deployment.
Current technical challenges for electrocatalytic methods include low faradaic efficiency, product selectivity issues, catalyst stability concerns, and scaling limitations. Most electrocatalysts struggle to maintain performance beyond several hundred hours of operation, presenting a significant barrier to industrial adoption. Additionally, current density achievements typically remain below the 300 mA/cm² threshold required for commercial viability.
Traditional methods benefit from technological maturity and established infrastructure but suffer from high energy demands and reliance on fossil-derived hydrogen sources. The thermodynamic limitations of these processes result in conversion efficiencies typically below 60%, with substantial heat losses and greenhouse gas emissions throughout the production chain.
Geographic distribution of technology development shows concentration of electrocatalytic innovation in North America, Western Europe, and East Asia, particularly in academic and research institutions. Traditional conversion technologies are more widely distributed but with significant industrial capacity centered in regions with established petrochemical infrastructure.
The economic gap between approaches remains substantial, with traditional methods benefiting from decades of process optimization and economies of scale. Current production costs for electrocatalytic pathways exceed traditional methods by factors of 2-5× depending on the target product, though this gap has narrowed significantly over the past five years as renewable electricity costs have declined.
Regulatory frameworks increasingly favor low-carbon production methods, creating potential advantages for electrocatalytic approaches despite their current cost premium. Carbon pricing mechanisms, when applied comprehensively, can significantly alter the competitive landscape between these technological approaches.
Current Electrocatalytic CO2 Conversion Methods
01 Metal-based catalysts for CO2 electroreduction
Metal-based catalysts play a crucial role in the electrochemical reduction of CO2 to valuable products. Various metals such as copper, silver, gold, and their alloys exhibit different selectivity and efficiency for converting CO2 into specific products like carbon monoxide, formate, or hydrocarbons. The catalyst structure, composition, and surface properties significantly influence the reaction pathways and product distribution in CO2 electroreduction processes.- Metal-based catalysts for CO2 electroreduction: Metal-based catalysts play a crucial role in the electrochemical reduction of CO2 to valuable products. Various metals such as copper, silver, gold, and their alloys exhibit different selectivity and efficiency for converting CO2 into specific products like carbon monoxide, formic acid, methanol, or hydrocarbons. The catalyst structure, morphology, and composition significantly influence the reaction pathways and product distribution, allowing for tailored approaches to CO2 valorization through electrocatalysis.
- Carbon-based materials for CO2 electroreduction: Carbon-based materials, including carbon nanotubes, graphene, and nitrogen-doped carbon structures, serve as effective electrocatalysts for CO2 reduction. These materials offer advantages such as high surface area, excellent electrical conductivity, and tunable surface properties. The incorporation of heteroatoms like nitrogen creates active sites that enhance catalytic activity and selectivity toward specific CO2 reduction products, making carbon-based catalysts promising alternatives to traditional metal catalysts.
- Reactor design and system optimization for CO2 electroreduction: The design of electrochemical reactors and system optimization are critical for efficient CO2 valorization. Innovations include flow cell configurations, gas diffusion electrodes, and membrane electrode assemblies that enhance mass transport and reaction kinetics. Advanced reactor designs address challenges such as CO2 solubility limitations, product separation, and scale-up considerations. Optimized systems integrate temperature control, pressure management, and electrolyte composition to maximize conversion efficiency and product selectivity.
- Electrolyte engineering for enhanced CO2 conversion: Electrolyte composition and properties significantly impact the performance of CO2 electroreduction processes. Researchers have developed specialized electrolytes containing ionic liquids, buffering agents, or co-catalysts that facilitate CO2 activation and conversion. Electrolyte engineering approaches focus on optimizing pH, conductivity, and CO2 solubility to enhance reaction rates and product selectivity. Novel electrolyte systems also address challenges related to catalyst stability and unwanted side reactions during the electrochemical process.
- Integration of renewable energy with CO2 electroreduction: The integration of renewable energy sources with CO2 electroreduction systems enables sustainable carbon utilization pathways. These integrated approaches couple intermittent renewable electricity from solar or wind with electrochemical CO2 conversion to produce valuable chemicals and fuels. Advanced control strategies and energy management systems optimize the operation under fluctuating power inputs. This integration creates a circular carbon economy where CO2 is transformed into useful products using renewable electricity, effectively storing renewable energy in chemical bonds.
02 Carbon-based electrocatalysts for CO2 conversion
Carbon-based materials serve as effective electrocatalysts for CO2 valorization. These include carbon nanotubes, graphene, carbon quantum dots, and nitrogen-doped carbon materials. The advantages of carbon-based catalysts include high surface area, excellent electrical conductivity, tunable surface chemistry, and cost-effectiveness. These materials can be functionalized or doped with heteroatoms to enhance their catalytic activity and selectivity toward specific CO2 reduction products.Expand Specific Solutions03 Reactor design and system optimization for CO2 electroreduction
The design of electrochemical reactors and system optimization are critical for efficient CO2 valorization. Various reactor configurations, including flow cells, membrane electrode assemblies, and gas diffusion electrodes, have been developed to overcome mass transport limitations and enhance reaction rates. Parameters such as electrode spacing, electrolyte composition, flow rate, and operating conditions (temperature, pressure, pH) significantly impact the performance and energy efficiency of CO2 electroreduction systems.Expand Specific Solutions04 Electrolyte engineering for enhanced CO2 conversion
Electrolyte composition and properties play a crucial role in CO2 electroreduction efficiency and selectivity. Researchers have explored various electrolyte systems including aqueous solutions with different pH values, ionic liquids, and organic solvents. The electrolyte affects CO2 solubility, mass transport, local pH near the electrode surface, and the stability of reaction intermediates. Additives and supporting electrolytes can be used to modify the local environment at the electrode-electrolyte interface to favor specific reaction pathways.Expand Specific Solutions05 Integration of renewable energy with CO2 electroreduction
Integrating renewable energy sources with CO2 electroreduction systems enables sustainable carbon capture and utilization. This approach involves coupling solar, wind, or other renewable energy technologies with electrochemical CO2 conversion processes to produce value-added chemicals and fuels. Such integrated systems can help balance the intermittent nature of renewable energy while simultaneously addressing CO2 emissions. Advanced control strategies and energy management systems are developed to optimize the performance of these integrated processes.Expand Specific Solutions
Leading Companies and Research Institutions in CO2 Valorization
Electrocatalytic CO2 valorization is emerging as a promising alternative to traditional carbon capture methods, currently positioned in the early growth phase of industry development. The global market for CO2 conversion technologies is expanding rapidly, projected to reach significant scale as decarbonization efforts intensify. While technical maturity varies across applications, research institutions like Dalian Institute of Chemical Physics, Zhejiang University, and TotalEnergies OneTech are making substantial advances in catalyst development and process efficiency. Academic players including Brown University and National University of Singapore are pioneering fundamental research, while commercial entities such as Topsoe A/S and Samsung Electronics are focusing on scalable implementation. The competitive landscape reflects a collaborative ecosystem where cross-sector partnerships between research institutions and industry are accelerating technology readiness for commercial deployment.
Dalian Institute of Chemical Physics of CAS
Technical Solution: Dalian Institute of Chemical Physics (DICP) has developed advanced electrocatalytic systems for CO2 valorization focusing on high-efficiency copper-based catalysts. Their approach involves precise control of copper catalyst morphology and electronic structure to enhance selectivity towards multi-carbon products like ethylene and ethanol. DICP's technology employs innovative electrode designs with hierarchical porous structures that facilitate mass transport and optimize the three-phase interface. Their system operates at industrially relevant current densities (>200 mA/cm²) while maintaining Faradaic efficiencies above 60% for C2+ products. Compared to traditional thermochemical methods, DICP's electrocatalytic approach operates at ambient conditions, significantly reducing energy requirements and enabling direct coupling with renewable electricity sources. Their integrated reactor designs incorporate membrane electrode assemblies that minimize the gap between cathode and anode, reducing ohmic losses and improving overall energy efficiency.
Strengths: Operates at ambient temperature and pressure, reducing energy consumption by approximately 40% compared to thermochemical routes. Direct utilization of renewable electricity enables carbon-neutral operation. Weaknesses: Current catalyst stability remains limited to several hundred hours, requiring improvement for industrial implementation. System scale-up faces challenges in maintaining performance metrics at larger dimensions.
Uchicago Argonne LLC
Technical Solution: Argonne National Laboratory has pioneered a hybrid approach to CO2 valorization that combines electrocatalytic reduction with subsequent thermochemical upgrading. Their system employs nanostructured silver and copper-based catalysts with precisely engineered interfaces to achieve high selectivity for CO and C2 products. The technology features a flow-cell configuration that addresses mass transport limitations inherent in traditional H-cell setups, enabling current densities exceeding 300 mA/cm². Argonne's approach includes innovative electrolyte engineering with ionic liquids and buffering agents to stabilize local pH and enhance product selectivity. Their comparative analysis demonstrates that electrocatalytic pathways can achieve carbon efficiency up to 90%, significantly higher than Fischer-Tropsch processes (typically 40-70%). The system incorporates in-situ spectroscopic monitoring techniques that allow real-time catalyst surface characterization and reaction mechanism elucidation, facilitating continuous process optimization. Argonne has also developed techno-economic models showing that their electrocatalytic approach becomes economically competitive with traditional methods when electricity prices fall below $0.04/kWh.
Strengths: Achieves higher atom economy and carbon efficiency than traditional thermochemical routes. Modular design allows for distributed implementation and flexible scaling. Weaknesses: Higher capital costs compared to established technologies. Current performance metrics require high-purity CO2 feedstock, limiting direct application to dilute sources like flue gas.
Key Patents and Breakthroughs in CO2 Electrocatalysis
Materials and methods for the electrochemical reduction of carbon dioxide
PatentPendingUS20240026551A1
Innovation
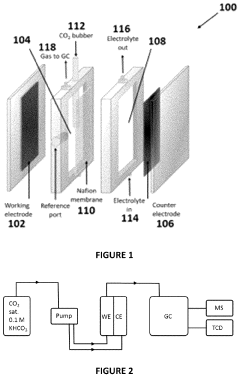
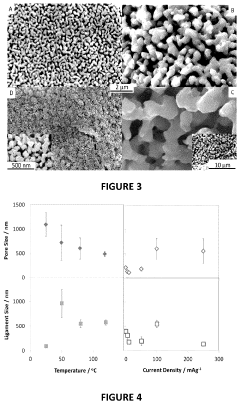
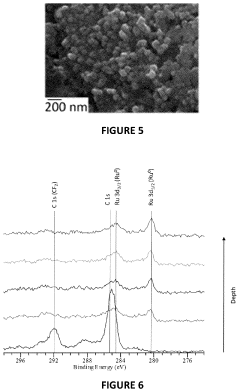
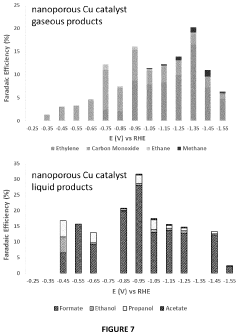
- The electrochemical reduction of carbon dioxide using a nanoporous Cu or Cu-M catalyst, such as Cu-Ru, in an electrochemical cell with a controlled potential, which selectively forms C2-C3 species like ethane, ethylene, ethanol, and propanol with higher Faradaic efficiency compared to methane and methanol.
Electrochemical conversion of carbon dioxide into value-added products
PatentActiveIN202442017597A
Innovation
- The method employs an electrochemical reactor with metallic Copper (Cu) or Cu-alloy symmetric electrodes and acidified water as electrolyte, achieving high FE and EE through asymmetric C-C coupling on the cathode surface, forming short-lived cation radical intermediates that react to produce ethanol and other value-added products.
Environmental Impact Assessment of CO2 Conversion Technologies
The environmental impact assessment of CO2 conversion technologies reveals significant differences between electrocatalytic CO2 valorization and traditional methods. Electrocatalytic approaches demonstrate considerable advantages in terms of carbon footprint reduction, with studies indicating potential greenhouse gas emissions reductions of 30-60% compared to conventional petrochemical routes when powered by renewable electricity sources.
Energy efficiency metrics show that electrocatalytic processes can operate at ambient temperatures and pressures, requiring substantially less energy input than thermochemical methods which typically demand high temperatures (300-900°C) and pressures (5-200 bar). This translates to reduced primary energy consumption, with recent life cycle assessments documenting energy savings of up to 45% for electrochemical CO2 reduction to formic acid compared to conventional synthesis pathways.
Water consumption represents another critical environmental parameter. Traditional CO2 conversion methods, particularly those involving Fischer-Tropsch processes, require significant water resources for cooling and separation processes. In contrast, electrocatalytic systems demonstrate 20-40% lower water intensity, though membrane-based systems still require water management considerations.
Land use impacts differ substantially between technologies. Traditional methods typically require large industrial complexes with extensive infrastructure, while electrocatalytic approaches can be modularly designed and distributed, potentially reducing land footprint by 25-50%. This distributed implementation model also offers advantages in reducing transportation emissions associated with centralized production facilities.
Waste generation profiles show electrocatalytic methods producing fewer toxic byproducts compared to traditional chemical synthesis routes. The absence of metal-organic catalysts and harsh solvents in many electrochemical approaches significantly reduces hazardous waste streams requiring specialized disposal procedures.
Biodiversity impacts must also be considered, particularly regarding resource extraction for catalyst materials. While traditional methods rely heavily on fossil resource extraction, electrocatalytic approaches require specific metals like copper, silver, and rare earth elements. Sustainable sourcing and recycling of these materials remains a critical challenge for ensuring the environmental benefits of electrocatalytic technologies are fully realized.
Air quality improvements represent a significant advantage of electrocatalytic methods, which produce virtually no NOx, SOx, or particulate emissions compared to combustion-based traditional processes. This benefit becomes particularly valuable when considering implementation in urban or industrially congested areas where air quality concerns are paramount.
Energy efficiency metrics show that electrocatalytic processes can operate at ambient temperatures and pressures, requiring substantially less energy input than thermochemical methods which typically demand high temperatures (300-900°C) and pressures (5-200 bar). This translates to reduced primary energy consumption, with recent life cycle assessments documenting energy savings of up to 45% for electrochemical CO2 reduction to formic acid compared to conventional synthesis pathways.
Water consumption represents another critical environmental parameter. Traditional CO2 conversion methods, particularly those involving Fischer-Tropsch processes, require significant water resources for cooling and separation processes. In contrast, electrocatalytic systems demonstrate 20-40% lower water intensity, though membrane-based systems still require water management considerations.
Land use impacts differ substantially between technologies. Traditional methods typically require large industrial complexes with extensive infrastructure, while electrocatalytic approaches can be modularly designed and distributed, potentially reducing land footprint by 25-50%. This distributed implementation model also offers advantages in reducing transportation emissions associated with centralized production facilities.
Waste generation profiles show electrocatalytic methods producing fewer toxic byproducts compared to traditional chemical synthesis routes. The absence of metal-organic catalysts and harsh solvents in many electrochemical approaches significantly reduces hazardous waste streams requiring specialized disposal procedures.
Biodiversity impacts must also be considered, particularly regarding resource extraction for catalyst materials. While traditional methods rely heavily on fossil resource extraction, electrocatalytic approaches require specific metals like copper, silver, and rare earth elements. Sustainable sourcing and recycling of these materials remains a critical challenge for ensuring the environmental benefits of electrocatalytic technologies are fully realized.
Air quality improvements represent a significant advantage of electrocatalytic methods, which produce virtually no NOx, SOx, or particulate emissions compared to combustion-based traditional processes. This benefit becomes particularly valuable when considering implementation in urban or industrially congested areas where air quality concerns are paramount.
Economic Viability and Scalability Analysis
The economic viability of electrocatalytic CO2 valorization compared to traditional methods presents a complex landscape of cost factors, efficiency metrics, and scalability considerations. Current economic analyses indicate that electrocatalytic approaches face significant challenges in competing with established industrial processes, primarily due to higher capital expenditure requirements and energy costs. The levelized cost of production for electrocatalytic CO2 conversion to valuable chemicals typically ranges from $50-200 per ton higher than conventional fossil-based routes, creating a substantial economic barrier to widespread adoption.
Energy efficiency remains a critical economic factor, with electrocatalytic systems currently achieving 40-65% energy conversion efficiency compared to 75-85% for optimized traditional methods. This efficiency gap directly translates to operational cost differences that impact long-term economic viability. Additionally, catalyst materials for electrocatalytic processes often incorporate precious metals like silver, gold, or platinum, contributing to higher production costs compared to conventional catalysts used in traditional CO2 conversion.
Scalability presents another significant challenge for electrocatalytic technologies. While traditional methods benefit from decades of industrial optimization and economies of scale, electrocatalytic systems are predominantly operating at laboratory or pilot scales. The transition to industrial scale faces technical hurdles including maintaining catalyst performance at scale, managing heat transfer in larger reactors, and addressing electrode degradation issues that emerge at commercial production volumes.
Infrastructure requirements further complicate the economic equation. Electrocatalytic processes demand integration with renewable electricity sources to achieve their full environmental benefits, necessitating substantial investments in grid connections or dedicated renewable generation capacity. Traditional methods, conversely, can utilize existing industrial infrastructure with minimal modifications, providing a significant economic advantage in the near term.
Recent techno-economic assessments suggest that electrocatalytic CO2 valorization could achieve cost parity with traditional methods under specific conditions: carbon pricing mechanisms exceeding $40-60 per ton CO2, renewable electricity costs below $0.04/kWh, and catalyst performance improvements that double current conversion efficiencies. Several demonstration projects in Europe and North America are approaching these thresholds, indicating potential economic viability within specialized market segments or regulatory environments.
The pathway to economic competitiveness will likely require a combination of technological advances, policy support, and strategic implementation in niche applications where electrocatalytic approaches offer unique advantages. Industries with access to low-cost renewable electricity, high-value product streams, or stringent carbon reduction requirements represent the most promising initial deployment opportunities for these emerging technologies.
Energy efficiency remains a critical economic factor, with electrocatalytic systems currently achieving 40-65% energy conversion efficiency compared to 75-85% for optimized traditional methods. This efficiency gap directly translates to operational cost differences that impact long-term economic viability. Additionally, catalyst materials for electrocatalytic processes often incorporate precious metals like silver, gold, or platinum, contributing to higher production costs compared to conventional catalysts used in traditional CO2 conversion.
Scalability presents another significant challenge for electrocatalytic technologies. While traditional methods benefit from decades of industrial optimization and economies of scale, electrocatalytic systems are predominantly operating at laboratory or pilot scales. The transition to industrial scale faces technical hurdles including maintaining catalyst performance at scale, managing heat transfer in larger reactors, and addressing electrode degradation issues that emerge at commercial production volumes.
Infrastructure requirements further complicate the economic equation. Electrocatalytic processes demand integration with renewable electricity sources to achieve their full environmental benefits, necessitating substantial investments in grid connections or dedicated renewable generation capacity. Traditional methods, conversely, can utilize existing industrial infrastructure with minimal modifications, providing a significant economic advantage in the near term.
Recent techno-economic assessments suggest that electrocatalytic CO2 valorization could achieve cost parity with traditional methods under specific conditions: carbon pricing mechanisms exceeding $40-60 per ton CO2, renewable electricity costs below $0.04/kWh, and catalyst performance improvements that double current conversion efficiencies. Several demonstration projects in Europe and North America are approaching these thresholds, indicating potential economic viability within specialized market segments or regulatory environments.
The pathway to economic competitiveness will likely require a combination of technological advances, policy support, and strategic implementation in niche applications where electrocatalytic approaches offer unique advantages. Industries with access to low-cost renewable electricity, high-value product streams, or stringent carbon reduction requirements represent the most promising initial deployment opportunities for these emerging technologies.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!