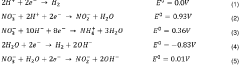Assessment of Coating Effects in Electrocatalytic CO2 Valorization
OCT 23, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Electrocatalytic CO2 Valorization Background and Objectives
Electrocatalytic CO2 valorization has emerged as a promising approach to address the dual challenges of climate change and sustainable energy production. The concept involves converting carbon dioxide, a major greenhouse gas, into value-added chemicals and fuels using electrical energy. This technology has evolved significantly since its inception in the 1980s, with major breakthroughs occurring in catalyst design, reactor engineering, and process optimization over the past decade.
The evolution of CO2 electroreduction technology has been marked by several key milestones, including the development of copper-based catalysts for multi-carbon product formation, the introduction of gas diffusion electrodes for enhanced mass transport, and the integration of renewable electricity sources for truly sustainable operation. Recent advances in nanomaterial synthesis and characterization techniques have further accelerated progress in this field.
Current technological trends point toward the increasing importance of surface modifications and coatings as critical factors in determining catalyst performance. These coatings can significantly alter the electronic structure, active site density, and stability of electrocatalysts, thereby influencing product selectivity, energy efficiency, and operational lifetime. The strategic application of coatings represents a promising frontier for overcoming persistent challenges in CO2 electroreduction.
The primary objective of this assessment is to comprehensively evaluate the effects of various coating strategies on electrocatalytic CO2 valorization performance. Specifically, we aim to understand how different coating materials, thicknesses, and deposition methods influence catalyst activity, selectivity toward high-value products, and long-term stability under industrially relevant conditions.
Additionally, this investigation seeks to establish correlations between coating properties and electrochemical performance parameters, with the goal of developing predictive models to guide future catalyst design. By systematically analyzing the structure-property-performance relationships of coated electrocatalysts, we intend to identify optimal coating configurations for specific target products and operating conditions.
The ultimate goal is to advance the fundamental understanding of interfacial phenomena in coated electrocatalysts while simultaneously providing practical guidelines for the rational design of next-generation CO2 conversion systems. This knowledge will contribute to accelerating the commercial deployment of CO2 electroreduction technology, supporting the transition toward a circular carbon economy where CO2 is viewed as a valuable feedstock rather than a waste product.
The evolution of CO2 electroreduction technology has been marked by several key milestones, including the development of copper-based catalysts for multi-carbon product formation, the introduction of gas diffusion electrodes for enhanced mass transport, and the integration of renewable electricity sources for truly sustainable operation. Recent advances in nanomaterial synthesis and characterization techniques have further accelerated progress in this field.
Current technological trends point toward the increasing importance of surface modifications and coatings as critical factors in determining catalyst performance. These coatings can significantly alter the electronic structure, active site density, and stability of electrocatalysts, thereby influencing product selectivity, energy efficiency, and operational lifetime. The strategic application of coatings represents a promising frontier for overcoming persistent challenges in CO2 electroreduction.
The primary objective of this assessment is to comprehensively evaluate the effects of various coating strategies on electrocatalytic CO2 valorization performance. Specifically, we aim to understand how different coating materials, thicknesses, and deposition methods influence catalyst activity, selectivity toward high-value products, and long-term stability under industrially relevant conditions.
Additionally, this investigation seeks to establish correlations between coating properties and electrochemical performance parameters, with the goal of developing predictive models to guide future catalyst design. By systematically analyzing the structure-property-performance relationships of coated electrocatalysts, we intend to identify optimal coating configurations for specific target products and operating conditions.
The ultimate goal is to advance the fundamental understanding of interfacial phenomena in coated electrocatalysts while simultaneously providing practical guidelines for the rational design of next-generation CO2 conversion systems. This knowledge will contribute to accelerating the commercial deployment of CO2 electroreduction technology, supporting the transition toward a circular carbon economy where CO2 is viewed as a valuable feedstock rather than a waste product.
Market Analysis for CO2 Conversion Technologies
The global market for CO2 conversion technologies is experiencing significant growth, driven by increasing environmental concerns and regulatory pressures to reduce carbon emissions. Current market valuations indicate that the CO2 utilization market reached approximately 2 billion USD in 2020, with projections suggesting growth to 550 billion USD by 2040 according to IEA estimates. This represents a compound annual growth rate of over 20%, positioning it as one of the fastest-growing segments within green technology.
Electrocatalytic CO2 valorization specifically has emerged as a promising subsector, with particular interest in coating technologies that enhance catalyst performance. Market research indicates that improved catalyst coatings can potentially reduce operational costs by 15-30% while increasing conversion efficiency by up to 40% compared to uncoated catalysts, creating substantial economic incentives for adoption.
Regionally, North America and Europe currently lead in research and commercial deployment of CO2 conversion technologies, collectively accounting for approximately 65% of the global market share. However, Asia-Pacific, particularly China and South Korea, is demonstrating the fastest growth rate at 25-30% annually, fueled by aggressive government initiatives and industrial investment in carbon neutrality technologies.
By application segment, the market for CO2-derived chemicals and fuels represents the largest share at 45%, followed by enhanced oil recovery applications at 30%. The remaining market is distributed among mineralization processes, biological conversion, and other emerging applications. Notably, the market for CO2-derived polymers and materials is experiencing particularly rapid growth, estimated at 35% annually.
Demand drivers include increasingly stringent carbon pricing mechanisms, with carbon prices in the EU ETS reaching record levels of over 90 EUR per ton in 2022. Additionally, corporate sustainability commitments have created a robust voluntary market for carbon utilization technologies, with major companies in energy, chemical, and manufacturing sectors investing heavily in these solutions to meet net-zero pledges.
Market challenges include high capital costs for implementation, with typical industrial-scale electrocatalytic systems requiring investments of 20-50 million USD. Technical barriers related to catalyst durability and selectivity also remain significant market constraints, with current systems typically requiring replacement or regeneration after 2,000-5,000 operating hours, representing a substantial operational expense.
Future market growth is expected to be particularly strong in sectors producing high-value chemicals from CO2, including methanol, formic acid, and ethylene, where profit margins can offset the currently higher production costs compared to conventional petrochemical routes.
Electrocatalytic CO2 valorization specifically has emerged as a promising subsector, with particular interest in coating technologies that enhance catalyst performance. Market research indicates that improved catalyst coatings can potentially reduce operational costs by 15-30% while increasing conversion efficiency by up to 40% compared to uncoated catalysts, creating substantial economic incentives for adoption.
Regionally, North America and Europe currently lead in research and commercial deployment of CO2 conversion technologies, collectively accounting for approximately 65% of the global market share. However, Asia-Pacific, particularly China and South Korea, is demonstrating the fastest growth rate at 25-30% annually, fueled by aggressive government initiatives and industrial investment in carbon neutrality technologies.
By application segment, the market for CO2-derived chemicals and fuels represents the largest share at 45%, followed by enhanced oil recovery applications at 30%. The remaining market is distributed among mineralization processes, biological conversion, and other emerging applications. Notably, the market for CO2-derived polymers and materials is experiencing particularly rapid growth, estimated at 35% annually.
Demand drivers include increasingly stringent carbon pricing mechanisms, with carbon prices in the EU ETS reaching record levels of over 90 EUR per ton in 2022. Additionally, corporate sustainability commitments have created a robust voluntary market for carbon utilization technologies, with major companies in energy, chemical, and manufacturing sectors investing heavily in these solutions to meet net-zero pledges.
Market challenges include high capital costs for implementation, with typical industrial-scale electrocatalytic systems requiring investments of 20-50 million USD. Technical barriers related to catalyst durability and selectivity also remain significant market constraints, with current systems typically requiring replacement or regeneration after 2,000-5,000 operating hours, representing a substantial operational expense.
Future market growth is expected to be particularly strong in sectors producing high-value chemicals from CO2, including methanol, formic acid, and ethylene, where profit margins can offset the currently higher production costs compared to conventional petrochemical routes.
Current Coating Technologies and Challenges in CO2 Electrocatalysis
The field of electrocatalytic CO2 valorization has witnessed significant advancements in coating technologies, which play a crucial role in enhancing catalyst performance. Currently, several coating methodologies dominate the landscape, each with distinct advantages and limitations. Physical vapor deposition (PVD) techniques, including magnetron sputtering and electron beam evaporation, offer precise control over coating thickness and composition but often struggle with uniform coverage on complex substrate geometries. Chemical vapor deposition (CVD) provides better conformality but typically requires elevated temperatures that may compromise substrate integrity.
Atomic layer deposition (ALD) has emerged as a powerful approach for creating ultrathin, conformal coatings with atomic-level precision. This technique enables exceptional control over coating thickness and composition, making it particularly valuable for creating protective layers that maintain electron transfer while preventing catalyst degradation. However, ALD faces challenges related to scalability and relatively slow deposition rates, limiting its industrial application despite its technical superiority.
Solution-based methods, including electrodeposition, sol-gel processing, and dip-coating, represent more economical alternatives with greater scalability potential. Electrodeposition, in particular, has gained traction for its ability to create tailored catalyst structures with controlled morphology. Nevertheless, these methods often struggle with reproducibility and precise thickness control compared to vacuum-based techniques.
A significant challenge across all coating technologies is achieving the optimal balance between protective functionality and catalytic activity. Coatings must be sufficiently robust to shield catalysts from degradation mechanisms while remaining permeable enough to facilitate efficient electron transfer and reactant access. This delicate balance remains difficult to achieve consistently, especially when scaling from laboratory to industrial applications.
Durability presents another major hurdle, as coatings must withstand harsh electrochemical conditions, including extreme pH environments and potential cycling. Current coating technologies frequently demonstrate promising initial performance but suffer from degradation over extended operation periods. This degradation manifests as delamination, cracking, or chemical transformation of the coating material, ultimately compromising catalyst longevity.
Integration challenges also persist when incorporating coated catalysts into practical electrolyzer designs. Issues related to electrical contact resistance, mass transport limitations, and mechanical stability during scale-up often diminish the performance advantages observed in laboratory settings. Additionally, the economic viability of advanced coating technologies remains questionable for large-scale CO2 conversion applications, where cost considerations frequently outweigh marginal performance improvements.
Recent research has begun exploring multifunctional coatings that simultaneously address multiple challenges, such as combining protective properties with enhanced selectivity or activity. These approaches include gradient compositions, multilayer architectures, and hybrid organic-inorganic coatings, which show promise but require further development before commercial implementation.
Atomic layer deposition (ALD) has emerged as a powerful approach for creating ultrathin, conformal coatings with atomic-level precision. This technique enables exceptional control over coating thickness and composition, making it particularly valuable for creating protective layers that maintain electron transfer while preventing catalyst degradation. However, ALD faces challenges related to scalability and relatively slow deposition rates, limiting its industrial application despite its technical superiority.
Solution-based methods, including electrodeposition, sol-gel processing, and dip-coating, represent more economical alternatives with greater scalability potential. Electrodeposition, in particular, has gained traction for its ability to create tailored catalyst structures with controlled morphology. Nevertheless, these methods often struggle with reproducibility and precise thickness control compared to vacuum-based techniques.
A significant challenge across all coating technologies is achieving the optimal balance between protective functionality and catalytic activity. Coatings must be sufficiently robust to shield catalysts from degradation mechanisms while remaining permeable enough to facilitate efficient electron transfer and reactant access. This delicate balance remains difficult to achieve consistently, especially when scaling from laboratory to industrial applications.
Durability presents another major hurdle, as coatings must withstand harsh electrochemical conditions, including extreme pH environments and potential cycling. Current coating technologies frequently demonstrate promising initial performance but suffer from degradation over extended operation periods. This degradation manifests as delamination, cracking, or chemical transformation of the coating material, ultimately compromising catalyst longevity.
Integration challenges also persist when incorporating coated catalysts into practical electrolyzer designs. Issues related to electrical contact resistance, mass transport limitations, and mechanical stability during scale-up often diminish the performance advantages observed in laboratory settings. Additionally, the economic viability of advanced coating technologies remains questionable for large-scale CO2 conversion applications, where cost considerations frequently outweigh marginal performance improvements.
Recent research has begun exploring multifunctional coatings that simultaneously address multiple challenges, such as combining protective properties with enhanced selectivity or activity. These approaches include gradient compositions, multilayer architectures, and hybrid organic-inorganic coatings, which show promise but require further development before commercial implementation.
State-of-the-Art Coating Solutions for CO2 Electrocatalysis
01 Metal-based coatings for enhanced electrocatalytic activity
Metal-based coatings can significantly enhance electrocatalytic performance by providing active sites for catalytic reactions. These coatings typically include noble metals (platinum, palladium), transition metals, and their alloys which offer excellent conductivity and catalytic properties. The coating structure and composition can be tailored to optimize electron transfer rates and increase catalytic efficiency for specific reactions such as oxygen reduction or hydrogen evolution.- Metal-based coatings for enhanced electrocatalytic activity: Metal-based coatings can significantly enhance electrocatalytic performance by providing active sites for catalytic reactions. These coatings typically include noble metals (platinum, palladium), transition metals, and their alloys which offer excellent conductivity and catalytic properties. The coating structure and composition can be tailored to optimize electron transfer rates and increase the surface area available for reactions, leading to improved catalytic efficiency and selectivity in electrochemical processes.
- Carbon-based materials as electrocatalytic coatings: Carbon-based materials such as graphene, carbon nanotubes, and doped carbon structures serve as effective electrocatalytic coatings. These materials offer high surface area, excellent electrical conductivity, and good chemical stability. When used as coatings in electrocatalysis, they can enhance electron transfer, provide numerous active sites, and improve catalyst durability. Additionally, carbon-based coatings can be functionalized or doped with heteroatoms to further tune their catalytic properties for specific electrochemical reactions.
- Nanostructured coatings for improved catalytic performance: Nanostructured coatings significantly enhance electrocatalytic performance through increased surface area and improved mass transport properties. These coatings feature controlled morphologies such as nanowires, nanoparticles, and nanoporous structures that expose more active sites for catalytic reactions. The nanoscale dimensions also facilitate faster electron transfer and reactant diffusion, leading to higher catalytic activity and efficiency. Various deposition techniques can be employed to create these nanostructured coatings with precise control over their architecture and composition.
- Composite and hybrid coating systems for synergistic catalytic effects: Composite and hybrid coating systems combine multiple materials to achieve synergistic catalytic effects in electrocatalysis. These systems typically integrate metals with metal oxides, polymers, or carbon-based materials to enhance overall performance. The combination of different components allows for complementary functionalities, such as improved conductivity from one component and enhanced catalytic activity from another. These hybrid coatings often demonstrate superior stability, selectivity, and activity compared to single-component catalysts, making them valuable for various electrochemical applications.
- Surface modification techniques for optimizing electrocatalytic coatings: Surface modification techniques play a crucial role in optimizing electrocatalytic coatings by altering their physical and chemical properties. These techniques include plasma treatment, chemical functionalization, atomic layer deposition, and thermal annealing. Such modifications can introduce specific functional groups, adjust surface energy, create defects that serve as active sites, or establish preferred crystallographic orientations. By carefully controlling surface properties, these methods enhance catalyst-substrate interactions, improve reactant adsorption, and ultimately boost the overall catalytic performance in electrochemical reactions.
02 Carbon-based materials as electrocatalytic coating substrates
Carbon-based materials serve as excellent substrates for electrocatalytic coatings due to their high surface area, good electrical conductivity, and chemical stability. Materials such as carbon nanotubes, graphene, and carbon fibers can be functionalized or doped to enhance their catalytic properties. When combined with active catalytic components, these carbon-based substrates create synergistic effects that improve reaction kinetics and overall catalytic performance in electrochemical applications.Expand Specific Solutions03 Nanostructured coatings for improved catalytic efficiency
Nanostructured coatings offer enhanced catalytic effects due to their high surface-to-volume ratio and unique electronic properties. These coatings can be engineered with specific morphologies such as nanowires, nanoparticles, or nanosheets to maximize active site exposure. The nanoscale architecture facilitates mass transport, reduces activation energy barriers, and improves reaction kinetics, resulting in superior electrocatalytic performance for applications including fuel cells, water splitting, and CO2 reduction.Expand Specific Solutions04 Composite and hybrid coating materials for synergistic catalytic effects
Composite and hybrid coating materials combine multiple components to achieve synergistic catalytic effects that exceed the performance of individual materials. These coatings typically integrate metals with metal oxides, sulfides, or carbon-based materials to create unique interfaces that enhance electron transfer and catalytic activity. The strategic combination of materials can provide complementary functionalities, improved stability, and tailored selectivity for specific electrocatalytic reactions.Expand Specific Solutions05 Surface modification techniques to enhance electrocatalytic performance
Various surface modification techniques can be employed to enhance the electrocatalytic performance of coatings. These include doping with heteroatoms, creating defects, controlling surface functional groups, and engineering interfaces. Such modifications alter the electronic structure, adsorption properties, and active site density of the catalyst surface. Advanced techniques like atomic layer deposition and plasma treatment enable precise control over surface properties, resulting in optimized catalytic activity, selectivity, and stability.Expand Specific Solutions
Leading Companies and Research Institutions in CO2 Valorization
The electrocatalytic CO2 valorization market is in its early growth phase, characterized by intensive R&D activities and emerging commercial applications. The global market size is projected to expand significantly as carbon utilization technologies gain traction amid climate change concerns. Technologically, coating effects represent a critical frontier, with varying degrees of maturity across applications. Leading players demonstrate diverse approaches: academic institutions (Dalian Institute of Chemical Physics, College de France, Brown University) focus on fundamental research; industrial entities (TotalEnergies, Industrie De Nora, Saudi Aramco) develop scalable solutions; while materials specialists (AGC, Panasonic, Covestro) contribute advanced coating technologies. The competitive landscape features strong collaboration between research institutions and industry partners, with Asian and European organizations particularly active in patent filings and technology commercialization.
Dalian Institute of Chemical Physics of CAS
Technical Solution: Dalian Institute of Chemical Physics (DICP) has developed innovative coating technologies for CO2 electroreduction catalysts, focusing on atomic layer deposition (ALD) techniques to create precisely controlled metal oxide overlayers on copper catalysts. Their approach involves depositing ultrathin (2-5 nm) TiO2, ZnO, or Al2O3 coatings that modify the electronic structure of the catalyst surface while maintaining permeability for reactants and products. DICP researchers have demonstrated that these coatings can significantly enhance C2+ product selectivity by up to 30% compared to uncoated catalysts, while suppressing the competing hydrogen evolution reaction. Their recent work has shown that TiO2-coated copper catalysts achieve Faradaic efficiencies exceeding 60% for ethylene production at industrially relevant current densities (>200 mA/cm²), representing a substantial improvement over conventional catalysts. Additionally, DICP has pioneered the use of in-situ characterization techniques to understand the dynamic changes in coating structures during electrocatalysis.
Strengths: Exceptional precision in coating thickness control down to sub-nanometer scale; demonstrated long-term stability improvements with catalysts maintaining performance for >100 hours; strong expertise in mechanistic understanding of coating effects. Weaknesses: Current coating methods are relatively expensive and challenging to scale up for industrial applications; some coating materials may dissolve under harsh reaction conditions.
Industrie De Nora SpA
Technical Solution: Industrie De Nora has developed proprietary coating technologies specifically optimized for industrial-scale CO2 electroreduction processes. Their approach centers on dimensionally stable electrode (DSE) technology, leveraging decades of expertise in electrochemical coatings for chlor-alkali and water treatment applications. De Nora's innovation involves multi-layer oxide coatings applied to titanium substrates, with carefully engineered compositions that balance catalytic activity, selectivity, and durability. Their most advanced systems utilize mixed metal oxide coatings containing copper, silver, and tin components dispersed in an iridium oxide matrix, providing synergistic catalytic effects for CO2 reduction while maintaining exceptional mechanical and chemical stability. These coatings are applied using proprietary thermal decomposition processes that ensure uniform coverage across large electrode surfaces (>1 m²), addressing a critical challenge in scaling up CO2 electroreduction technology. De Nora has demonstrated that their coated electrodes can achieve current densities exceeding 300 mA/cm² with Faradaic efficiencies of >70% toward CO and formate production in continuous operation for thousands of hours. Their systems have been successfully integrated into pilot-scale CO2 electrolyzers operating at the 10-100 kW scale, representing some of the most advanced demonstrations of this technology in industrial settings.
Strengths: Unparalleled expertise in industrial-scale electrode coating manufacturing; demonstrated long-term stability under harsh operating conditions; established manufacturing infrastructure capable of producing coated electrodes at commercial scale. Weaknesses: Current coating technologies are more focused on CO/formate production rather than higher-value C2+ products; relatively high precious metal content in some coating formulations may impact economic viability.
Critical Patents and Research on Catalyst Coating Effects
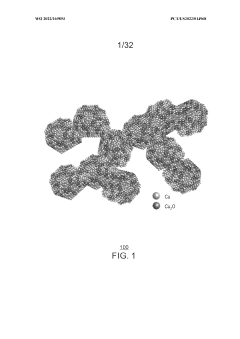
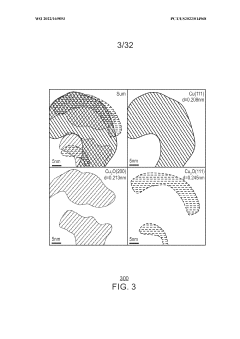
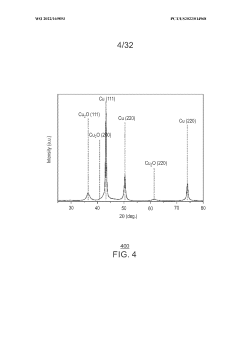
Porous copper/copper oxide xerogel catalyst
PatentWO2022169893A1
Innovation
- A porous copper/copper oxide xerogel catalyst is synthesized through wet-chemistry, featuring a high surface area and dense Cu°-Cu+ interfaces, which enhances CO2 activation and C-C dimerization, resulting in improved Faradaic efficiency and partial current density for ethanol production.
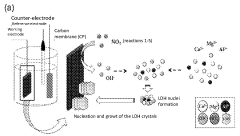
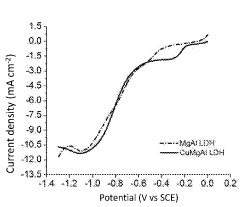
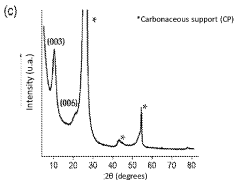
Layered double-hydroxide catalytic coatings containing: CU, mg e al, achievable electro¬ chemically, for uses such as electrochemical reduction of carbon dioxide
PatentWO2024127296A1
Innovation
- A nanostructured composite material with a layered hydrotalcite-type ternary structure of CuMgAl LDH, containing metal copper and cuprous ion as a redox couple, is developed, which is electrochemically deposited on a carbon gas diffusion membrane to enhance catalytic activity and selectivity for C2 products.
Sustainability Impact and Carbon Footprint Assessment
The electrocatalytic valorization of CO2 represents a promising approach to mitigate climate change while producing valuable chemicals. However, the sustainability credentials of this technology must be rigorously assessed to ensure its implementation delivers genuine environmental benefits. The coating processes used in catalyst preparation can significantly impact the overall sustainability profile of CO2 conversion technologies.
Life Cycle Assessment (LCA) studies indicate that coating materials and methods contribute substantially to the carbon footprint of electrocatalytic systems. For instance, precious metal coatings like platinum and gold, while highly effective catalytically, require energy-intensive mining and refining processes that generate significant greenhouse gas emissions. Alternative coating materials such as transition metal oxides or carbon-based materials generally exhibit lower environmental impacts during production.
The manufacturing processes for catalyst coatings also merit careful consideration. Physical vapor deposition and chemical vapor deposition techniques typically consume substantial energy, whereas solution-based methods like electrodeposition or sol-gel processes often present lower energy requirements and reduced environmental impacts. Recent research indicates that atomic layer deposition, despite its precision, may have a higher carbon footprint per unit area compared to conventional wet chemistry approaches.
Durability of coatings directly influences sustainability metrics. Longer-lasting coatings reduce the frequency of catalyst replacement, thereby decreasing material consumption and waste generation over the system lifetime. Studies demonstrate that robust coatings can extend catalyst lifespans by 200-300%, significantly improving the sustainability profile of CO2 valorization processes.
Water consumption represents another critical sustainability parameter. Hydrothermal coating methods typically require substantial water inputs, whereas dry coating techniques minimize water usage but may increase energy demands. This trade-off necessitates context-specific optimization based on local resource availability and constraints.
End-of-life considerations for catalyst coatings reveal additional sustainability implications. Recyclability varies significantly among coating materials, with precious metals offering excellent recovery potential despite energy-intensive separation processes. Conversely, many composite coatings present recycling challenges due to complex material integration.
When evaluating the net environmental impact, the carbon emissions avoided through CO2 conversion must be balanced against emissions generated during catalyst production and operation. Current best practices in coating technology can achieve carbon payback periods ranging from 6 months to 3 years, depending on system efficiency and operational parameters. Emerging coating innovations focusing on earth-abundant materials and low-energy deposition techniques promise to further improve this sustainability equation.
Life Cycle Assessment (LCA) studies indicate that coating materials and methods contribute substantially to the carbon footprint of electrocatalytic systems. For instance, precious metal coatings like platinum and gold, while highly effective catalytically, require energy-intensive mining and refining processes that generate significant greenhouse gas emissions. Alternative coating materials such as transition metal oxides or carbon-based materials generally exhibit lower environmental impacts during production.
The manufacturing processes for catalyst coatings also merit careful consideration. Physical vapor deposition and chemical vapor deposition techniques typically consume substantial energy, whereas solution-based methods like electrodeposition or sol-gel processes often present lower energy requirements and reduced environmental impacts. Recent research indicates that atomic layer deposition, despite its precision, may have a higher carbon footprint per unit area compared to conventional wet chemistry approaches.
Durability of coatings directly influences sustainability metrics. Longer-lasting coatings reduce the frequency of catalyst replacement, thereby decreasing material consumption and waste generation over the system lifetime. Studies demonstrate that robust coatings can extend catalyst lifespans by 200-300%, significantly improving the sustainability profile of CO2 valorization processes.
Water consumption represents another critical sustainability parameter. Hydrothermal coating methods typically require substantial water inputs, whereas dry coating techniques minimize water usage but may increase energy demands. This trade-off necessitates context-specific optimization based on local resource availability and constraints.
End-of-life considerations for catalyst coatings reveal additional sustainability implications. Recyclability varies significantly among coating materials, with precious metals offering excellent recovery potential despite energy-intensive separation processes. Conversely, many composite coatings present recycling challenges due to complex material integration.
When evaluating the net environmental impact, the carbon emissions avoided through CO2 conversion must be balanced against emissions generated during catalyst production and operation. Current best practices in coating technology can achieve carbon payback periods ranging from 6 months to 3 years, depending on system efficiency and operational parameters. Emerging coating innovations focusing on earth-abundant materials and low-energy deposition techniques promise to further improve this sustainability equation.
Scalability and Industrial Implementation Considerations
The scalability of electrocatalytic CO2 valorization technologies from laboratory scale to industrial implementation presents significant engineering challenges that must be addressed systematically. When considering coating effects specifically, the transition to larger scales requires careful evaluation of coating uniformity, durability, and performance consistency across larger electrode surfaces. Current laboratory-scale coating methods such as spin coating, electrodeposition, and physical vapor deposition often face limitations when applied to industrial dimensions.
Material consumption and cost efficiency become critical factors at scale. While precious metal catalysts like gold and platinum demonstrate excellent performance in laboratory settings, their economic viability at industrial scale remains questionable. Alternative approaches utilizing earth-abundant materials with specialized coatings have shown promising results, potentially offering more cost-effective solutions for large-scale implementation.
Process integration represents another crucial consideration, as electrocatalytic CO2 conversion systems must be seamlessly incorporated into existing industrial infrastructure. This includes compatibility with upstream CO2 capture technologies and downstream product separation processes. The coating technologies selected must withstand the operational conditions of integrated systems, including potential contaminants from industrial CO2 streams that may poison or degrade catalyst coatings over time.
Energy efficiency at scale directly impacts the economic viability of these technologies. Coating designs that minimize overpotential requirements while maintaining high current densities become increasingly important as systems scale up. Recent advances in hierarchical coating architectures that optimize both mass transport and electron transfer show particular promise for industrial applications, though their long-term stability under continuous operation requires further investigation.
Manufacturing reproducibility presents significant challenges when scaling coating technologies. Techniques that work precisely in controlled laboratory environments may exhibit variability when implemented in industrial production lines. Advanced quality control methodologies, including in-line characterization techniques and real-time monitoring systems, are being developed to ensure coating consistency across large production batches.
Regulatory considerations also influence industrial implementation pathways. Coatings containing nanomaterials or potentially hazardous components face additional scrutiny regarding environmental impact and worker safety. Developing environmentally benign coating formulations that maintain high catalytic performance represents an active area of research with significant implications for commercial deployment timelines.
Material consumption and cost efficiency become critical factors at scale. While precious metal catalysts like gold and platinum demonstrate excellent performance in laboratory settings, their economic viability at industrial scale remains questionable. Alternative approaches utilizing earth-abundant materials with specialized coatings have shown promising results, potentially offering more cost-effective solutions for large-scale implementation.
Process integration represents another crucial consideration, as electrocatalytic CO2 conversion systems must be seamlessly incorporated into existing industrial infrastructure. This includes compatibility with upstream CO2 capture technologies and downstream product separation processes. The coating technologies selected must withstand the operational conditions of integrated systems, including potential contaminants from industrial CO2 streams that may poison or degrade catalyst coatings over time.
Energy efficiency at scale directly impacts the economic viability of these technologies. Coating designs that minimize overpotential requirements while maintaining high current densities become increasingly important as systems scale up. Recent advances in hierarchical coating architectures that optimize both mass transport and electron transfer show particular promise for industrial applications, though their long-term stability under continuous operation requires further investigation.
Manufacturing reproducibility presents significant challenges when scaling coating technologies. Techniques that work precisely in controlled laboratory environments may exhibit variability when implemented in industrial production lines. Advanced quality control methodologies, including in-line characterization techniques and real-time monitoring systems, are being developed to ensure coating consistency across large production batches.
Regulatory considerations also influence industrial implementation pathways. Coatings containing nanomaterials or potentially hazardous components face additional scrutiny regarding environmental impact and worker safety. Developing environmentally benign coating formulations that maintain high catalytic performance represents an active area of research with significant implications for commercial deployment timelines.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!