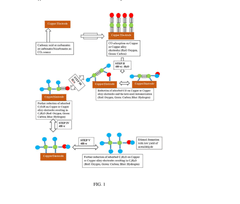
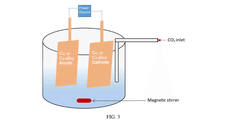
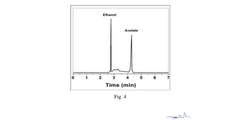
Electrocatalytic CO2 Valorization in Compliant Pharmaceutical Processes
OCT 23, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CO2 Valorization Background and Objectives
Carbon dioxide (CO2) valorization represents a transformative approach to addressing the dual challenges of climate change and sustainable chemical production. The concept involves converting waste CO2 emissions into valuable chemical products, effectively turning an environmental liability into an economic asset. This approach has gained significant momentum over the past decade as global efforts to reduce carbon footprints have intensified, particularly in carbon-intensive industries such as pharmaceuticals.
The pharmaceutical sector, traditionally characterized by high carbon emissions and waste generation, presents a unique opportunity for CO2 valorization technologies. Historical approaches to pharmaceutical manufacturing have prioritized product quality and yield over environmental considerations, resulting in processes with substantial carbon footprints. Recent regulatory pressures and corporate sustainability commitments have catalyzed a shift toward greener manufacturing practices.
Electrocatalytic CO2 valorization specifically refers to the use of electrical energy and catalysts to convert CO2 into value-added compounds that can serve as building blocks for pharmaceutical products. This technology has evolved from early theoretical concepts in the 1980s to increasingly practical applications in the 2010s, with significant breakthroughs in catalyst design and electrochemical cell configurations occurring in recent years.
The primary objective of electrocatalytic CO2 valorization in pharmaceutical processes is to develop economically viable and environmentally sustainable methods for incorporating waste CO2 into pharmaceutical supply chains. This includes creating pathways for CO2 conversion to common pharmaceutical precursors such as formic acid, methanol, ethylene, and more complex carboxylic acids and carbonates.
Secondary objectives include reducing the carbon intensity of pharmaceutical manufacturing, decreasing dependence on fossil fuel-derived feedstocks, and creating closed-loop carbon systems within production facilities. These objectives align with broader industry goals of achieving carbon neutrality while maintaining compliance with stringent pharmaceutical quality standards.
The technical evolution trajectory suggests a convergence of electrochemical engineering, catalyst science, and pharmaceutical process design. Early research focused primarily on fundamental catalyst performance, while current efforts emphasize integration with pharmaceutical manufacturing constraints, including purity requirements, scale-up challenges, and regulatory compliance.
Looking forward, the field aims to develop selective catalysts capable of producing pharmaceutical-grade intermediates directly from CO2, design energy-efficient electrochemical cells suitable for GMP (Good Manufacturing Practice) environments, and create integrated systems that can operate within the complex regulatory framework of pharmaceutical production.
The pharmaceutical sector, traditionally characterized by high carbon emissions and waste generation, presents a unique opportunity for CO2 valorization technologies. Historical approaches to pharmaceutical manufacturing have prioritized product quality and yield over environmental considerations, resulting in processes with substantial carbon footprints. Recent regulatory pressures and corporate sustainability commitments have catalyzed a shift toward greener manufacturing practices.
Electrocatalytic CO2 valorization specifically refers to the use of electrical energy and catalysts to convert CO2 into value-added compounds that can serve as building blocks for pharmaceutical products. This technology has evolved from early theoretical concepts in the 1980s to increasingly practical applications in the 2010s, with significant breakthroughs in catalyst design and electrochemical cell configurations occurring in recent years.
The primary objective of electrocatalytic CO2 valorization in pharmaceutical processes is to develop economically viable and environmentally sustainable methods for incorporating waste CO2 into pharmaceutical supply chains. This includes creating pathways for CO2 conversion to common pharmaceutical precursors such as formic acid, methanol, ethylene, and more complex carboxylic acids and carbonates.
Secondary objectives include reducing the carbon intensity of pharmaceutical manufacturing, decreasing dependence on fossil fuel-derived feedstocks, and creating closed-loop carbon systems within production facilities. These objectives align with broader industry goals of achieving carbon neutrality while maintaining compliance with stringent pharmaceutical quality standards.
The technical evolution trajectory suggests a convergence of electrochemical engineering, catalyst science, and pharmaceutical process design. Early research focused primarily on fundamental catalyst performance, while current efforts emphasize integration with pharmaceutical manufacturing constraints, including purity requirements, scale-up challenges, and regulatory compliance.
Looking forward, the field aims to develop selective catalysts capable of producing pharmaceutical-grade intermediates directly from CO2, design energy-efficient electrochemical cells suitable for GMP (Good Manufacturing Practice) environments, and create integrated systems that can operate within the complex regulatory framework of pharmaceutical production.
Pharmaceutical Industry Demand Analysis
The pharmaceutical industry is experiencing a significant shift towards sustainable manufacturing practices, driven by both regulatory pressures and corporate environmental commitments. Market analysis indicates that pharmaceutical companies are increasingly seeking technologies that can reduce their carbon footprint while maintaining compliance with stringent quality standards. Electrocatalytic CO2 valorization represents a promising solution that aligns with these emerging market demands.
Recent surveys show that over 80% of major pharmaceutical companies have established sustainability targets that include carbon neutrality goals within the next decade. This creates a substantial market opportunity for technologies that can convert waste CO2 into valuable pharmaceutical intermediates or active pharmaceutical ingredients (APIs).
The demand for green chemistry solutions in pharmaceutical manufacturing is particularly strong in regions with strict environmental regulations, such as the European Union, where the European Green Deal has set ambitious targets for industrial decarbonization. North American pharmaceutical companies are following suit, with many adopting voluntary sustainability frameworks that exceed regulatory requirements.
Market research indicates that pharmaceutical manufacturers are willing to invest in technologies that offer dual benefits: environmental sustainability and economic advantages. Electrocatalytic CO2 valorization potentially delivers both by transforming a waste product into valuable chemical building blocks while reducing dependence on petroleum-derived feedstocks.
The COVID-19 pandemic has accelerated this trend, highlighting vulnerabilities in pharmaceutical supply chains and increasing interest in localized, sustainable manufacturing processes. Companies are now more receptive to innovative technologies that can enhance supply chain resilience while addressing environmental concerns.
Specific market segments showing the strongest demand include manufacturers of common analgesics, antibiotics, and cardiovascular medications, where production volumes are high and environmental impact is significant. These segments represent early adoption opportunities for electrocatalytic CO2 valorization technologies.
Financial analysis suggests that pharmaceutical companies are allocating increasing portions of their R&D budgets to green chemistry initiatives, with sustainability-focused projects receiving priority funding. This trend is expected to continue as regulatory pressures intensify and consumer awareness of pharmaceutical environmental impacts grows.
Market forecasts predict that technologies enabling carbon-neutral pharmaceutical manufacturing could capture substantial market share within the next five years, particularly as carbon pricing mechanisms become more widespread and stringent across global markets.
Recent surveys show that over 80% of major pharmaceutical companies have established sustainability targets that include carbon neutrality goals within the next decade. This creates a substantial market opportunity for technologies that can convert waste CO2 into valuable pharmaceutical intermediates or active pharmaceutical ingredients (APIs).
The demand for green chemistry solutions in pharmaceutical manufacturing is particularly strong in regions with strict environmental regulations, such as the European Union, where the European Green Deal has set ambitious targets for industrial decarbonization. North American pharmaceutical companies are following suit, with many adopting voluntary sustainability frameworks that exceed regulatory requirements.
Market research indicates that pharmaceutical manufacturers are willing to invest in technologies that offer dual benefits: environmental sustainability and economic advantages. Electrocatalytic CO2 valorization potentially delivers both by transforming a waste product into valuable chemical building blocks while reducing dependence on petroleum-derived feedstocks.
The COVID-19 pandemic has accelerated this trend, highlighting vulnerabilities in pharmaceutical supply chains and increasing interest in localized, sustainable manufacturing processes. Companies are now more receptive to innovative technologies that can enhance supply chain resilience while addressing environmental concerns.
Specific market segments showing the strongest demand include manufacturers of common analgesics, antibiotics, and cardiovascular medications, where production volumes are high and environmental impact is significant. These segments represent early adoption opportunities for electrocatalytic CO2 valorization technologies.
Financial analysis suggests that pharmaceutical companies are allocating increasing portions of their R&D budgets to green chemistry initiatives, with sustainability-focused projects receiving priority funding. This trend is expected to continue as regulatory pressures intensify and consumer awareness of pharmaceutical environmental impacts grows.
Market forecasts predict that technologies enabling carbon-neutral pharmaceutical manufacturing could capture substantial market share within the next five years, particularly as carbon pricing mechanisms become more widespread and stringent across global markets.
Electrocatalytic CO2 Conversion: Status and Barriers
The current state of electrocatalytic CO2 conversion technology presents a complex landscape of achievements and persistent challenges. Globally, research has accelerated significantly over the past decade, with notable breakthroughs in catalyst design and reaction efficiency. Laboratory-scale demonstrations have successfully converted CO2 into valuable products such as carbon monoxide, formic acid, methanol, and ethylene with improving Faradaic efficiencies. However, the transition from laboratory success to industrial implementation remains problematic, particularly for pharmaceutical applications where product purity requirements are exceptionally stringent.
A primary technical barrier is catalyst selectivity. Current catalysts often produce multiple products simultaneously, necessitating costly downstream separation processes that are particularly challenging in pharmaceutical manufacturing where impurity profiles must be tightly controlled. Even advanced copper-based catalysts, while showing promise for C2+ product formation, struggle to achieve the >99% selectivity often required in pharmaceutical processes.
Energy efficiency represents another significant hurdle. Most electrocatalytic systems require substantial overpotentials to drive CO2 reduction reactions, resulting in energy consumption levels that make processes economically unviable at commercial scale. This is particularly problematic when considering the pharmaceutical industry's increasing focus on sustainability metrics and carbon footprint reduction.
Catalyst stability under continuous operation conditions presents a third major challenge. Deactivation mechanisms including poisoning, leaching, and structural degradation limit catalyst lifetimes to hours or days rather than the months required for industrial viability. This instability introduces variability in product quality that is incompatible with pharmaceutical quality standards.
Scale-up challenges further complicate implementation. Mass transport limitations become increasingly problematic at larger scales, with CO2 solubility in aqueous electrolytes creating a fundamental constraint. Current reactor designs struggle to maintain consistent reaction conditions across larger electrode surfaces, leading to heterogeneous product distributions.
Geographically, research leadership in this field is concentrated in North America, Western Europe, and East Asia, with China, the United States, and Germany producing the highest volume of publications and patents. However, industrial implementation remains limited worldwide, with only a handful of pilot plants demonstrating continuous operation beyond laboratory scale.
Regulatory considerations add another layer of complexity specific to pharmaceutical applications. Electrocatalytic processes must demonstrate compliance with Good Manufacturing Practice (GMP) standards, which current technology has not yet achieved. The pharmaceutical industry's conservative approach to process innovation further slows adoption of these emerging technologies despite their potential sustainability benefits.
A primary technical barrier is catalyst selectivity. Current catalysts often produce multiple products simultaneously, necessitating costly downstream separation processes that are particularly challenging in pharmaceutical manufacturing where impurity profiles must be tightly controlled. Even advanced copper-based catalysts, while showing promise for C2+ product formation, struggle to achieve the >99% selectivity often required in pharmaceutical processes.
Energy efficiency represents another significant hurdle. Most electrocatalytic systems require substantial overpotentials to drive CO2 reduction reactions, resulting in energy consumption levels that make processes economically unviable at commercial scale. This is particularly problematic when considering the pharmaceutical industry's increasing focus on sustainability metrics and carbon footprint reduction.
Catalyst stability under continuous operation conditions presents a third major challenge. Deactivation mechanisms including poisoning, leaching, and structural degradation limit catalyst lifetimes to hours or days rather than the months required for industrial viability. This instability introduces variability in product quality that is incompatible with pharmaceutical quality standards.
Scale-up challenges further complicate implementation. Mass transport limitations become increasingly problematic at larger scales, with CO2 solubility in aqueous electrolytes creating a fundamental constraint. Current reactor designs struggle to maintain consistent reaction conditions across larger electrode surfaces, leading to heterogeneous product distributions.
Geographically, research leadership in this field is concentrated in North America, Western Europe, and East Asia, with China, the United States, and Germany producing the highest volume of publications and patents. However, industrial implementation remains limited worldwide, with only a handful of pilot plants demonstrating continuous operation beyond laboratory scale.
Regulatory considerations add another layer of complexity specific to pharmaceutical applications. Electrocatalytic processes must demonstrate compliance with Good Manufacturing Practice (GMP) standards, which current technology has not yet achieved. The pharmaceutical industry's conservative approach to process innovation further slows adoption of these emerging technologies despite their potential sustainability benefits.
Current Electrocatalytic Solutions for Pharmaceuticals
01 Metal-based catalysts for CO2 electroreduction
Metal-based catalysts play a crucial role in the electrochemical reduction of CO2 to valuable products. These catalysts, including transition metals, metal alloys, and metal oxides, offer selective pathways for CO2 conversion to specific products such as carbon monoxide, formic acid, methanol, or hydrocarbons. The catalytic performance can be tuned by controlling the metal composition, structure, and surface properties to enhance activity, selectivity, and stability during the electrocatalytic CO2 valorization process.- Metal-based catalysts for CO2 electroreduction: Metal-based catalysts play a crucial role in the electrochemical reduction of CO2 to valuable products. Various metals such as copper, silver, gold, and their alloys exhibit different selectivity toward specific products like carbon monoxide, formic acid, or hydrocarbons. The catalytic performance can be enhanced by controlling the morphology, particle size, and surface structure of these metal catalysts, leading to improved efficiency and selectivity in CO2 valorization processes.
- Carbon-based electrocatalysts for CO2 conversion: Carbon-based materials serve as effective electrocatalysts for CO2 reduction. These include carbon nanotubes, graphene, and nitrogen-doped carbon structures that offer high surface area, excellent conductivity, and tunable surface properties. The incorporation of heteroatoms like nitrogen or boron into carbon frameworks creates active sites that facilitate CO2 adsorption and activation, enhancing the overall catalytic performance for converting CO2 into value-added chemicals and fuels.
- Reactor design and system optimization for CO2 electroreduction: The design of electrochemical reactors significantly impacts the efficiency of CO2 valorization processes. Advanced reactor configurations, including flow cells, gas diffusion electrodes, and microfluidic systems, enhance mass transport and reaction kinetics. System optimization involves controlling parameters such as electrolyte composition, pH, temperature, and applied potential to maximize product yield and energy efficiency. Integrated systems that combine CO2 capture with electrochemical conversion represent a promising approach for sustainable carbon utilization.
- Bimetallic and multi-component catalysts for selective CO2 reduction: Bimetallic and multi-component catalysts offer enhanced selectivity and activity for CO2 electroreduction compared to single-metal catalysts. These systems combine the complementary properties of different metals or incorporate metal oxides and other functional materials to create synergistic effects. The interface between different components often serves as active sites for CO2 activation, while the composition and structure can be tailored to direct the reaction pathway toward specific high-value products like ethylene, ethanol, or multi-carbon compounds.
- Membrane and electrolyte innovations for CO2 electroreduction: Advanced membranes and electrolyte systems are critical for improving the performance of CO2 electroreduction processes. Ion-exchange membranes with high conductivity and selectivity help separate reaction products and maintain optimal pH conditions. Novel electrolytes, including ionic liquids and deep eutectic solvents, enhance CO2 solubility and stabilize reaction intermediates. These innovations address key challenges in CO2 valorization by reducing energy consumption, increasing product concentration, and enabling continuous operation of electrochemical systems.
02 Carbon-based materials for CO2 electroreduction
Carbon-based materials serve as effective electrocatalysts or catalyst supports for CO2 reduction. These materials include carbon nanotubes, graphene, carbon fibers, and doped carbon structures that provide high surface area, excellent electrical conductivity, and tunable surface chemistry. The incorporation of heteroatoms (N, S, P) into carbon frameworks creates active sites that facilitate CO2 adsorption and activation, leading to improved catalytic performance for converting CO2 into value-added chemicals and fuels.Expand Specific Solutions03 Reactor design and system optimization for CO2 electroreduction
The design of electrochemical reactors and system optimization are critical for efficient CO2 valorization. Advanced reactor configurations, including flow cells, gas diffusion electrodes, and membrane electrode assemblies, enhance mass transport and reaction kinetics. System parameters such as electrolyte composition, pH, temperature, pressure, and applied potential significantly influence the performance of CO2 electroreduction. Optimized systems aim to achieve high current densities, energy efficiency, and product selectivity while minimizing side reactions.Expand Specific Solutions04 Hybrid and composite catalysts for enhanced CO2 conversion
Hybrid and composite catalysts combine different materials to create synergistic effects for improved CO2 electroreduction. These catalysts often integrate metals with carbon materials, metal-organic frameworks, polymers, or ionic liquids to enhance catalytic activity and selectivity. The composite structure provides multiple active sites, facilitates electron transfer, and optimizes CO2 adsorption and activation. These advanced catalyst designs aim to overcome limitations of single-component catalysts and achieve higher efficiency in converting CO2 to valuable products.Expand Specific Solutions05 Process integration and product separation techniques
Effective integration of CO2 electroreduction with capture systems and downstream processing is essential for practical applications. This includes coupling electrochemical cells with CO2 capture technologies, renewable energy sources, and product separation methods. Advanced separation techniques such as membrane separation, distillation, and adsorption are employed to isolate and purify the valuable products from the reaction mixture. Integrated systems aim to achieve continuous operation, high product purity, and overall process sustainability for industrial-scale CO2 valorization.Expand Specific Solutions
Leading Organizations in CO2 Valorization Research
Electrocatalytic CO2 valorization in pharmaceutical processes is emerging as a promising field at the intersection of green chemistry and sustainable manufacturing. The market is in its early growth phase, with an estimated size of $2-3 billion and projected annual growth of 15-20%. Key players represent diverse sectors: energy giants (TotalEnergies, Sinopec), academic institutions (Zhejiang University, Dalian University of Technology), and specialized research organizations (CNRS, Dalian Institute of Chemical Physics). Technology maturity varies significantly across applications, with industrial leaders like TotalEnergies and Sinopec focusing on scalable solutions, while academic institutions contribute fundamental research. The competitive landscape is characterized by strategic partnerships between industry and academia, with increasing patent activity signaling the transition from research to commercial applications.
The Regents of the University of California
Technical Solution: The University of California has developed advanced electrocatalytic systems for CO2 reduction that integrate with pharmaceutical manufacturing processes. Their approach utilizes novel copper-based catalysts with precisely engineered nanostructures that achieve high faradaic efficiency (>85%) for converting CO2 to value-added pharmaceutical precursors. The technology employs a flow-cell design that allows for continuous operation and easy integration with existing pharmaceutical production lines. Their system incorporates real-time monitoring and control mechanisms to ensure compliance with Good Manufacturing Practice (GMP) standards, making it particularly suitable for pharmaceutical applications. The catalyst design minimizes side reactions and unwanted byproducts, addressing a key challenge in pharmaceutical-grade synthesis.
Strengths: Superior catalyst selectivity and efficiency in producing pharmaceutical-relevant compounds; robust compliance with pharmaceutical regulatory standards; scalable continuous flow process design. Weaknesses: Higher implementation costs compared to traditional methods; requires specialized expertise for operation and maintenance; catalyst longevity issues under industrial conditions.
Centre National de la Recherche Scientifique
Technical Solution: CNRS has pioneered a comprehensive approach to electrocatalytic CO2 valorization specifically tailored for pharmaceutical applications. Their technology centers on innovative ionic liquid-mediated electrocatalysis that operates at near-ambient conditions, significantly reducing energy requirements. The system achieves selective conversion of CO2 to carboxylic acids and carbonyl compounds that serve as key building blocks in pharmaceutical synthesis. Their process integrates seamlessly with continuous flow chemistry platforms, enabling precise control over reaction parameters and product purity. CNRS researchers have developed proprietary electrode materials with enhanced stability in the presence of pharmaceutical intermediates and solvents commonly used in drug manufacturing. The technology includes advanced separation techniques that facilitate the isolation of high-purity products suitable for pharmaceutical applications.
Strengths: Low energy consumption; high selectivity toward pharmaceutical intermediates; compatibility with existing pharmaceutical manufacturing equipment; minimal waste generation. Weaknesses: Scale-up challenges for certain electrode materials; sensitivity to trace impurities in CO2 feedstock; higher initial capital investment compared to conventional methods.
Key Patents and Innovations in CO2 Electrocatalysis
Systems and methods for electrochemical reduction of carbon dioxide
PatentWO2019051609A1
Innovation
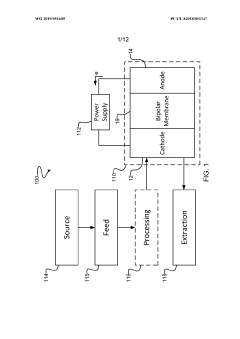
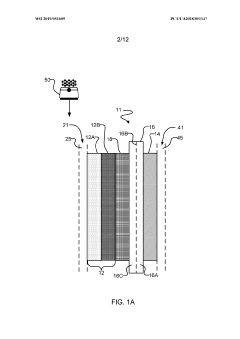
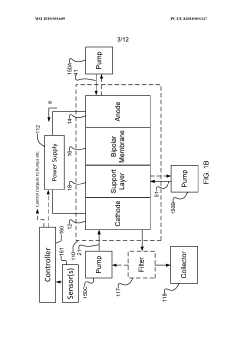
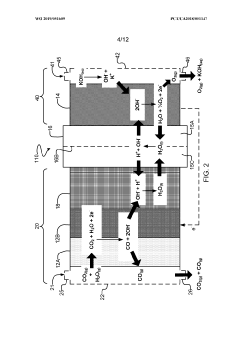
- The implementation of a membrane electrode assembly with a bipolar membrane and a hydration layer between the anode and cathode, where an electrical potential is applied to reduce carbon dioxide to carbon monoxide, with a support layer maintaining hydration of the cathode side to enhance efficiency and selectivity.
Electrochemical conversion of carbon dioxide into value-added products
PatentActiveIN202442017597A
Innovation
- The method employs an electrochemical reactor with metallic Copper (Cu) or Cu-alloy symmetric electrodes and acidified water as electrolyte, achieving high FE and EE through asymmetric C-C coupling on the cathode surface, forming short-lived cation radical intermediates that react to produce ethanol and other value-added products.
Regulatory Compliance in Pharmaceutical Manufacturing
Pharmaceutical manufacturing operates within one of the most stringent regulatory frameworks globally, necessitating careful consideration when implementing novel technologies such as electrocatalytic CO2 valorization. The integration of such processes must align with established regulatory standards including Good Manufacturing Practices (GMP), International Conference on Harmonisation (ICH) guidelines, and region-specific regulations like FDA requirements in the US and EMA directives in Europe.
The pharmaceutical industry faces unique compliance challenges when adopting sustainable technologies. Electrocatalytic CO2 conversion processes must demonstrate consistent quality, safety, and efficacy while maintaining traceability throughout the production lifecycle. Regulatory bodies require extensive validation protocols and documentation for any new manufacturing process, with particular scrutiny on novel catalytic systems that may introduce unfamiliar impurity profiles.
Quality by Design (QbD) principles have become increasingly important in regulatory compliance frameworks, requiring pharmaceutical manufacturers to understand critical process parameters that may affect product quality. For electrocatalytic CO2 valorization, this necessitates comprehensive characterization of catalyst performance, reaction conditions, and potential degradation pathways to establish appropriate control strategies.
Environmental regulations present both challenges and opportunities for CO2 valorization technologies. While carbon capture and utilization align with sustainability goals encouraged by regulatory bodies, the processes must still comply with emissions standards, waste management regulations, and chemical safety requirements. Companies implementing these technologies may benefit from regulatory incentives for green chemistry initiatives while simultaneously navigating complex compliance requirements.
Validation protocols for electrocatalytic processes require demonstration of reproducibility, robustness, and scalability. This includes process validation, cleaning validation, and analytical method validation specific to the catalytic systems employed. Regulatory authorities will expect evidence that the electrocatalytic process consistently produces pharmaceutical intermediates or active ingredients meeting predetermined quality specifications.
Change management represents a significant regulatory consideration when implementing new technologies in pharmaceutical manufacturing. Established facilities seeking to incorporate electrocatalytic CO2 valorization must follow formal change control procedures, potentially including prior approval supplements for major manufacturing changes. The regulatory pathway may be streamlined for new facilities designed with these technologies from inception.
International harmonization efforts continue to evolve regarding sustainable manufacturing technologies. Organizations such as the International Council for Harmonisation (ICH) are developing guidance documents addressing green chemistry approaches, potentially creating more standardized regulatory pathways for technologies like electrocatalytic CO2 valorization across different jurisdictions.
The pharmaceutical industry faces unique compliance challenges when adopting sustainable technologies. Electrocatalytic CO2 conversion processes must demonstrate consistent quality, safety, and efficacy while maintaining traceability throughout the production lifecycle. Regulatory bodies require extensive validation protocols and documentation for any new manufacturing process, with particular scrutiny on novel catalytic systems that may introduce unfamiliar impurity profiles.
Quality by Design (QbD) principles have become increasingly important in regulatory compliance frameworks, requiring pharmaceutical manufacturers to understand critical process parameters that may affect product quality. For electrocatalytic CO2 valorization, this necessitates comprehensive characterization of catalyst performance, reaction conditions, and potential degradation pathways to establish appropriate control strategies.
Environmental regulations present both challenges and opportunities for CO2 valorization technologies. While carbon capture and utilization align with sustainability goals encouraged by regulatory bodies, the processes must still comply with emissions standards, waste management regulations, and chemical safety requirements. Companies implementing these technologies may benefit from regulatory incentives for green chemistry initiatives while simultaneously navigating complex compliance requirements.
Validation protocols for electrocatalytic processes require demonstration of reproducibility, robustness, and scalability. This includes process validation, cleaning validation, and analytical method validation specific to the catalytic systems employed. Regulatory authorities will expect evidence that the electrocatalytic process consistently produces pharmaceutical intermediates or active ingredients meeting predetermined quality specifications.
Change management represents a significant regulatory consideration when implementing new technologies in pharmaceutical manufacturing. Established facilities seeking to incorporate electrocatalytic CO2 valorization must follow formal change control procedures, potentially including prior approval supplements for major manufacturing changes. The regulatory pathway may be streamlined for new facilities designed with these technologies from inception.
International harmonization efforts continue to evolve regarding sustainable manufacturing technologies. Organizations such as the International Council for Harmonisation (ICH) are developing guidance documents addressing green chemistry approaches, potentially creating more standardized regulatory pathways for technologies like electrocatalytic CO2 valorization across different jurisdictions.
Sustainability Metrics and Carbon Accounting
Sustainability metrics and carbon accounting are critical components in evaluating the environmental impact of electrocatalytic CO2 valorization processes within the pharmaceutical industry. The implementation of standardized measurement frameworks enables companies to quantify their carbon footprint accurately and track progress toward sustainability goals.
Life Cycle Assessment (LCA) represents the primary methodology for comprehensive evaluation of environmental impacts across the entire value chain of pharmaceutical processes utilizing CO2 conversion technologies. These assessments typically measure carbon intensity in terms of CO2-equivalent emissions per unit of pharmaceutical product manufactured, providing a standardized basis for comparison between traditional and electrocatalytic approaches.
The Greenhouse Gas Protocol offers a structured framework for carbon accounting in pharmaceutical operations, categorizing emissions into three scopes: direct emissions from owned sources (Scope 1), indirect emissions from purchased electricity (Scope 2), and all other indirect emissions occurring in the value chain (Scope 3). Electrocatalytic CO2 valorization processes can potentially reduce Scope 1 emissions by utilizing waste CO2 streams and may impact Scope 2 emissions depending on the electricity sources powering the electrocatalytic systems.
Key performance indicators (KPIs) for sustainability in pharmaceutical CO2 valorization include carbon capture efficiency, energy intensity per unit of CO2 converted, renewable energy percentage in the process, and carbon utilization factor. These metrics allow for benchmarking against industry standards and tracking improvements over time.
Carbon accounting methodologies must address the unique challenges of electrocatalytic processes, including allocation of emissions between CO2 feedstock sourcing, conversion process, and final pharmaceutical product. The temporal dimension of carbon sequestration in pharmaceutical products must also be considered, as the duration of carbon storage varies significantly depending on the product lifecycle.
Emerging frameworks such as the Science-Based Targets initiative (SBTi) provide pharmaceutical companies with guidelines for setting emissions reduction targets aligned with global climate goals. These frameworks increasingly recognize carbon utilization technologies as legitimate strategies for emissions reduction when properly quantified and verified.
Regulatory compliance adds another dimension to sustainability metrics, with frameworks like the EU Emissions Trading System and emerging carbon border adjustment mechanisms potentially affecting the economic viability of electrocatalytic CO2 valorization processes. Companies implementing these technologies must navigate evolving reporting requirements while demonstrating genuine environmental benefits rather than engaging in greenwashing practices.
Life Cycle Assessment (LCA) represents the primary methodology for comprehensive evaluation of environmental impacts across the entire value chain of pharmaceutical processes utilizing CO2 conversion technologies. These assessments typically measure carbon intensity in terms of CO2-equivalent emissions per unit of pharmaceutical product manufactured, providing a standardized basis for comparison between traditional and electrocatalytic approaches.
The Greenhouse Gas Protocol offers a structured framework for carbon accounting in pharmaceutical operations, categorizing emissions into three scopes: direct emissions from owned sources (Scope 1), indirect emissions from purchased electricity (Scope 2), and all other indirect emissions occurring in the value chain (Scope 3). Electrocatalytic CO2 valorization processes can potentially reduce Scope 1 emissions by utilizing waste CO2 streams and may impact Scope 2 emissions depending on the electricity sources powering the electrocatalytic systems.
Key performance indicators (KPIs) for sustainability in pharmaceutical CO2 valorization include carbon capture efficiency, energy intensity per unit of CO2 converted, renewable energy percentage in the process, and carbon utilization factor. These metrics allow for benchmarking against industry standards and tracking improvements over time.
Carbon accounting methodologies must address the unique challenges of electrocatalytic processes, including allocation of emissions between CO2 feedstock sourcing, conversion process, and final pharmaceutical product. The temporal dimension of carbon sequestration in pharmaceutical products must also be considered, as the duration of carbon storage varies significantly depending on the product lifecycle.
Emerging frameworks such as the Science-Based Targets initiative (SBTi) provide pharmaceutical companies with guidelines for setting emissions reduction targets aligned with global climate goals. These frameworks increasingly recognize carbon utilization technologies as legitimate strategies for emissions reduction when properly quantified and verified.
Regulatory compliance adds another dimension to sustainability metrics, with frameworks like the EU Emissions Trading System and emerging carbon border adjustment mechanisms potentially affecting the economic viability of electrocatalytic CO2 valorization processes. Companies implementing these technologies must navigate evolving reporting requirements while demonstrating genuine environmental benefits rather than engaging in greenwashing practices.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!