Why Electrocatalytic CO2 Valorization Matters for Pharmaceuticals
OCT 23, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CO2 Valorization Background and Objectives
Carbon dioxide (CO2) valorization represents a transformative approach to addressing two critical global challenges simultaneously: reducing greenhouse gas emissions and creating valuable chemical products. The concept has evolved significantly over the past decades, from theoretical possibilities to practical applications across various industries. In the pharmaceutical sector specifically, electrocatalytic CO2 valorization has emerged as a promising frontier for sustainable manufacturing processes.
The historical trajectory of CO2 utilization began in the early 20th century with basic chemical conversions, but has accelerated dramatically since the 1990s with the growing recognition of climate change impacts. Early approaches focused primarily on physical utilization methods, while recent advances in electrocatalysis have opened new pathways for chemical transformation of this abundant carbon source.
Electrocatalytic CO2 valorization involves the conversion of carbon dioxide into higher-value compounds through electrochemical processes, utilizing renewable electricity. This approach offers significant advantages over traditional chemical synthesis routes, particularly for pharmaceutical manufacturing, which has historically relied on petroleum-derived feedstocks and energy-intensive processes.
The pharmaceutical industry faces mounting pressure to reduce its environmental footprint while maintaining stringent quality standards. Current manufacturing processes for active pharmaceutical ingredients (APIs) often involve multiple steps with low atom economy and significant waste generation. The carbon intensity of pharmaceutical products can be 25-100 times higher than that of bulk chemicals on a per-kilogram basis, highlighting the urgent need for greener synthesis routes.
The primary objectives of electrocatalytic CO2 valorization in pharmaceutical applications include developing selective catalysts for complex molecule synthesis, establishing scalable electrochemical processes compatible with pharmaceutical manufacturing requirements, and creating economically viable pathways that can compete with conventional synthesis routes.
Technical goals extend to achieving high faradaic efficiencies, developing stable catalyst systems that can operate continuously for extended periods, and designing modular electrochemical systems that can be integrated into existing pharmaceutical manufacturing infrastructure. Additionally, there is significant focus on expanding the range of accessible molecular structures through CO2-derived building blocks.
The evolution of this technology is increasingly driven by convergence with other emerging fields, including artificial intelligence for catalyst discovery, advanced materials science for electrode development, and process intensification strategies. These synergistic developments are accelerating progress toward practical implementation in pharmaceutical manufacturing contexts.
The historical trajectory of CO2 utilization began in the early 20th century with basic chemical conversions, but has accelerated dramatically since the 1990s with the growing recognition of climate change impacts. Early approaches focused primarily on physical utilization methods, while recent advances in electrocatalysis have opened new pathways for chemical transformation of this abundant carbon source.
Electrocatalytic CO2 valorization involves the conversion of carbon dioxide into higher-value compounds through electrochemical processes, utilizing renewable electricity. This approach offers significant advantages over traditional chemical synthesis routes, particularly for pharmaceutical manufacturing, which has historically relied on petroleum-derived feedstocks and energy-intensive processes.
The pharmaceutical industry faces mounting pressure to reduce its environmental footprint while maintaining stringent quality standards. Current manufacturing processes for active pharmaceutical ingredients (APIs) often involve multiple steps with low atom economy and significant waste generation. The carbon intensity of pharmaceutical products can be 25-100 times higher than that of bulk chemicals on a per-kilogram basis, highlighting the urgent need for greener synthesis routes.
The primary objectives of electrocatalytic CO2 valorization in pharmaceutical applications include developing selective catalysts for complex molecule synthesis, establishing scalable electrochemical processes compatible with pharmaceutical manufacturing requirements, and creating economically viable pathways that can compete with conventional synthesis routes.
Technical goals extend to achieving high faradaic efficiencies, developing stable catalyst systems that can operate continuously for extended periods, and designing modular electrochemical systems that can be integrated into existing pharmaceutical manufacturing infrastructure. Additionally, there is significant focus on expanding the range of accessible molecular structures through CO2-derived building blocks.
The evolution of this technology is increasingly driven by convergence with other emerging fields, including artificial intelligence for catalyst discovery, advanced materials science for electrode development, and process intensification strategies. These synergistic developments are accelerating progress toward practical implementation in pharmaceutical manufacturing contexts.
Pharmaceutical Industry Demand for Green Chemistry
The pharmaceutical industry has been increasingly focused on adopting green chemistry principles over the past decade, driven by regulatory pressures, environmental concerns, and economic incentives. Traditional pharmaceutical manufacturing processes are notoriously inefficient, with E-factors (waste-to-product ratios) often exceeding 100, significantly higher than other chemical industries. This inefficiency translates to substantial waste generation, resource consumption, and environmental impact that contradicts modern sustainability goals.
Market research indicates that pharmaceutical companies implementing green chemistry initiatives have realized cost reductions of 10-15% in manufacturing operations through decreased waste management expenses and improved resource efficiency. Additionally, these companies have reported enhanced brand reputation and competitive advantage in an increasingly environmentally conscious marketplace.
The demand for green chemistry solutions in pharmaceuticals is further accelerated by stringent regulatory frameworks worldwide. The European Medicines Agency has established guidelines for environmental risk assessment of medicinal products, while the FDA encourages green chemistry through its Quality by Design initiative. These regulatory developments have created a market pull for sustainable manufacturing technologies.
Consumer and investor pressure represents another significant driver. Sustainable investment funds managing trillions in assets now routinely evaluate pharmaceutical companies based on environmental performance metrics. Market analysis shows that pharmaceutical companies with robust sustainability programs have outperformed industry averages in stock performance by approximately 5-7% annually over the past five years.
The COVID-19 pandemic has further highlighted supply chain vulnerabilities in pharmaceutical manufacturing, prompting companies to reconsider localized, sustainable production methods. This trend aligns perfectly with green chemistry approaches that minimize hazardous reagents and reduce dependence on globally distributed supply chains.
Electrocatalytic CO2 valorization presents a particularly attractive green chemistry solution for the pharmaceutical sector. By utilizing carbon dioxide as a feedstock and renewable electricity as an energy source, this technology addresses multiple sustainability challenges simultaneously. The potential to replace petroleum-derived building blocks with CO2-derived alternatives could significantly reduce the carbon footprint of pharmaceutical products while creating economic value from a greenhouse gas.
Market projections suggest that green chemistry technologies in pharmaceuticals represent a rapidly growing segment, with compound annual growth rates exceeding 8%. Early adopters of technologies like electrocatalytic CO2 valorization stand to capture significant market share and establish technological leadership in this evolving landscape.
Market research indicates that pharmaceutical companies implementing green chemistry initiatives have realized cost reductions of 10-15% in manufacturing operations through decreased waste management expenses and improved resource efficiency. Additionally, these companies have reported enhanced brand reputation and competitive advantage in an increasingly environmentally conscious marketplace.
The demand for green chemistry solutions in pharmaceuticals is further accelerated by stringent regulatory frameworks worldwide. The European Medicines Agency has established guidelines for environmental risk assessment of medicinal products, while the FDA encourages green chemistry through its Quality by Design initiative. These regulatory developments have created a market pull for sustainable manufacturing technologies.
Consumer and investor pressure represents another significant driver. Sustainable investment funds managing trillions in assets now routinely evaluate pharmaceutical companies based on environmental performance metrics. Market analysis shows that pharmaceutical companies with robust sustainability programs have outperformed industry averages in stock performance by approximately 5-7% annually over the past five years.
The COVID-19 pandemic has further highlighted supply chain vulnerabilities in pharmaceutical manufacturing, prompting companies to reconsider localized, sustainable production methods. This trend aligns perfectly with green chemistry approaches that minimize hazardous reagents and reduce dependence on globally distributed supply chains.
Electrocatalytic CO2 valorization presents a particularly attractive green chemistry solution for the pharmaceutical sector. By utilizing carbon dioxide as a feedstock and renewable electricity as an energy source, this technology addresses multiple sustainability challenges simultaneously. The potential to replace petroleum-derived building blocks with CO2-derived alternatives could significantly reduce the carbon footprint of pharmaceutical products while creating economic value from a greenhouse gas.
Market projections suggest that green chemistry technologies in pharmaceuticals represent a rapidly growing segment, with compound annual growth rates exceeding 8%. Early adopters of technologies like electrocatalytic CO2 valorization stand to capture significant market share and establish technological leadership in this evolving landscape.
Electrocatalytic CO2 Conversion: Status and Barriers
Electrocatalytic CO2 conversion has emerged as a promising approach to address both environmental concerns and resource utilization challenges. Currently, the technology has advanced from laboratory proof-of-concept to pilot-scale demonstrations, with several key products including carbon monoxide, formic acid, methanol, and ethylene being successfully produced through various catalytic pathways. However, despite these achievements, widespread industrial implementation remains limited due to significant technical barriers.
The primary challenge facing electrocatalytic CO2 conversion is catalyst performance. Current catalysts suffer from low selectivity, with many systems producing multiple products simultaneously, complicating downstream separation processes. Additionally, catalyst stability remains inadequate for long-term industrial operation, with performance degradation occurring over relatively short timeframes. Most catalysts also require high overpotentials, reducing energy efficiency and economic viability.
Mass transport limitations represent another critical barrier. CO2 has limited solubility in aqueous electrolytes, creating concentration gradients that hinder reaction rates. The competition between CO2 reduction and hydrogen evolution further complicates the process, especially at higher current densities where hydrogen production often becomes dominant.
System integration challenges persist across the technology landscape. Current reactor designs struggle to balance efficient CO2 delivery, product separation, and heat management. The scale-up from laboratory to industrial implementation introduces additional complexities related to electrode surface area, electrolyte distribution, and maintaining consistent reaction conditions across larger systems.
From a geographical perspective, research leadership is concentrated in North America, Europe, and East Asia, with the United States, Germany, China, and Japan hosting the most advanced research facilities. This distribution reflects both historical expertise in electrochemistry and current investment priorities in sustainable technologies.
Economic barriers further impede commercialization efforts. The cost of electricity significantly impacts operational expenses, while capital costs for specialized electrodes and membranes remain high. Current production rates and efficiencies have not yet reached the thresholds necessary for cost-competitive manufacturing of pharmaceutical precursors compared to conventional petrochemical routes.
Regulatory frameworks present additional challenges, as standards for electrochemical processes in pharmaceutical manufacturing are still evolving. The pharmaceutical industry's stringent purity requirements necessitate additional purification steps for electrocatalytically produced compounds, further affecting economic viability.
Despite these barriers, recent technological breakthroughs, including novel catalyst designs incorporating nanostructured materials and improved reactor configurations, suggest pathways toward overcoming current limitations. The integration of renewable electricity sources also continues to improve the sustainability profile and potential economic competitiveness of these processes.
The primary challenge facing electrocatalytic CO2 conversion is catalyst performance. Current catalysts suffer from low selectivity, with many systems producing multiple products simultaneously, complicating downstream separation processes. Additionally, catalyst stability remains inadequate for long-term industrial operation, with performance degradation occurring over relatively short timeframes. Most catalysts also require high overpotentials, reducing energy efficiency and economic viability.
Mass transport limitations represent another critical barrier. CO2 has limited solubility in aqueous electrolytes, creating concentration gradients that hinder reaction rates. The competition between CO2 reduction and hydrogen evolution further complicates the process, especially at higher current densities where hydrogen production often becomes dominant.
System integration challenges persist across the technology landscape. Current reactor designs struggle to balance efficient CO2 delivery, product separation, and heat management. The scale-up from laboratory to industrial implementation introduces additional complexities related to electrode surface area, electrolyte distribution, and maintaining consistent reaction conditions across larger systems.
From a geographical perspective, research leadership is concentrated in North America, Europe, and East Asia, with the United States, Germany, China, and Japan hosting the most advanced research facilities. This distribution reflects both historical expertise in electrochemistry and current investment priorities in sustainable technologies.
Economic barriers further impede commercialization efforts. The cost of electricity significantly impacts operational expenses, while capital costs for specialized electrodes and membranes remain high. Current production rates and efficiencies have not yet reached the thresholds necessary for cost-competitive manufacturing of pharmaceutical precursors compared to conventional petrochemical routes.
Regulatory frameworks present additional challenges, as standards for electrochemical processes in pharmaceutical manufacturing are still evolving. The pharmaceutical industry's stringent purity requirements necessitate additional purification steps for electrocatalytically produced compounds, further affecting economic viability.
Despite these barriers, recent technological breakthroughs, including novel catalyst designs incorporating nanostructured materials and improved reactor configurations, suggest pathways toward overcoming current limitations. The integration of renewable electricity sources also continues to improve the sustainability profile and potential economic competitiveness of these processes.
Current Electrocatalytic Approaches for CO2 Utilization
01 Metal-based catalysts for CO2 electroreduction
Metal-based catalysts play a crucial role in the electrochemical reduction of CO2 to valuable products. Various metals such as copper, silver, gold, and their alloys exhibit different selectivity toward specific products like carbon monoxide, formate, or hydrocarbons. The catalytic performance can be enhanced by controlling the morphology, particle size, and surface structure of these metal catalysts. Nanostructured metal catalysts with high surface area and abundant active sites show improved efficiency for CO2 valorization.- Metal-based catalysts for CO2 electroreduction: Metal-based catalysts play a crucial role in the electrochemical reduction of CO2 to valuable products. Various metals such as copper, silver, gold, and their alloys exhibit different selectivity toward specific products like carbon monoxide, formate, or hydrocarbons. The catalytic performance can be enhanced by controlling the morphology, particle size, and surface structure of these metal catalysts. Nanostructured metal catalysts with high surface area and abundant active sites show improved efficiency for CO2 valorization.
- Carbon-based materials as electrocatalysts: Carbon-based materials, including carbon nanotubes, graphene, and nitrogen-doped carbon, serve as effective electrocatalysts for CO2 reduction. These materials offer advantages such as high conductivity, large surface area, and tunable electronic properties. The incorporation of heteroatoms like nitrogen, boron, or sulfur into carbon frameworks creates active sites for CO2 adsorption and activation. Carbon-based catalysts are particularly promising due to their abundance, low cost, and environmental friendliness compared to precious metal catalysts.
- Reactor design and system optimization: The design of electrochemical reactors significantly impacts the efficiency of CO2 valorization processes. Advanced reactor configurations, including flow cells, gas diffusion electrodes, and membrane electrode assemblies, enhance mass transfer and reduce energy consumption. System optimization involves controlling parameters such as electrolyte composition, pH, temperature, and applied potential to maximize product selectivity and conversion rates. Continuous flow systems and integrated approaches that combine electrochemical reduction with product separation show promise for industrial-scale CO2 utilization.
- Bimetallic and multi-component catalysts: Bimetallic and multi-component catalysts offer synergistic effects that enhance CO2 electroreduction performance. By combining metals with complementary properties, these catalysts can overcome limitations of single-metal systems, improving both activity and selectivity. The interface between different metals creates unique active sites that facilitate CO2 activation and conversion. Strategic design of these catalysts involves controlling composition ratios, atomic arrangements, and electronic interactions to direct the reaction pathway toward desired products like ethanol, ethylene, or multi-carbon compounds.
- Electrolyte engineering for enhanced CO2 reduction: Electrolyte composition and properties significantly influence the electrochemical reduction of CO2. Ionic liquids, deep eutectic solvents, and modified aqueous electrolytes can enhance CO2 solubility and stabilize reaction intermediates. The addition of specific ions, pH buffers, or co-catalysts to the electrolyte can direct selectivity toward valuable products. Advanced electrolyte engineering approaches focus on reducing the competing hydrogen evolution reaction and improving the overall energy efficiency of the CO2 valorization process.
02 Carbon-based materials as electrocatalysts
Carbon-based materials, including graphene, carbon nanotubes, and nitrogen-doped carbon, serve as effective electrocatalysts for CO2 reduction. These materials offer advantages such as high conductivity, large surface area, and tunable electronic properties. Doping with heteroatoms like nitrogen, boron, or sulfur can create active sites that enhance catalytic activity and selectivity. Carbon-based catalysts are particularly attractive due to their abundance, low cost, and environmental friendliness compared to precious metal catalysts.Expand Specific Solutions03 Reactor design and system optimization
The design of electrochemical reactors significantly impacts the efficiency of CO2 valorization processes. Various reactor configurations, including flow cells, membrane electrode assemblies, and microfluidic systems, have been developed to enhance mass transfer, reduce energy consumption, and improve product selectivity. System optimization involves controlling parameters such as electrolyte composition, pH, temperature, pressure, and applied potential to achieve higher conversion rates and faradaic efficiency. Advanced reactor designs also address challenges related to gas diffusion, bubble formation, and product separation.Expand Specific Solutions04 Hybrid and composite catalysts
Hybrid and composite catalysts combine different materials to achieve synergistic effects in CO2 electroreduction. These may include metal-organic frameworks (MOFs), metal/metal oxide composites, and catalyst-support combinations. The integration of multiple components can enhance catalytic activity, stability, and selectivity by providing complementary functionalities. For example, combining metals with conductive supports improves electron transfer, while incorporating molecular catalysts into heterogeneous systems can offer site-specific reactivity. These hybrid approaches often result in improved performance compared to single-component catalysts.Expand Specific Solutions05 Process integration and product valorization
Integrating CO2 electroreduction with other processes enhances overall efficiency and economic viability. This includes coupling with renewable energy sources, combining electrochemical and biological systems, and developing cascade processes for further product upgrading. The valorization of CO2 reduction products involves converting primary products like CO, formate, or ethylene into higher-value chemicals and fuels through additional processing steps. Techno-economic analysis and life cycle assessment are essential for evaluating the sustainability and commercial potential of these integrated approaches to CO2 utilization.Expand Specific Solutions
Leading Companies in CO2 Valorization for Pharmaceuticals
Electrocatalytic CO2 valorization for pharmaceuticals is emerging as a strategic technology at the intersection of sustainability and high-value chemical production. The market is in early growth phase with increasing investment, projected to expand significantly as pharmaceutical companies seek greener synthesis routes. Technologically, research institutions like CNRS, California Institute of Technology, and Zhejiang University lead fundamental research, while companies such as Air Liquide and B. Braun Surgical are exploring commercial applications. Chinese universities (Shandong, Huazhong, Xiamen) show strong publication activity, while European institutions (ETH Zurich, Technical University of Denmark) focus on catalyst development. The field remains pre-commercial but rapidly maturing as industrial players recognize the potential for carbon-neutral pharmaceutical manufacturing processes.
Centre National de la Recherche Scientifique
Technical Solution: CNRS has developed innovative electrocatalytic systems for CO2 valorization in pharmaceutical applications, focusing on metal-organic frameworks (MOFs) as catalysts. Their approach combines copper-based MOFs with tailored ligands to achieve selective conversion of CO2 to valuable pharmaceutical precursors such as formic acid, methanol, and ethylene. The research team has demonstrated continuous-flow electrochemical cells that maintain high Faradaic efficiency (>80%) and stability over 100+ hours of operation. CNRS's technology enables direct integration of captured CO2 into pharmaceutical synthesis pathways, reducing the carbon footprint of drug manufacturing while creating value from waste CO2. Their latest catalysts operate at near-ambient conditions (temperature <40°C, atmospheric pressure), making the process energetically favorable compared to traditional thermochemical routes that require high temperatures and pressures.
Strengths: Superior catalyst selectivity for pharmaceutical-relevant molecules, operates under mild conditions reducing energy requirements, and demonstrates excellent long-term stability. Weaknesses: Still faces challenges in scaling up from laboratory to industrial production, and requires further optimization to reduce precious metal content in catalysts.
Dalian Institute of Chemical Physics of CAS
Technical Solution: Dalian Institute has pioneered a comprehensive electrocatalytic CO2 conversion platform specifically designed for pharmaceutical applications. Their technology employs bimetallic nanoalloy catalysts (primarily Cu-Sn and Cu-Ag systems) with precisely controlled surface structures to enable selective CO2 reduction to carboxylic acids, aldehydes, and alcohols - key building blocks for pharmaceutical synthesis. The institute has developed proprietary electrode architectures featuring hierarchical porosity that enhances mass transport while maintaining high active site density. Their system achieves partial current densities exceeding 300 mA/cm² for target products while operating at potentials below -1.0V vs. RHE. Notably, they've demonstrated direct electrochemical carboxylation reactions to produce pharmaceutical intermediates like 2-phenylpropionic acid derivatives with yields above 70%. The technology incorporates in-situ product separation systems to overcome product inhibition issues common in electrocatalytic processes, enabling continuous operation for pharmaceutical manufacturing.
Strengths: Exceptional selectivity toward complex organic molecules relevant to pharmaceuticals, integrated system design addressing both catalysis and separation challenges, and demonstrated scalability in pilot projects. Weaknesses: Higher energy consumption compared to some competing technologies and dependence on specialized electrode materials that may increase production costs.
Key Catalytic Mechanisms and Materials Science Innovations
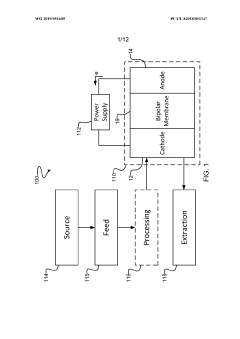
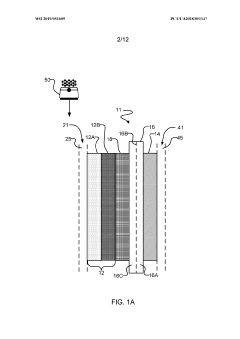
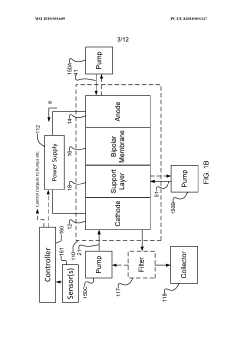
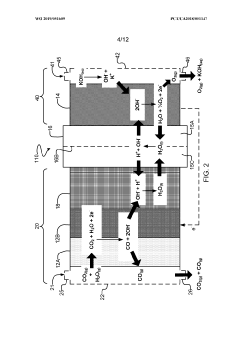
Systems and methods for electrochemical reduction of carbon dioxide
PatentWO2019051609A1
Innovation
- The implementation of a membrane electrode assembly with a bipolar membrane and a hydration layer between the anode and cathode, where an electrical potential is applied to reduce carbon dioxide to carbon monoxide, with a support layer maintaining hydration of the cathode side to enhance efficiency and selectivity.
Electrocatalytic conversion of carbon dioxide in liquids expanded by carbon dioxide
PatentInactiveUS20210262103A1
Innovation
- The use of CO2 expanded liquid media at elevated pressures, which increases CO2 solubility and enables high CO2 concentrations and fast electron transfer, allowing for sustainable and commercially viable pathways to convert CO2 into fuels and chemicals, by optimizing the combination of organic liquid solvents and electrolytes to maintain a single phase and facilitate pressure-tunable CO2 conversion.
Sustainability Metrics and Life Cycle Assessment
Sustainability metrics and life cycle assessment (LCA) are critical frameworks for evaluating the environmental impact of electrocatalytic CO2 valorization in pharmaceutical manufacturing. These methodologies provide quantitative measures to assess whether CO2 conversion technologies genuinely deliver environmental benefits compared to conventional pharmaceutical synthesis routes.
Traditional pharmaceutical manufacturing processes typically generate 25-100 kg of waste per kilogram of active pharmaceutical ingredient (API) produced, resulting in a high environmental footprint. Electrocatalytic CO2 valorization offers potential improvements by utilizing waste CO2 as a feedstock while employing renewable electricity, potentially reducing both carbon emissions and resource consumption.
Key sustainability metrics for evaluating these processes include carbon intensity (kg CO2 equivalent per kg of product), energy efficiency (kWh per kg of product), atom economy, E-factor (kg waste per kg product), and process mass intensity. When applied to electrocatalytic CO2 valorization for pharmaceutical intermediates, these metrics often reveal significant improvements over traditional petroleum-based synthesis routes, particularly when renewable electricity sources are employed.
Life cycle assessment provides a more comprehensive evaluation by examining environmental impacts across the entire value chain. For pharmaceutical applications of CO2 valorization, LCA typically encompasses raw material extraction, catalyst production, electricity generation, reaction processes, separation/purification, and end-of-life considerations. Recent LCA studies have demonstrated that CO2-derived pharmaceutical intermediates can achieve carbon footprint reductions of 30-60% compared to conventional routes when powered by renewable electricity.
However, challenges in sustainability assessment remain. The environmental benefits of electrocatalytic CO2 valorization are highly dependent on electricity sources, with coal-powered electricity potentially negating any environmental advantages. Additionally, catalyst production often involves rare metals with their own environmental footprint, and separation processes for pharmaceutical-grade purity can be energy-intensive.
Standardization of sustainability metrics specific to CO2 valorization in pharmaceuticals remains underdeveloped. Industry stakeholders are increasingly advocating for harmonized assessment frameworks that account for the unique aspects of these technologies, including temporal and spatial variations in electricity carbon intensity, catalyst lifetime considerations, and integration with existing pharmaceutical manufacturing infrastructure.
Traditional pharmaceutical manufacturing processes typically generate 25-100 kg of waste per kilogram of active pharmaceutical ingredient (API) produced, resulting in a high environmental footprint. Electrocatalytic CO2 valorization offers potential improvements by utilizing waste CO2 as a feedstock while employing renewable electricity, potentially reducing both carbon emissions and resource consumption.
Key sustainability metrics for evaluating these processes include carbon intensity (kg CO2 equivalent per kg of product), energy efficiency (kWh per kg of product), atom economy, E-factor (kg waste per kg product), and process mass intensity. When applied to electrocatalytic CO2 valorization for pharmaceutical intermediates, these metrics often reveal significant improvements over traditional petroleum-based synthesis routes, particularly when renewable electricity sources are employed.
Life cycle assessment provides a more comprehensive evaluation by examining environmental impacts across the entire value chain. For pharmaceutical applications of CO2 valorization, LCA typically encompasses raw material extraction, catalyst production, electricity generation, reaction processes, separation/purification, and end-of-life considerations. Recent LCA studies have demonstrated that CO2-derived pharmaceutical intermediates can achieve carbon footprint reductions of 30-60% compared to conventional routes when powered by renewable electricity.
However, challenges in sustainability assessment remain. The environmental benefits of electrocatalytic CO2 valorization are highly dependent on electricity sources, with coal-powered electricity potentially negating any environmental advantages. Additionally, catalyst production often involves rare metals with their own environmental footprint, and separation processes for pharmaceutical-grade purity can be energy-intensive.
Standardization of sustainability metrics specific to CO2 valorization in pharmaceuticals remains underdeveloped. Industry stakeholders are increasingly advocating for harmonized assessment frameworks that account for the unique aspects of these technologies, including temporal and spatial variations in electricity carbon intensity, catalyst lifetime considerations, and integration with existing pharmaceutical manufacturing infrastructure.
Regulatory Framework for Green Pharmaceutical Manufacturing
The pharmaceutical industry is increasingly subject to stringent environmental regulations that directly impact manufacturing processes. As electrocatalytic CO2 valorization emerges as a promising green technology, understanding the regulatory landscape becomes essential for pharmaceutical companies seeking to implement these innovative approaches.
The European Union leads with its comprehensive Green Deal framework, which includes specific provisions for pharmaceutical manufacturing under the Industrial Emissions Directive (IED). Recent amendments to this directive have established more rigorous carbon emission standards, with pharmaceutical companies required to reduce their carbon footprint by 30% by 2030 compared to 2020 levels. The EU Emissions Trading System (ETS) further incentivizes adoption of carbon capture and utilization technologies like electrocatalytic CO2 valorization.
In the United States, the FDA has introduced the Green Chemistry Initiative specifically targeting pharmaceutical manufacturing. This program offers expedited review processes for drug applications that incorporate sustainable manufacturing methods, including CO2 utilization technologies. The EPA's Clean Air Act regulations have also been updated to include more stringent carbon emission standards for pharmaceutical facilities, with specific provisions encouraging carbon recycling technologies.
International standards such as ISO 14001 for Environmental Management Systems have been revised to include specific guidelines for pharmaceutical manufacturing, with new sections addressing carbon capture and utilization technologies. The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) has developed the Q14 guideline on sustainable pharmaceutical manufacturing, which explicitly recognizes electrocatalytic processes as preferred green chemistry approaches.
Financial incentives play a crucial role in the regulatory framework. Many jurisdictions offer tax credits for implementation of green technologies in pharmaceutical manufacturing. For instance, the US Inflation Reduction Act provides tax incentives of up to 45% for investments in carbon capture and utilization technologies, while the EU Innovation Fund allocates substantial grants for pharmaceutical companies implementing electrocatalytic CO2 valorization systems.
Regulatory reporting requirements have also evolved, with major markets now requiring pharmaceutical companies to disclose their carbon footprint and sustainability initiatives in annual reports. The Task Force on Climate-related Financial Disclosures (TCFD) framework has been widely adopted, requiring pharmaceutical companies to report on climate-related risks and opportunities, including their strategies for carbon utilization and reduction.
The European Union leads with its comprehensive Green Deal framework, which includes specific provisions for pharmaceutical manufacturing under the Industrial Emissions Directive (IED). Recent amendments to this directive have established more rigorous carbon emission standards, with pharmaceutical companies required to reduce their carbon footprint by 30% by 2030 compared to 2020 levels. The EU Emissions Trading System (ETS) further incentivizes adoption of carbon capture and utilization technologies like electrocatalytic CO2 valorization.
In the United States, the FDA has introduced the Green Chemistry Initiative specifically targeting pharmaceutical manufacturing. This program offers expedited review processes for drug applications that incorporate sustainable manufacturing methods, including CO2 utilization technologies. The EPA's Clean Air Act regulations have also been updated to include more stringent carbon emission standards for pharmaceutical facilities, with specific provisions encouraging carbon recycling technologies.
International standards such as ISO 14001 for Environmental Management Systems have been revised to include specific guidelines for pharmaceutical manufacturing, with new sections addressing carbon capture and utilization technologies. The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) has developed the Q14 guideline on sustainable pharmaceutical manufacturing, which explicitly recognizes electrocatalytic processes as preferred green chemistry approaches.
Financial incentives play a crucial role in the regulatory framework. Many jurisdictions offer tax credits for implementation of green technologies in pharmaceutical manufacturing. For instance, the US Inflation Reduction Act provides tax incentives of up to 45% for investments in carbon capture and utilization technologies, while the EU Innovation Fund allocates substantial grants for pharmaceutical companies implementing electrocatalytic CO2 valorization systems.
Regulatory reporting requirements have also evolved, with major markets now requiring pharmaceutical companies to disclose their carbon footprint and sustainability initiatives in annual reports. The Task Force on Climate-related Financial Disclosures (TCFD) framework has been widely adopted, requiring pharmaceutical companies to report on climate-related risks and opportunities, including their strategies for carbon utilization and reduction.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!