Research on Electrocatalytic CO2 Valorization Material Innovations
OCT 23, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CO2 Valorization Background and Objectives
Carbon dioxide (CO2) valorization represents a critical frontier in addressing the dual challenges of climate change and sustainable resource utilization. The atmospheric concentration of CO2 has surpassed 415 ppm, marking a 50% increase since pre-industrial levels and contributing significantly to global warming. Electrocatalytic CO2 conversion offers a promising approach to transform this greenhouse gas into value-added chemicals and fuels, effectively closing the carbon cycle while potentially creating economic opportunities.
The evolution of CO2 valorization technologies has progressed through several distinct phases. Initial research in the 1980s demonstrated basic feasibility but suffered from low efficiency and selectivity. The 2000s witnessed significant advances in catalyst design and electrochemical cell configurations, while the past decade has seen remarkable progress in nanomaterial catalysts and operando characterization techniques that have dramatically improved conversion efficiencies.
Current technological objectives focus on developing advanced electrocatalytic materials capable of converting CO2 with high energy efficiency, product selectivity, and long-term stability. Specifically, researchers aim to achieve Faradaic efficiencies exceeding 90% for target products, reduce overpotentials below 0.5V, and maintain catalyst performance for thousands of hours under industrial conditions. Additionally, there is growing emphasis on catalyst designs that can operate effectively in flow-cell configurations suitable for industrial scaling.
The field is trending toward multifunctional catalyst systems that can direct CO2 conversion toward specific high-value products such as ethylene, ethanol, and multi-carbon compounds rather than simple reduction to carbon monoxide or formate. This shift reflects both scientific progress and market demands for chemicals that can replace fossil-based feedstocks in existing value chains.
Beyond technical performance, emerging objectives include developing catalysts from earth-abundant materials to ensure economic viability and sustainability. The integration of renewable electricity sources with CO2 conversion systems represents another critical goal, potentially enabling carbon-negative production pathways when powered by solar, wind, or other low-carbon energy sources.
The convergence of advanced materials science, electrochemistry, and computational modeling is accelerating innovation in this field. Machine learning approaches are increasingly being applied to predict catalyst performance and guide experimental design, while in-situ and operando characterization techniques provide unprecedented insights into reaction mechanisms at the molecular level.
The evolution of CO2 valorization technologies has progressed through several distinct phases. Initial research in the 1980s demonstrated basic feasibility but suffered from low efficiency and selectivity. The 2000s witnessed significant advances in catalyst design and electrochemical cell configurations, while the past decade has seen remarkable progress in nanomaterial catalysts and operando characterization techniques that have dramatically improved conversion efficiencies.
Current technological objectives focus on developing advanced electrocatalytic materials capable of converting CO2 with high energy efficiency, product selectivity, and long-term stability. Specifically, researchers aim to achieve Faradaic efficiencies exceeding 90% for target products, reduce overpotentials below 0.5V, and maintain catalyst performance for thousands of hours under industrial conditions. Additionally, there is growing emphasis on catalyst designs that can operate effectively in flow-cell configurations suitable for industrial scaling.
The field is trending toward multifunctional catalyst systems that can direct CO2 conversion toward specific high-value products such as ethylene, ethanol, and multi-carbon compounds rather than simple reduction to carbon monoxide or formate. This shift reflects both scientific progress and market demands for chemicals that can replace fossil-based feedstocks in existing value chains.
Beyond technical performance, emerging objectives include developing catalysts from earth-abundant materials to ensure economic viability and sustainability. The integration of renewable electricity sources with CO2 conversion systems represents another critical goal, potentially enabling carbon-negative production pathways when powered by solar, wind, or other low-carbon energy sources.
The convergence of advanced materials science, electrochemistry, and computational modeling is accelerating innovation in this field. Machine learning approaches are increasingly being applied to predict catalyst performance and guide experimental design, while in-situ and operando characterization techniques provide unprecedented insights into reaction mechanisms at the molecular level.
Market Analysis for CO2 Conversion Technologies
The global market for CO2 conversion technologies is experiencing significant growth, driven by increasing environmental concerns and regulatory pressures to reduce carbon emissions. The market was valued at approximately $1.8 billion in 2022 and is projected to reach $4.2 billion by 2030, representing a compound annual growth rate (CAGR) of 11.2%. This growth trajectory reflects the urgent need for sustainable solutions to address climate change challenges.
Electrocatalytic CO2 valorization represents a particularly promising segment within this market, with potential applications across multiple industries including chemicals, fuels, and materials. The demand for converted CO2 products is strongest in regions with stringent carbon regulations, notably the European Union, North America, and increasingly in parts of Asia, particularly China and Japan.
Industrial sectors driving market demand include chemical manufacturing, which seeks sustainable feedstocks; energy production, looking for carbon-neutral fuel alternatives; and consumer goods industries pursuing greener material inputs. The market is further segmented by end products, with CO2-derived fuels (methanol, formic acid) currently dominating at 45% market share, followed by chemicals (30%) and materials (25%).
Key market drivers include tightening carbon regulations globally, with over 60 countries implementing carbon pricing mechanisms as of 2023. Corporate sustainability commitments represent another significant driver, with more than 300 major global corporations pledging net-zero emissions by 2050. Additionally, government incentives for carbon capture and utilization technologies have increased substantially, with the EU's Innovation Fund allocating €10 billion for low-carbon technologies and the US Inflation Reduction Act providing tax credits for carbon utilization.
Market barriers include high capital costs for implementation, with typical industrial-scale electrocatalytic systems requiring investments of $50-100 million. Technical challenges in scaling laboratory breakthroughs to commercial applications remain significant, with energy efficiency limitations being particularly problematic. Competitive pricing against conventional petrochemical products also presents challenges, though this gap is narrowing as carbon prices increase.
Regional market analysis shows Europe leading with approximately 38% market share, followed by North America (32%) and Asia-Pacific (25%). The fastest growth is anticipated in Asia-Pacific markets, particularly China, which has made substantial investments in green technology development as part of its carbon neutrality goals.
Electrocatalytic CO2 valorization represents a particularly promising segment within this market, with potential applications across multiple industries including chemicals, fuels, and materials. The demand for converted CO2 products is strongest in regions with stringent carbon regulations, notably the European Union, North America, and increasingly in parts of Asia, particularly China and Japan.
Industrial sectors driving market demand include chemical manufacturing, which seeks sustainable feedstocks; energy production, looking for carbon-neutral fuel alternatives; and consumer goods industries pursuing greener material inputs. The market is further segmented by end products, with CO2-derived fuels (methanol, formic acid) currently dominating at 45% market share, followed by chemicals (30%) and materials (25%).
Key market drivers include tightening carbon regulations globally, with over 60 countries implementing carbon pricing mechanisms as of 2023. Corporate sustainability commitments represent another significant driver, with more than 300 major global corporations pledging net-zero emissions by 2050. Additionally, government incentives for carbon capture and utilization technologies have increased substantially, with the EU's Innovation Fund allocating €10 billion for low-carbon technologies and the US Inflation Reduction Act providing tax credits for carbon utilization.
Market barriers include high capital costs for implementation, with typical industrial-scale electrocatalytic systems requiring investments of $50-100 million. Technical challenges in scaling laboratory breakthroughs to commercial applications remain significant, with energy efficiency limitations being particularly problematic. Competitive pricing against conventional petrochemical products also presents challenges, though this gap is narrowing as carbon prices increase.
Regional market analysis shows Europe leading with approximately 38% market share, followed by North America (32%) and Asia-Pacific (25%). The fastest growth is anticipated in Asia-Pacific markets, particularly China, which has made substantial investments in green technology development as part of its carbon neutrality goals.
Electrocatalytic CO2 Reduction: Status and Barriers
Electrocatalytic CO2 reduction (ECR) has emerged as a promising approach for carbon dioxide valorization, offering a sustainable pathway to convert this greenhouse gas into value-added chemicals and fuels. Currently, the technology has reached laboratory demonstration scale with several catalyst systems showing promising performance metrics. However, significant barriers remain before widespread commercial implementation becomes viable.
The state-of-the-art catalysts for ECR include copper-based materials for multi-carbon product formation, silver and gold for CO production, and tin or bismuth for formate generation. Recent advances have pushed Faradaic efficiencies above 90% for certain products, while current densities have improved to 100-300 mA/cm² in optimized systems. Despite these achievements, industrial relevance typically requires current densities exceeding 500 mA/cm² with sustained performance.
A fundamental challenge in the field remains product selectivity. Most catalysts produce multiple products simultaneously, necessitating costly downstream separation. Even the most selective catalysts struggle to maintain performance when scaled beyond laboratory dimensions, with selectivity often decreasing dramatically at industrially relevant current densities.
Stability presents another critical barrier, with many high-performing catalysts showing significant degradation over time. State-of-the-art systems typically demonstrate stability for hundreds of hours, whereas commercial viability would require thousands of hours of consistent operation. Catalyst poisoning, structural reorganization, and leaching contribute to performance losses over extended operation.
Energy efficiency remains suboptimal, with significant overpotentials required to drive desired reactions. This translates to higher energy consumption, undermining the sustainability benefits of the technology. Current systems typically operate at 30-60% energy efficiency, whereas >80% would be desirable for commercial implementation.
Scale-up challenges persist throughout the field. Laboratory demonstrations typically utilize small electrodes (cm²), while industrial applications would require m² scale. As systems scale up, mass transport limitations, heat management, and uniform current distribution become increasingly problematic. Gas diffusion electrode technologies have shown promise in addressing some of these issues but introduce new challenges in mechanical stability and flooding control.
The economic viability of ECR technologies faces competition from established chemical processes. Production costs for ECR-derived chemicals remain 2-5 times higher than conventional routes, primarily due to capital costs and electricity prices. Without significant carbon pricing or regulatory incentives, the economic case for widespread adoption remains challenging.
Geographically, research leadership in ECR is concentrated in North America, Western Europe, China, and Japan, with emerging contributions from South Korea and Singapore. This distribution largely follows patterns of investment in renewable energy and carbon mitigation technologies.
The state-of-the-art catalysts for ECR include copper-based materials for multi-carbon product formation, silver and gold for CO production, and tin or bismuth for formate generation. Recent advances have pushed Faradaic efficiencies above 90% for certain products, while current densities have improved to 100-300 mA/cm² in optimized systems. Despite these achievements, industrial relevance typically requires current densities exceeding 500 mA/cm² with sustained performance.
A fundamental challenge in the field remains product selectivity. Most catalysts produce multiple products simultaneously, necessitating costly downstream separation. Even the most selective catalysts struggle to maintain performance when scaled beyond laboratory dimensions, with selectivity often decreasing dramatically at industrially relevant current densities.
Stability presents another critical barrier, with many high-performing catalysts showing significant degradation over time. State-of-the-art systems typically demonstrate stability for hundreds of hours, whereas commercial viability would require thousands of hours of consistent operation. Catalyst poisoning, structural reorganization, and leaching contribute to performance losses over extended operation.
Energy efficiency remains suboptimal, with significant overpotentials required to drive desired reactions. This translates to higher energy consumption, undermining the sustainability benefits of the technology. Current systems typically operate at 30-60% energy efficiency, whereas >80% would be desirable for commercial implementation.
Scale-up challenges persist throughout the field. Laboratory demonstrations typically utilize small electrodes (cm²), while industrial applications would require m² scale. As systems scale up, mass transport limitations, heat management, and uniform current distribution become increasingly problematic. Gas diffusion electrode technologies have shown promise in addressing some of these issues but introduce new challenges in mechanical stability and flooding control.
The economic viability of ECR technologies faces competition from established chemical processes. Production costs for ECR-derived chemicals remain 2-5 times higher than conventional routes, primarily due to capital costs and electricity prices. Without significant carbon pricing or regulatory incentives, the economic case for widespread adoption remains challenging.
Geographically, research leadership in ECR is concentrated in North America, Western Europe, China, and Japan, with emerging contributions from South Korea and Singapore. This distribution largely follows patterns of investment in renewable energy and carbon mitigation technologies.
Current Electrocatalytic Material Solutions
01 Metal-based catalysts for CO2 electroreduction
Metal-based catalysts, particularly transition metals and their alloys, demonstrate significant activity for CO2 electroreduction. These catalysts can be engineered with specific morphologies, such as nanoparticles, nanosheets, or porous structures, to increase active sites and enhance catalytic efficiency. The selectivity and efficiency of these catalysts can be tuned by controlling their composition, crystal structure, and surface properties to favor specific reaction pathways in CO2 valorization.- Metal-based catalysts for CO2 electroreduction: Metal-based catalysts, particularly transition metals and their alloys, demonstrate significant efficiency in CO2 electroreduction. These materials offer tunable selectivity toward different carbon products through composition and structure optimization. Metals such as copper, silver, and zinc show distinct product selectivity, with copper being particularly effective for multi-carbon product formation. Nanostructuring these catalysts enhances active site exposure and improves catalytic performance by optimizing binding energies of reaction intermediates.
- Carbon-based materials as CO2 reduction catalysts: Carbon-based materials, including carbon nanotubes, graphene, and doped carbon structures, serve as effective catalysts or catalyst supports for CO2 electroreduction. These materials offer advantages such as high conductivity, large surface area, and tunable electronic properties. Heteroatom doping (N, B, S, P) creates active sites by modifying electronic structures and local charge distributions. Carbon-based catalysts demonstrate good stability under electrochemical conditions and can be produced from sustainable precursors, making them environmentally friendly alternatives to metal-based systems.
- Metal-organic frameworks and hybrid materials: Metal-organic frameworks (MOFs) and hybrid materials combine the benefits of both organic and inorganic components for CO2 electroreduction. These materials feature well-defined structures with tunable pore sizes that can concentrate CO2 near catalytic sites. The metal nodes serve as active centers while organic linkers provide structural stability and can influence product selectivity. Post-synthetic modification and derivatization strategies can further enhance catalytic performance. MOF-derived catalysts, obtained through controlled pyrolysis, often exhibit improved conductivity while maintaining high density of active sites.
- Catalyst interface engineering and electrolyte effects: Interface engineering between catalyst surfaces and electrolytes significantly impacts CO2 electroreduction efficiency. Strategies include creating hydrophobic/hydrophilic interfaces to control CO2 concentration and water availability, modifying local pH through buffer effects, and introducing specific functional groups to stabilize intermediates. Electrolyte composition, including ion type, concentration, and pH, directly affects reaction pathways and product distribution. Advanced interface designs incorporate multiple phases (solid-liquid-gas) to overcome mass transport limitations and enhance CO2 availability at active sites.
- Reactor design and system integration for improved efficiency: Reactor design and system integration play crucial roles in maximizing the efficiency of CO2 electroreduction processes. Advanced reactor configurations include flow cells, gas diffusion electrodes, and membrane electrode assemblies that enhance mass transport and reduce energy losses. System-level approaches integrate CO2 capture with electroreduction to improve overall process efficiency. Continuous flow systems enable better control of reaction conditions and facilitate scale-up. Innovations in electrode architecture, such as 3D structures and hierarchical porosity, optimize catalyst utilization and improve current density while maintaining selectivity.
02 Carbon-based materials as electrocatalysts
Carbon-based materials, including graphene, carbon nanotubes, and doped carbon structures, serve as effective electrocatalysts for CO2 reduction. These materials can be functionalized or doped with heteroatoms (N, S, P) to create active sites that enhance catalytic performance. Carbon-based catalysts offer advantages such as high conductivity, large surface area, and structural stability, making them promising candidates for sustainable CO2 valorization processes with improved efficiency.Expand Specific Solutions03 Metal-organic frameworks and hybrid materials
Metal-organic frameworks (MOFs) and hybrid materials combine the benefits of both organic and inorganic components to create highly efficient CO2 electrocatalysts. These materials feature tunable pore structures, high surface areas, and abundant active sites that facilitate CO2 adsorption and conversion. By engineering the coordination environment and incorporating functional groups, these catalysts can achieve enhanced selectivity and efficiency for specific CO2 reduction products while maintaining stability under electrochemical conditions.Expand Specific Solutions04 Reactor design and system optimization
Advanced reactor designs and system optimization strategies significantly impact the efficiency of electrocatalytic CO2 valorization processes. Innovations include flow-cell configurations, gas diffusion electrodes, and membrane-electrode assemblies that improve mass transport and reduce energy losses. Optimized electrolyte compositions, operating conditions (temperature, pressure, pH), and electrode structures can enhance catalyst utilization and product selectivity, leading to higher conversion rates and energy efficiency in CO2 electroreduction systems.Expand Specific Solutions05 Single-atom catalysts and atomically dispersed active sites
Single-atom catalysts (SACs) and atomically dispersed active sites represent a frontier in electrocatalytic CO2 valorization. These catalysts feature isolated metal atoms anchored on various supports, maximizing atom utilization efficiency and providing well-defined, uniform active sites. The unique electronic structure and coordination environment of single atoms enable precise tuning of binding energies for reaction intermediates, resulting in enhanced activity, selectivity, and stability for CO2 electroreduction to valuable products like carbon monoxide, formate, or hydrocarbons.Expand Specific Solutions
Leading Institutions and Companies in CO2 Valorization
Electrocatalytic CO2 valorization technology is currently in a growth phase, with the market expanding rapidly due to increasing focus on carbon neutrality goals. The global market size is projected to reach significant scale as industries seek sustainable carbon utilization solutions. From a technological maturity perspective, academic institutions like Zhejiang University, Dalian Institute of Chemical Physics, and Beijing University of Chemical Technology are leading fundamental research, while commercial entities such as Sinopec, Faraday Technology, and Siemens Energy are advancing practical applications. The competitive landscape shows strong collaboration between academia and industry, with Chinese institutions demonstrating particular strength in catalyst development. Western players like Brown University and CNRS contribute significant innovations in reaction mechanisms and system integration, creating a globally distributed innovation ecosystem.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed an integrated approach to CO2 valorization that combines electrocatalytic reduction with their existing petrochemical infrastructure. Their technology focuses on practical implementation at industrial scales, with emphasis on robust catalyst systems that can operate under real-world conditions. Sinopec has engineered copper-zinc oxide composite catalysts supported on carbon nanotubes that demonstrate remarkable stability over thousands of hours of operation while maintaining high selectivity toward syngas and methanol production. Their innovation includes a proprietary electrode design that minimizes ohmic losses and optimizes mass transport in large-format electrochemical cells. Sinopec has also developed a hybrid process that integrates CO2 electroreduction with conventional thermocatalytic processes, allowing for more efficient energy utilization across their production facilities[5]. A key aspect of their technology is the development of specialized membranes that enable selective ion transport while preventing product crossover, significantly improving overall system efficiency and product purity.
Strengths: Strong focus on industrial applicability and system integration; robust catalyst formulations designed for long-term stability; significant infrastructure advantages for scaling and implementation. Weaknesses: Less emphasis on fundamental breakthroughs in catalyst design; technology heavily optimized for integration with existing petrochemical processes rather than standalone applications; economic viability currently depends on government carbon policies.
Dalian University of Technology
Technical Solution: Dalian University of Technology (DUT) has developed advanced electrocatalytic materials for CO2 reduction based on atomic-level engineering of transition metal compounds. Their approach focuses on creating defect-rich nanostructures that serve as active sites for CO2 activation and conversion. DUT researchers have pioneered a series of copper-based catalysts with precisely controlled oxidation states that achieve high selectivity toward C2+ products. Their technology incorporates innovative synthesis methods including electrodeposition under controlled potential conditions and plasma-assisted fabrication to create unique surface structures with optimized binding energies for reaction intermediates. A significant breakthrough from DUT is their development of 3D porous electrodes with hierarchical porosity that addresses mass transport limitations while maintaining high catalyst utilization[4]. The university has also developed in-operando characterization techniques that provide real-time information about catalyst structure and reaction mechanisms during CO2 electroreduction, enabling rational design principles for next-generation materials.
Strengths: Exceptional control over catalyst structure at the atomic level; innovative synthesis methods that create unique active sites; strong fundamental understanding of reaction mechanisms through advanced characterization. Weaknesses: Some materials rely on precious metals or complex synthesis procedures that may limit commercial viability; catalyst stability under industrial conditions needs further improvement; scaling up laboratory prototypes remains challenging.
Key Patents and Breakthroughs in Catalyst Design
Doped carbon material for the electrocatalytic conversion of co 2 into hydrocarbons, uses of the material and conversion method using said material
PatentWO2013004882A1
Innovation
- Doped carbon materials, specifically carbon gels with transition metals like Ni, Cu, or Fe, are used as electro-catalysts, offering high surface area and porosity, minimizing metal leaching, and enabling efficient CO2 transformation into hydrocarbons at atmospheric pressure.
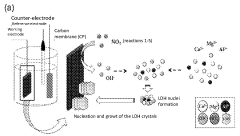
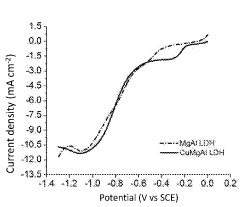
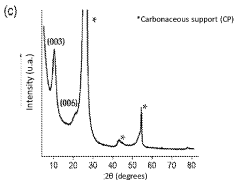
Layered double-hydroxide catalytic coatings containing: CU, mg e al, achievable electro¬ chemically, for uses such as electrochemical reduction of carbon dioxide
PatentWO2024127296A1
Innovation
- A nanostructured composite material with a layered hydrotalcite-type ternary structure of CuMgAl LDH, containing metal copper and cuprous ion as a redox couple, is developed, which is electrochemically deposited on a carbon gas diffusion membrane to enhance catalytic activity and selectivity for C2 products.
Techno-economic Assessment of CO2 Valorization
The techno-economic assessment of CO2 valorization reveals significant potential for transforming carbon dioxide from an environmental liability into valuable products. Current economic analyses indicate that electrochemical CO2 reduction processes can achieve production costs between $0.75-2.50 per kilogram for various C1-C3 products, depending on electricity prices, catalyst efficiency, and system scale.
Capital expenditure for industrial-scale CO2 valorization facilities ranges from $50-150 million, with electrolyzer units representing 35-45% of total investment. Operating costs are primarily driven by electricity consumption (40-60%), followed by maintenance (15-20%) and catalyst replacement (10-15%). Renewable electricity integration can substantially improve both economic viability and environmental benefits, with levelized costs potentially decreasing by 30-40% when utilizing off-peak renewable power.
Market analysis demonstrates growing demand for CO2-derived products, particularly in chemical intermediates, synthetic fuels, and polymer precursors. The global market for CO2-derived products is projected to reach $7.5 billion by 2030, with compound annual growth rates exceeding 25% in certain segments. Carbon pricing mechanisms further enhance economic feasibility, with each $50/ton CO2 price improving project economics by approximately 15-20%.
Sensitivity analyses highlight electricity cost as the most critical economic factor, with each $0.01/kWh change affecting production costs by 8-12%. Catalyst performance improvements, particularly selectivity and stability enhancements, could reduce operational costs by 20-30% over current baseline technologies. System integration with existing industrial infrastructure can reduce capital requirements by 15-25% through shared utilities and carbon capture synergies.
Life cycle assessments indicate that electrocatalytic CO2 valorization can achieve carbon reduction benefits of 0.6-2.5 tons CO2-equivalent per ton of product, depending on electricity sources and process efficiency. The economic breakeven point for most CO2 valorization pathways currently requires either carbon pricing support ($40-80/ton CO2) or premium product pricing (15-30% above conventional alternatives).
Policy incentives significantly impact economic viability, with production tax credits, carbon pricing, and renewable content mandates potentially improving internal rates of return by 5-15 percentage points. Technology learning curves suggest cost reductions of 40-60% are achievable over the next decade through scale-up, catalyst improvements, and system optimization.
Capital expenditure for industrial-scale CO2 valorization facilities ranges from $50-150 million, with electrolyzer units representing 35-45% of total investment. Operating costs are primarily driven by electricity consumption (40-60%), followed by maintenance (15-20%) and catalyst replacement (10-15%). Renewable electricity integration can substantially improve both economic viability and environmental benefits, with levelized costs potentially decreasing by 30-40% when utilizing off-peak renewable power.
Market analysis demonstrates growing demand for CO2-derived products, particularly in chemical intermediates, synthetic fuels, and polymer precursors. The global market for CO2-derived products is projected to reach $7.5 billion by 2030, with compound annual growth rates exceeding 25% in certain segments. Carbon pricing mechanisms further enhance economic feasibility, with each $50/ton CO2 price improving project economics by approximately 15-20%.
Sensitivity analyses highlight electricity cost as the most critical economic factor, with each $0.01/kWh change affecting production costs by 8-12%. Catalyst performance improvements, particularly selectivity and stability enhancements, could reduce operational costs by 20-30% over current baseline technologies. System integration with existing industrial infrastructure can reduce capital requirements by 15-25% through shared utilities and carbon capture synergies.
Life cycle assessments indicate that electrocatalytic CO2 valorization can achieve carbon reduction benefits of 0.6-2.5 tons CO2-equivalent per ton of product, depending on electricity sources and process efficiency. The economic breakeven point for most CO2 valorization pathways currently requires either carbon pricing support ($40-80/ton CO2) or premium product pricing (15-30% above conventional alternatives).
Policy incentives significantly impact economic viability, with production tax credits, carbon pricing, and renewable content mandates potentially improving internal rates of return by 5-15 percentage points. Technology learning curves suggest cost reductions of 40-60% are achievable over the next decade through scale-up, catalyst improvements, and system optimization.
Environmental Impact and Carbon Neutrality Implications
Electrocatalytic CO2 valorization technologies represent a critical pathway toward achieving carbon neutrality goals globally. By converting waste CO2 into valuable products, these processes offer a dual benefit of reducing greenhouse gas emissions while producing useful chemicals and fuels. The environmental impact assessment of these technologies reveals significant potential for carbon footprint reduction across multiple industrial sectors, particularly in energy-intensive industries where CO2 emissions are highest.
When implemented at scale, electrocatalytic CO2 conversion systems could potentially reduce global CO2 emissions by 5-8% by 2050, according to recent climate modeling studies. This reduction becomes even more significant when considering the avoided emissions from conventional manufacturing processes for the same chemical products. Life cycle assessments indicate that CO2-derived products can achieve carbon intensity reductions of 30-70% compared to their fossil-based counterparts, depending on the electricity source and process efficiency.
The carbon neutrality implications extend beyond direct emission reductions. By creating a circular carbon economy, these technologies transform the linear "take-make-dispose" model into a regenerative system where carbon is continuously recycled. This paradigm shift aligns perfectly with sustainable development goals and provides a technological bridge during the transition to a fully renewable energy system.
Energy source considerations remain paramount for true environmental benefits. When powered by renewable electricity, electrocatalytic CO2 valorization becomes a genuinely carbon-negative process. Recent grid decarbonization trends in many countries enhance the viability of these technologies from a carbon neutrality perspective, with projections suggesting that by 2030, sufficient renewable capacity could be available to power large-scale CO2 conversion facilities in major industrial regions.
Water consumption represents another important environmental consideration, as many electrocatalytic processes require significant water inputs. Advanced system designs incorporating water recycling can reduce freshwater requirements by up to 80%, minimizing the environmental footprint beyond carbon emissions alone.
Policy frameworks increasingly recognize these technologies as essential components of carbon neutrality strategies. Carbon pricing mechanisms, clean energy incentives, and regulatory frameworks for carbon capture utilization are evolving to support the commercial deployment of electrocatalytic CO2 valorization. The inclusion of these technologies in national determined contributions under the Paris Agreement signals their growing importance in global climate action plans.
When implemented at scale, electrocatalytic CO2 conversion systems could potentially reduce global CO2 emissions by 5-8% by 2050, according to recent climate modeling studies. This reduction becomes even more significant when considering the avoided emissions from conventional manufacturing processes for the same chemical products. Life cycle assessments indicate that CO2-derived products can achieve carbon intensity reductions of 30-70% compared to their fossil-based counterparts, depending on the electricity source and process efficiency.
The carbon neutrality implications extend beyond direct emission reductions. By creating a circular carbon economy, these technologies transform the linear "take-make-dispose" model into a regenerative system where carbon is continuously recycled. This paradigm shift aligns perfectly with sustainable development goals and provides a technological bridge during the transition to a fully renewable energy system.
Energy source considerations remain paramount for true environmental benefits. When powered by renewable electricity, electrocatalytic CO2 valorization becomes a genuinely carbon-negative process. Recent grid decarbonization trends in many countries enhance the viability of these technologies from a carbon neutrality perspective, with projections suggesting that by 2030, sufficient renewable capacity could be available to power large-scale CO2 conversion facilities in major industrial regions.
Water consumption represents another important environmental consideration, as many electrocatalytic processes require significant water inputs. Advanced system designs incorporating water recycling can reduce freshwater requirements by up to 80%, minimizing the environmental footprint beyond carbon emissions alone.
Policy frameworks increasingly recognize these technologies as essential components of carbon neutrality strategies. Carbon pricing mechanisms, clean energy incentives, and regulatory frameworks for carbon capture utilization are evolving to support the commercial deployment of electrocatalytic CO2 valorization. The inclusion of these technologies in national determined contributions under the Paris Agreement signals their growing importance in global climate action plans.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!