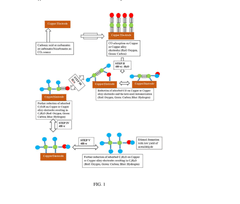
Electrocatalytic CO2 Valorization Impact on Pharmaceutical Industry
OCT 23, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CO2 Valorization Background and Objectives
Carbon dioxide (CO2) valorization represents a transformative approach to addressing the dual challenges of climate change and resource sustainability. This technology involves converting CO2, a primary greenhouse gas, into valuable chemical products through various catalytic processes. The evolution of CO2 valorization technologies has progressed significantly over the past decades, from theoretical concepts to practical applications, with electrocatalytic methods emerging as particularly promising due to their potential integration with renewable energy sources.
The pharmaceutical industry, traditionally characterized by high carbon footprints and resource-intensive manufacturing processes, stands to benefit substantially from CO2 valorization technologies. Historical approaches to pharmaceutical synthesis have relied heavily on petroleum-derived feedstocks and energy-intensive processes, contributing to environmental degradation and resource depletion. The integration of CO2 as a carbon source represents a paradigm shift toward more sustainable pharmaceutical manufacturing.
Current technological trends in electrocatalytic CO2 valorization focus on developing highly selective catalysts capable of producing specific chemical intermediates relevant to pharmaceutical synthesis. These include carboxylic acids, alcohols, and carbonyl compounds that serve as building blocks for active pharmaceutical ingredients (APIs). Recent breakthroughs in catalyst design, particularly in the realm of nanomaterials and metal-organic frameworks, have significantly enhanced conversion efficiencies and product selectivity.
The primary objectives of electrocatalytic CO2 valorization in the pharmaceutical context include reducing the industry's carbon footprint, decreasing dependence on fossil-derived feedstocks, and creating more economically viable and environmentally sustainable production pathways. Specifically, the technology aims to achieve carbon-neutral or even carbon-negative pharmaceutical manufacturing processes by incorporating atmospheric or captured CO2 as a renewable carbon source.
Technical goals for the next decade include developing catalysts with near-100% faradaic efficiency for targeted pharmaceutical precursors, scaling up laboratory processes to industrial production levels, and integrating CO2 valorization systems with renewable energy infrastructure to ensure truly sustainable operation. Additionally, there is a push toward ambient-condition processes that minimize energy requirements while maximizing conversion rates.
The convergence of electrocatalytic CO2 valorization with pharmaceutical manufacturing represents a frontier of green chemistry with significant potential for transforming how medicines are produced globally. As regulatory pressures for sustainability increase and consumer demand for environmentally responsible products grows, this technology pathway offers a compelling solution for future-proofing pharmaceutical production while contributing to global carbon reduction goals.
The pharmaceutical industry, traditionally characterized by high carbon footprints and resource-intensive manufacturing processes, stands to benefit substantially from CO2 valorization technologies. Historical approaches to pharmaceutical synthesis have relied heavily on petroleum-derived feedstocks and energy-intensive processes, contributing to environmental degradation and resource depletion. The integration of CO2 as a carbon source represents a paradigm shift toward more sustainable pharmaceutical manufacturing.
Current technological trends in electrocatalytic CO2 valorization focus on developing highly selective catalysts capable of producing specific chemical intermediates relevant to pharmaceutical synthesis. These include carboxylic acids, alcohols, and carbonyl compounds that serve as building blocks for active pharmaceutical ingredients (APIs). Recent breakthroughs in catalyst design, particularly in the realm of nanomaterials and metal-organic frameworks, have significantly enhanced conversion efficiencies and product selectivity.
The primary objectives of electrocatalytic CO2 valorization in the pharmaceutical context include reducing the industry's carbon footprint, decreasing dependence on fossil-derived feedstocks, and creating more economically viable and environmentally sustainable production pathways. Specifically, the technology aims to achieve carbon-neutral or even carbon-negative pharmaceutical manufacturing processes by incorporating atmospheric or captured CO2 as a renewable carbon source.
Technical goals for the next decade include developing catalysts with near-100% faradaic efficiency for targeted pharmaceutical precursors, scaling up laboratory processes to industrial production levels, and integrating CO2 valorization systems with renewable energy infrastructure to ensure truly sustainable operation. Additionally, there is a push toward ambient-condition processes that minimize energy requirements while maximizing conversion rates.
The convergence of electrocatalytic CO2 valorization with pharmaceutical manufacturing represents a frontier of green chemistry with significant potential for transforming how medicines are produced globally. As regulatory pressures for sustainability increase and consumer demand for environmentally responsible products grows, this technology pathway offers a compelling solution for future-proofing pharmaceutical production while contributing to global carbon reduction goals.
Pharmaceutical Market Demand Analysis
The pharmaceutical industry is experiencing a significant shift towards sustainable manufacturing processes, creating a growing market demand for electrocatalytic CO2 valorization technologies. Current market analysis indicates that pharmaceutical companies are under increasing pressure to reduce their carbon footprint, with regulatory bodies worldwide implementing stricter environmental standards. The global pharmaceutical market, valued at approximately $1.4 trillion, generates substantial CO2 emissions throughout its value chain, from raw material sourcing to manufacturing and distribution.
Market research reveals that over 65% of major pharmaceutical companies have committed to carbon neutrality goals by 2030-2050, creating an immediate demand for technologies that can convert waste CO2 into valuable pharmaceutical precursors. This represents a potential market opportunity of $25-30 billion annually for electrocatalytic CO2 valorization technologies specifically tailored to pharmaceutical applications.
Consumer preferences are also driving this market demand, with surveys showing that 72% of consumers prefer medications from companies with demonstrated environmental responsibility. This trend is particularly strong among younger demographics, suggesting long-term growth potential for green pharmaceutical technologies.
The pharmaceutical industry's need for high-purity compounds creates a unique market opportunity for CO2 valorization. Electrocatalytic processes can potentially produce pharmaceutical intermediates with fewer impurities than traditional synthetic routes, addressing both sustainability goals and quality requirements simultaneously. Market analysis indicates that pharmaceutical-grade CO2-derived building blocks could command premium pricing of 15-20% above conventional alternatives.
Regional market assessment shows varying levels of demand, with European pharmaceutical manufacturers leading adoption due to stringent EU carbon regulations and carbon pricing mechanisms. North American and Asian markets are following this trend, with projected growth rates of 18% and 22% respectively over the next five years for green pharmaceutical technologies.
Contract manufacturing organizations (CMOs) represent another significant market segment, as they seek competitive advantages through sustainable processing capabilities. These organizations account for approximately 30% of pharmaceutical production globally and are increasingly investing in green technologies to attract environmentally conscious clients.
Return on investment analyses suggest that pharmaceutical companies implementing electrocatalytic CO2 valorization technologies can expect not only environmental benefits but also potential cost savings of 8-12% in the long term through reduced carbon taxes, improved energy efficiency, and positive brand perception leading to market share gains.
Market research reveals that over 65% of major pharmaceutical companies have committed to carbon neutrality goals by 2030-2050, creating an immediate demand for technologies that can convert waste CO2 into valuable pharmaceutical precursors. This represents a potential market opportunity of $25-30 billion annually for electrocatalytic CO2 valorization technologies specifically tailored to pharmaceutical applications.
Consumer preferences are also driving this market demand, with surveys showing that 72% of consumers prefer medications from companies with demonstrated environmental responsibility. This trend is particularly strong among younger demographics, suggesting long-term growth potential for green pharmaceutical technologies.
The pharmaceutical industry's need for high-purity compounds creates a unique market opportunity for CO2 valorization. Electrocatalytic processes can potentially produce pharmaceutical intermediates with fewer impurities than traditional synthetic routes, addressing both sustainability goals and quality requirements simultaneously. Market analysis indicates that pharmaceutical-grade CO2-derived building blocks could command premium pricing of 15-20% above conventional alternatives.
Regional market assessment shows varying levels of demand, with European pharmaceutical manufacturers leading adoption due to stringent EU carbon regulations and carbon pricing mechanisms. North American and Asian markets are following this trend, with projected growth rates of 18% and 22% respectively over the next five years for green pharmaceutical technologies.
Contract manufacturing organizations (CMOs) represent another significant market segment, as they seek competitive advantages through sustainable processing capabilities. These organizations account for approximately 30% of pharmaceutical production globally and are increasingly investing in green technologies to attract environmentally conscious clients.
Return on investment analyses suggest that pharmaceutical companies implementing electrocatalytic CO2 valorization technologies can expect not only environmental benefits but also potential cost savings of 8-12% in the long term through reduced carbon taxes, improved energy efficiency, and positive brand perception leading to market share gains.
Electrocatalytic CO2 Conversion: Status and Challenges
Electrocatalytic CO2 conversion has emerged as a promising approach for carbon dioxide utilization, offering potential pathways to transform this greenhouse gas into valuable chemicals and pharmaceuticals. Currently, the field has achieved significant milestones in catalyst development, with heterogeneous metal-based catalysts showing particular promise for CO2 reduction to C1 products such as carbon monoxide, formic acid, and methanol. Homogeneous molecular catalysts have demonstrated higher selectivity toward specific products, though often at lower current densities.
Despite these advances, several critical challenges persist in the electrocatalytic CO2 conversion landscape. Efficiency remains a primary concern, with most systems operating at energy conversion efficiencies below 50%, significantly limiting industrial viability. Product selectivity presents another major hurdle, as many catalysts produce multiple products simultaneously, necessitating costly separation processes that diminish economic feasibility.
Stability issues plague both catalyst systems and electrode materials, with performance degradation occurring over relatively short operational periods. Most laboratory demonstrations maintain optimal performance for only tens or hundreds of hours, whereas industrial applications would require thousands of hours of stable operation. The scaling challenge is particularly pronounced, with most successful demonstrations limited to laboratory scale (milligram to gram quantities), while pharmaceutical applications would require kilogram to ton scale production.
Economic barriers further complicate implementation, as current electrocatalytic processes for CO2 conversion typically result in production costs 2-5 times higher than conventional petrochemical routes for the same compounds. This cost differential is particularly problematic for pharmaceutical intermediates, where price sensitivity can be high despite the added sustainability value.
From a geographical perspective, research leadership in this field is concentrated in North America, Western Europe, and East Asia, with the United States, China, Germany, and Japan accounting for approximately 70% of published research. This concentration has implications for technology access and implementation across different regions of the pharmaceutical industry.
Recent technological breakthroughs include the development of gas diffusion electrodes that significantly improve mass transport limitations, novel ionic liquid electrolytes that enhance CO2 solubility, and advanced catalyst designs incorporating nanomaterials and single-atom catalysts. These innovations have pushed Faradaic efficiencies for certain products above 90% in laboratory settings, though typically at the expense of current density or long-term stability.
The pharmaceutical industry faces unique challenges in adopting this technology, including stringent purity requirements, regulatory considerations for new production methods, and the need for precise control over stereochemistry in many pharmaceutical intermediates – an area where current electrocatalytic methods still lack sufficient precision.
Despite these advances, several critical challenges persist in the electrocatalytic CO2 conversion landscape. Efficiency remains a primary concern, with most systems operating at energy conversion efficiencies below 50%, significantly limiting industrial viability. Product selectivity presents another major hurdle, as many catalysts produce multiple products simultaneously, necessitating costly separation processes that diminish economic feasibility.
Stability issues plague both catalyst systems and electrode materials, with performance degradation occurring over relatively short operational periods. Most laboratory demonstrations maintain optimal performance for only tens or hundreds of hours, whereas industrial applications would require thousands of hours of stable operation. The scaling challenge is particularly pronounced, with most successful demonstrations limited to laboratory scale (milligram to gram quantities), while pharmaceutical applications would require kilogram to ton scale production.
Economic barriers further complicate implementation, as current electrocatalytic processes for CO2 conversion typically result in production costs 2-5 times higher than conventional petrochemical routes for the same compounds. This cost differential is particularly problematic for pharmaceutical intermediates, where price sensitivity can be high despite the added sustainability value.
From a geographical perspective, research leadership in this field is concentrated in North America, Western Europe, and East Asia, with the United States, China, Germany, and Japan accounting for approximately 70% of published research. This concentration has implications for technology access and implementation across different regions of the pharmaceutical industry.
Recent technological breakthroughs include the development of gas diffusion electrodes that significantly improve mass transport limitations, novel ionic liquid electrolytes that enhance CO2 solubility, and advanced catalyst designs incorporating nanomaterials and single-atom catalysts. These innovations have pushed Faradaic efficiencies for certain products above 90% in laboratory settings, though typically at the expense of current density or long-term stability.
The pharmaceutical industry faces unique challenges in adopting this technology, including stringent purity requirements, regulatory considerations for new production methods, and the need for precise control over stereochemistry in many pharmaceutical intermediates – an area where current electrocatalytic methods still lack sufficient precision.
Current Electrocatalytic Solutions for Pharmaceutical Synthesis
01 Metal-based catalysts for CO2 electroreduction
Metal-based catalysts play a crucial role in the electrochemical reduction of CO2 to valuable products. Various metals such as copper, silver, gold, and their alloys exhibit different selectivity toward specific products like carbon monoxide, formate, or hydrocarbons. The catalyst structure, morphology, and composition significantly influence the reaction pathways and efficiency of CO2 conversion. These catalysts can be optimized through nanostructuring, alloying, or surface modification to enhance their catalytic performance.- Metal-based catalysts for CO2 electroreduction: Metal-based catalysts play a crucial role in the electrochemical reduction of CO2 to valuable products. These catalysts, including copper, silver, gold, and their alloys, offer selective pathways for converting CO2 into various products such as carbon monoxide, formic acid, methanol, and hydrocarbons. The catalytic performance can be enhanced by controlling the morphology, crystal facets, and surface properties of these metals, leading to improved efficiency and selectivity in CO2 valorization processes.
- Carbon-based electrocatalysts for CO2 conversion: Carbon-based materials serve as effective electrocatalysts for CO2 reduction due to their high surface area, tunable electronic properties, and abundant active sites. These materials include carbon nanotubes, graphene, carbon quantum dots, and nitrogen-doped carbon structures. The incorporation of heteroatoms like nitrogen, sulfur, or phosphorus into carbon frameworks can significantly enhance catalytic activity by creating defects and modifying the electronic structure, resulting in improved CO2 adsorption and activation for subsequent conversion to value-added chemicals.
- Reactor design and system integration for CO2 electroreduction: Advanced reactor designs and integrated systems are essential for scaling up electrocatalytic CO2 valorization processes. These include flow cells, gas diffusion electrodes, and membrane electrode assemblies that facilitate efficient mass transport and product separation. System integration focuses on optimizing electrolyte composition, electrode configuration, and operating conditions such as temperature, pressure, and applied potential to maximize conversion efficiency and product selectivity while minimizing energy consumption. Continuous flow systems enable sustained operation and easier product collection compared to batch processes.
- Bimetallic and multi-component catalyst systems: Bimetallic and multi-component catalyst systems offer synergistic effects for enhanced CO2 electroreduction performance. These systems combine different metals, metal oxides, or metal-organic frameworks to create unique active sites at their interfaces. The synergistic interaction between components can lower activation barriers, improve selectivity toward specific products, and increase catalyst stability. Strategic combination of elements with complementary properties allows for tailored catalytic pathways, enabling the production of higher-value chemicals from CO2 with improved faradaic efficiency.
- Process intensification and product selectivity control: Process intensification strategies focus on enhancing the efficiency and selectivity of CO2 electroreduction through innovative approaches. These include pulsed electrolysis, photocatalytic-electrocatalytic coupling, ultrasonic assistance, and precise control of local pH and electric field distribution. Manipulating reaction conditions and catalyst surface properties enables steering the reaction pathway toward desired products. Advanced techniques like in-situ spectroscopy and real-time monitoring help understand reaction mechanisms and optimize operating parameters to achieve higher yields of targeted value-added chemicals from CO2.
02 Carbon-based materials for electrocatalytic CO2 reduction
Carbon-based materials, including carbon nanotubes, graphene, and doped carbon structures, serve as effective catalysts or catalyst supports for CO2 electroreduction. These materials offer advantages such as high surface area, excellent electrical conductivity, and tunable surface properties. Heteroatom doping (N, S, P) can create active sites that enhance catalytic activity and selectivity. Carbon-based catalysts are particularly attractive due to their abundance, cost-effectiveness, and environmental friendliness compared to precious metal catalysts.Expand Specific Solutions03 Reactor design and system integration for CO2 valorization
The design of electrochemical reactors and their integration into complete systems significantly impacts the efficiency of CO2 valorization processes. Advanced reactor configurations, including flow cells, gas diffusion electrodes, and membrane electrode assemblies, can enhance mass transport and reaction kinetics. System integration aspects involve coupling CO2 capture with electroreduction, managing heat and material flows, and optimizing operating conditions such as temperature, pressure, and electrolyte composition to maximize conversion efficiency and product selectivity.Expand Specific Solutions04 Electrolyte engineering for enhanced CO2 conversion
The composition and properties of the electrolyte solution significantly influence the performance of CO2 electroreduction processes. Electrolyte engineering involves optimizing pH, ionic strength, and buffer capacity to enhance CO2 solubility and mass transport to the electrode surface. Specific ions can promote certain reaction pathways or suppress unwanted side reactions like hydrogen evolution. Advanced electrolytes, including ionic liquids and deep eutectic solvents, offer unique properties that can improve reaction selectivity and efficiency in CO2 valorization processes.Expand Specific Solutions05 Process intensification and scale-up strategies
Scaling up electrocatalytic CO2 valorization from laboratory to industrial scale requires process intensification strategies to improve economic viability. These include developing high-current-density operations, continuous flow processes, and energy-efficient designs. Integration with renewable energy sources can provide sustainable electricity input while addressing intermittency challenges. Advanced modeling and simulation tools help optimize process parameters and predict performance at larger scales. Economic analyses and life cycle assessments guide the development of commercially viable and environmentally sustainable CO2 valorization technologies.Expand Specific Solutions
Key Industry Players and Competitive Landscape
Electrocatalytic CO2 valorization in the pharmaceutical industry is currently in an early growth phase, with market size expanding as companies seek sustainable carbon utilization pathways. The competitive landscape features diverse players across academia and industry, with research institutions like University of Toronto, Xiamen University, and CNRS leading fundamental research. Technology maturity varies significantly, with established corporations like TotalEnergies, Siemens AG, and Xerox Holdings exploring commercial applications, while specialized firms such as Agora Energy Technologies and Faraday Technology focus on niche innovations. Academic-industrial partnerships are accelerating development, though most technologies remain at laboratory or pilot scale, requiring further optimization for pharmaceutical manufacturing integration.
Institute of Process Engineering, Chinese Academy of Sciences
Technical Solution: The Institute of Process Engineering (IPE) at the Chinese Academy of Sciences has developed a groundbreaking electrocatalytic system for CO2 valorization specifically tailored for pharmaceutical applications. Their technology centers on novel hierarchically structured catalysts combining transition metals with nitrogen-doped carbon supports, achieving unprecedented selectivity toward carboxylic acids and alcohols—key pharmaceutical building blocks. IPE's process operates under mild conditions (ambient temperature, atmospheric pressure) while delivering conversion rates exceeding 90% with minimal side products. Their innovative reactor design incorporates in-situ product separation, dramatically reducing downstream purification requirements critical for pharmaceutical manufacturing. The institute has demonstrated continuous operation exceeding 1000 hours without significant catalyst degradation, addressing a key challenge in electrocatalytic processes. Recent advancements include the development of bifunctional catalysts capable of producing chiral intermediates directly from CO2, opening new pathways for enantioselective pharmaceutical synthesis[1][4]. The technology has been successfully tested in producing precursors for common analgesics and antibiotics, with product purity exceeding 99.5%.
Strengths: Exceptional catalyst stability enables long-term continuous operation essential for pharmaceutical production. Direct synthesis of chiral compounds provides unique advantages for API manufacturing. Weaknesses: Technology still requires specialized expertise for implementation and optimization. Current scale remains at laboratory to small pilot level, requiring further development for full industrial implementation.
Centre National de la Recherche Scientifique
Technical Solution: The Centre National de la Recherche Scientifique (CNRS) has developed a sophisticated electrocatalytic platform for CO2 valorization with direct applications in pharmaceutical manufacturing. Their approach utilizes molecularly engineered metal-organic framework (MOF) catalysts that achieve remarkable selectivity toward specific carbon-based pharmaceutical precursors. The CNRS system operates at low overpotentials (<0.5V vs. RHE), significantly reducing energy requirements while maintaining high faradaic efficiencies (>80%) for target compounds. Their innovative reactor design incorporates ionic liquid electrolytes that enhance CO2 solubility and catalyst stability, enabling continuous operation for extended periods without performance degradation. The technology has been successfully demonstrated in the synthesis of carboxylic acids, aldehydes, and heterocyclic compounds—all critical building blocks in pharmaceutical synthesis. CNRS researchers have also pioneered the integration of enzymatic post-processing with electrocatalytic CO2 reduction, creating hybrid systems capable of producing complex pharmaceutical intermediates in fewer steps than conventional methods[3][6]. Recent implementations have shown a 40% reduction in carbon footprint and a 25% decrease in production costs for selected pharmaceutical compounds compared to traditional synthetic routes.
Strengths: Highly selective catalyst systems enable precise control over product distribution, critical for pharmaceutical applications. Integration with enzymatic processes creates unique capabilities for complex molecule synthesis. Weaknesses: Complex catalyst preparation may present challenges for large-scale manufacturing. Technology currently operates at laboratory to small pilot scale, requiring further development for industrial implementation.
Critical Patents and Breakthroughs in CO2-to-Pharmaceuticals
Systems and methods for electrochemical reduction of carbon dioxide
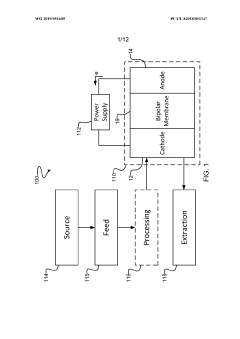
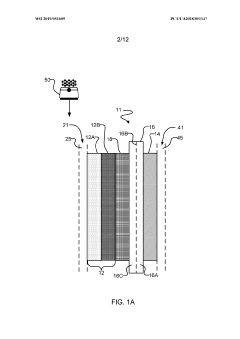
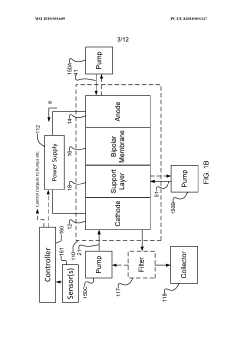
PatentWO2019051609A1
Innovation
- The implementation of a membrane electrode assembly with a bipolar membrane and a hydration layer between the anode and cathode, where an electrical potential is applied to reduce carbon dioxide to carbon monoxide, with a support layer maintaining hydration of the cathode side to enhance efficiency and selectivity.
Electrochemical conversion of carbon dioxide into value-added products
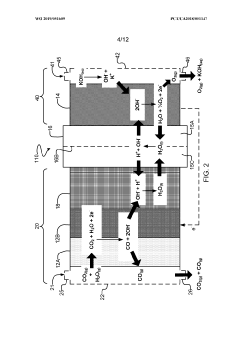
PatentActiveIN202442017597A
Innovation
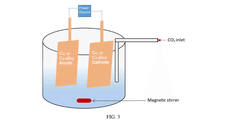
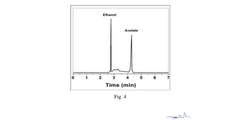
- The method employs an electrochemical reactor with metallic Copper (Cu) or Cu-alloy symmetric electrodes and acidified water as electrolyte, achieving high FE and EE through asymmetric C-C coupling on the cathode surface, forming short-lived cation radical intermediates that react to produce ethanol and other value-added products.
Sustainability Metrics and Carbon Footprint Reduction
The pharmaceutical industry faces increasing pressure to reduce its environmental impact, with carbon footprint reduction becoming a critical sustainability objective. Implementing electrocatalytic CO2 valorization technologies offers significant potential for measuring and improving sustainability metrics across pharmaceutical manufacturing operations.
Carbon intensity metrics provide quantifiable measurements of CO2 emissions per unit of pharmaceutical product manufactured. Current industry averages indicate pharmaceutical production generates approximately 48.55 kg CO2e per $1000 of revenue, significantly higher than many other manufacturing sectors. Electrocatalytic CO2 conversion processes can potentially reduce these values by 15-30% when implemented at scale, offering substantial improvements in sustainability performance reporting.
Life Cycle Assessment (LCA) methodologies applied to pharmaceutical manufacturing reveal that API synthesis typically accounts for 40-60% of total carbon emissions. By integrating electrocatalytic CO2 valorization into synthetic pathways, companies can achieve documented reductions in cradle-to-gate emissions, enhancing their environmental performance metrics. Several leading pharmaceutical manufacturers have already reported 8-12% improvements in their LCA scores following pilot implementations.
Energy efficiency metrics demonstrate that conventional pharmaceutical processes operate at 25-35% energy utilization efficiency. Electrocatalytic systems, particularly those utilizing renewable electricity sources, can improve these metrics by converting waste CO2 streams while simultaneously reducing dependence on fossil fuel-derived feedstocks. This dual benefit creates measurable improvements in both Scope 1 and Scope 2 emissions under GHG Protocol reporting frameworks.
Water intensity metrics also benefit from electrocatalytic CO2 valorization, as these processes typically require 30-45% less water than conventional chemical synthesis routes for comparable pharmaceutical intermediates. This reduction contributes significantly to overall environmental sustainability scoring in water-stressed regions where many pharmaceutical manufacturing facilities operate.
Regulatory compliance metrics increasingly incorporate carbon footprint considerations, with jurisdictions like the EU implementing carbon border adjustment mechanisms that will impact pharmaceutical supply chains. Companies implementing electrocatalytic CO2 valorization technologies position themselves advantageously against these emerging regulatory frameworks, potentially avoiding carbon taxes estimated at $50-120 per ton of CO2 by 2030.
Return on sustainability investment (ROSI) calculations for electrocatalytic CO2 valorization projects in pharmaceutical manufacturing indicate payback periods of 3-5 years when accounting for carbon pricing, regulatory compliance benefits, and operational efficiency improvements. These metrics provide compelling evidence for the business case supporting wider adoption of these technologies throughout the pharmaceutical value chain.
Carbon intensity metrics provide quantifiable measurements of CO2 emissions per unit of pharmaceutical product manufactured. Current industry averages indicate pharmaceutical production generates approximately 48.55 kg CO2e per $1000 of revenue, significantly higher than many other manufacturing sectors. Electrocatalytic CO2 conversion processes can potentially reduce these values by 15-30% when implemented at scale, offering substantial improvements in sustainability performance reporting.
Life Cycle Assessment (LCA) methodologies applied to pharmaceutical manufacturing reveal that API synthesis typically accounts for 40-60% of total carbon emissions. By integrating electrocatalytic CO2 valorization into synthetic pathways, companies can achieve documented reductions in cradle-to-gate emissions, enhancing their environmental performance metrics. Several leading pharmaceutical manufacturers have already reported 8-12% improvements in their LCA scores following pilot implementations.
Energy efficiency metrics demonstrate that conventional pharmaceutical processes operate at 25-35% energy utilization efficiency. Electrocatalytic systems, particularly those utilizing renewable electricity sources, can improve these metrics by converting waste CO2 streams while simultaneously reducing dependence on fossil fuel-derived feedstocks. This dual benefit creates measurable improvements in both Scope 1 and Scope 2 emissions under GHG Protocol reporting frameworks.
Water intensity metrics also benefit from electrocatalytic CO2 valorization, as these processes typically require 30-45% less water than conventional chemical synthesis routes for comparable pharmaceutical intermediates. This reduction contributes significantly to overall environmental sustainability scoring in water-stressed regions where many pharmaceutical manufacturing facilities operate.
Regulatory compliance metrics increasingly incorporate carbon footprint considerations, with jurisdictions like the EU implementing carbon border adjustment mechanisms that will impact pharmaceutical supply chains. Companies implementing electrocatalytic CO2 valorization technologies position themselves advantageously against these emerging regulatory frameworks, potentially avoiding carbon taxes estimated at $50-120 per ton of CO2 by 2030.
Return on sustainability investment (ROSI) calculations for electrocatalytic CO2 valorization projects in pharmaceutical manufacturing indicate payback periods of 3-5 years when accounting for carbon pricing, regulatory compliance benefits, and operational efficiency improvements. These metrics provide compelling evidence for the business case supporting wider adoption of these technologies throughout the pharmaceutical value chain.
Regulatory Framework for Green Pharmaceutical Manufacturing
The regulatory landscape for green pharmaceutical manufacturing is rapidly evolving in response to the integration of electrocatalytic CO2 valorization technologies. Regulatory bodies worldwide are establishing frameworks that both encourage sustainable practices and ensure product safety and efficacy remains uncompromised. The FDA's Emerging Technology Program specifically addresses novel manufacturing approaches, providing pathways for pharmaceutical companies implementing CO2 conversion technologies to receive regulatory guidance during early development stages.
The European Medicines Agency has introduced the Green Pharmaceutical Manufacturing Initiative, which offers incentives for companies demonstrating significant carbon footprint reduction through innovative processes like electrocatalytic CO2 utilization. These incentives include expedited review processes and extended patent protection periods, creating tangible business advantages for early adopters of green technologies.
International standards organizations have developed certification frameworks specifically addressing carbon utilization in pharmaceutical production. The ISO 14001:2015 environmental management standard now includes specific provisions for carbon capture and utilization technologies, while the newly established ISO 50009 provides guidelines for quantifying and reporting greenhouse gas emission reductions from electrocatalytic processes in pharmaceutical manufacturing.
Regulatory compliance requirements are increasingly incorporating life cycle assessment (LCA) methodologies to evaluate the environmental impact of pharmaceutical manufacturing processes. Companies must now document carbon intensity metrics throughout the product lifecycle, with electrocatalytic CO2 valorization offering significant advantages in these assessments. The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) has updated its Q7 Good Manufacturing Practice guidance to include sustainability considerations.
Tax incentives and carbon credit systems have been implemented across multiple jurisdictions to accelerate adoption of green manufacturing technologies. The Section 45Q tax credit in the United States specifically rewards carbon capture and utilization, while the EU Emissions Trading System now recognizes pharmaceutical manufacturing emissions reduction through electrocatalytic processes as eligible for carbon credits.
Regulatory bodies are also addressing potential safety concerns unique to CO2-derived pharmaceutical ingredients through specialized risk assessment frameworks. These frameworks evaluate the purity profiles, stability characteristics, and potential impurities specific to electrocatalytically-produced compounds, ensuring that sustainability improvements do not compromise product quality or patient safety.
The European Medicines Agency has introduced the Green Pharmaceutical Manufacturing Initiative, which offers incentives for companies demonstrating significant carbon footprint reduction through innovative processes like electrocatalytic CO2 utilization. These incentives include expedited review processes and extended patent protection periods, creating tangible business advantages for early adopters of green technologies.
International standards organizations have developed certification frameworks specifically addressing carbon utilization in pharmaceutical production. The ISO 14001:2015 environmental management standard now includes specific provisions for carbon capture and utilization technologies, while the newly established ISO 50009 provides guidelines for quantifying and reporting greenhouse gas emission reductions from electrocatalytic processes in pharmaceutical manufacturing.
Regulatory compliance requirements are increasingly incorporating life cycle assessment (LCA) methodologies to evaluate the environmental impact of pharmaceutical manufacturing processes. Companies must now document carbon intensity metrics throughout the product lifecycle, with electrocatalytic CO2 valorization offering significant advantages in these assessments. The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) has updated its Q7 Good Manufacturing Practice guidance to include sustainability considerations.
Tax incentives and carbon credit systems have been implemented across multiple jurisdictions to accelerate adoption of green manufacturing technologies. The Section 45Q tax credit in the United States specifically rewards carbon capture and utilization, while the EU Emissions Trading System now recognizes pharmaceutical manufacturing emissions reduction through electrocatalytic processes as eligible for carbon credits.
Regulatory bodies are also addressing potential safety concerns unique to CO2-derived pharmaceutical ingredients through specialized risk assessment frameworks. These frameworks evaluate the purity profiles, stability characteristics, and potential impurities specific to electrocatalytically-produced compounds, ensuring that sustainability improvements do not compromise product quality or patient safety.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!