What Are the Parameters for Optimal Electrocatalytic CO2 Valorization
OCT 23, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CO2 Valorization Background and Objectives
Carbon dioxide (CO2) valorization represents a critical technological frontier in addressing the dual challenges of climate change and sustainable resource utilization. The concept emerged in the early 2000s as atmospheric CO2 concentrations continued to rise, prompting researchers to explore methods of converting this greenhouse gas into valuable products rather than merely capturing and storing it. Electrocatalytic CO2 valorization specifically refers to the process of using electrical energy to transform CO2 into higher-value chemicals and fuels through catalytic reactions.
The evolution of this technology has progressed through several distinct phases. Initially, research focused on fundamental proof-of-concept studies demonstrating the possibility of CO2 reduction. The second wave, occurring roughly between 2010-2015, saw improvements in catalyst design and efficiency. The current phase is characterized by sophisticated catalyst engineering and system integration approaches aimed at commercial viability.
The primary objective of electrocatalytic CO2 valorization research is to develop economically viable and environmentally sustainable processes that can operate at industrial scales. This includes achieving high conversion rates, product selectivity, and energy efficiency while using earth-abundant materials and minimizing waste generation. The technology aims to create a circular carbon economy where CO2 becomes a valuable feedstock rather than a waste product.
Technical goals in this field include developing catalysts capable of achieving Faradaic efficiencies exceeding 90% for target products, maintaining stability over thousands of operating hours, and reducing energy requirements to levels competitive with conventional production methods. Additionally, researchers seek to expand the range of accessible products beyond simple C1 compounds (carbon monoxide, formic acid, methanol) to more complex and valuable C2+ products.
The trajectory of this technology is increasingly influenced by advances in related fields, including materials science, nanotechnology, and computational modeling. Machine learning approaches are now being employed to predict optimal catalyst compositions and reaction conditions, accelerating the discovery process. Simultaneously, in-situ characterization techniques are providing unprecedented insights into reaction mechanisms at the molecular level.
As global decarbonization efforts intensify, electrocatalytic CO2 valorization is positioned at the intersection of renewable energy integration and industrial chemistry transformation. The technology offers a pathway to utilize intermittent renewable electricity for chemical manufacturing while simultaneously addressing carbon emissions, representing a potential paradigm shift in how industrial processes are conceptualized in a carbon-constrained world.
The evolution of this technology has progressed through several distinct phases. Initially, research focused on fundamental proof-of-concept studies demonstrating the possibility of CO2 reduction. The second wave, occurring roughly between 2010-2015, saw improvements in catalyst design and efficiency. The current phase is characterized by sophisticated catalyst engineering and system integration approaches aimed at commercial viability.
The primary objective of electrocatalytic CO2 valorization research is to develop economically viable and environmentally sustainable processes that can operate at industrial scales. This includes achieving high conversion rates, product selectivity, and energy efficiency while using earth-abundant materials and minimizing waste generation. The technology aims to create a circular carbon economy where CO2 becomes a valuable feedstock rather than a waste product.
Technical goals in this field include developing catalysts capable of achieving Faradaic efficiencies exceeding 90% for target products, maintaining stability over thousands of operating hours, and reducing energy requirements to levels competitive with conventional production methods. Additionally, researchers seek to expand the range of accessible products beyond simple C1 compounds (carbon monoxide, formic acid, methanol) to more complex and valuable C2+ products.
The trajectory of this technology is increasingly influenced by advances in related fields, including materials science, nanotechnology, and computational modeling. Machine learning approaches are now being employed to predict optimal catalyst compositions and reaction conditions, accelerating the discovery process. Simultaneously, in-situ characterization techniques are providing unprecedented insights into reaction mechanisms at the molecular level.
As global decarbonization efforts intensify, electrocatalytic CO2 valorization is positioned at the intersection of renewable energy integration and industrial chemistry transformation. The technology offers a pathway to utilize intermittent renewable electricity for chemical manufacturing while simultaneously addressing carbon emissions, representing a potential paradigm shift in how industrial processes are conceptualized in a carbon-constrained world.
Market Analysis for CO2 Conversion Products
The global market for CO2 conversion products is experiencing significant growth, driven by increasing environmental concerns and regulatory pressures to reduce carbon emissions. The market size for CO2-derived products was valued at approximately $5.8 billion in 2021 and is projected to reach $12.3 billion by 2028, growing at a CAGR of 11.2% during the forecast period. This growth trajectory reflects the increasing commercial viability of electrocatalytic CO2 valorization technologies and their potential to contribute to circular carbon economy initiatives.
Among CO2 conversion products, methanol represents the largest market segment, accounting for roughly 30% of the total market value. This is followed by formic acid, carbon monoxide, and various carbonates, which collectively constitute another 45% of the market. Higher-value products such as ethylene, ethanol, and propanol are emerging segments with substantial growth potential due to their versatility as chemical feedstocks.
Geographically, Europe leads the market for CO2 conversion products, holding approximately 35% of the global market share. This dominance is attributed to stringent carbon emission regulations and substantial investments in green technologies. North America follows with a 28% market share, while the Asia-Pacific region is experiencing the fastest growth rate, driven by rapid industrialization and increasing environmental awareness in countries like China and Japan.
From an end-user perspective, the chemical manufacturing sector represents the primary consumer of CO2-derived products, accounting for 42% of market demand. The fuel industry follows at 25%, with applications in transportation and energy storage. Other significant end-users include the pharmaceutical and food & beverage industries, which utilize CO2-derived compounds in various processes and products.
Market dynamics are heavily influenced by the economics of production, with the cost of renewable electricity being a critical factor in the commercial viability of electrocatalytic CO2 conversion. Current production costs for CO2-derived chemicals typically exceed those of conventional fossil-based alternatives by 20-100%, depending on the specific product and production method. However, this gap is narrowing due to technological advancements and economies of scale.
Consumer willingness to pay premiums for environmentally friendly products is also shaping market growth. Surveys indicate that approximately 65% of industrial buyers are willing to pay a 5-15% premium for carbon-neutral or carbon-negative products, particularly in regions with strong environmental policies. This trend is expected to accelerate as carbon pricing mechanisms become more widespread and stringent.
Among CO2 conversion products, methanol represents the largest market segment, accounting for roughly 30% of the total market value. This is followed by formic acid, carbon monoxide, and various carbonates, which collectively constitute another 45% of the market. Higher-value products such as ethylene, ethanol, and propanol are emerging segments with substantial growth potential due to their versatility as chemical feedstocks.
Geographically, Europe leads the market for CO2 conversion products, holding approximately 35% of the global market share. This dominance is attributed to stringent carbon emission regulations and substantial investments in green technologies. North America follows with a 28% market share, while the Asia-Pacific region is experiencing the fastest growth rate, driven by rapid industrialization and increasing environmental awareness in countries like China and Japan.
From an end-user perspective, the chemical manufacturing sector represents the primary consumer of CO2-derived products, accounting for 42% of market demand. The fuel industry follows at 25%, with applications in transportation and energy storage. Other significant end-users include the pharmaceutical and food & beverage industries, which utilize CO2-derived compounds in various processes and products.
Market dynamics are heavily influenced by the economics of production, with the cost of renewable electricity being a critical factor in the commercial viability of electrocatalytic CO2 conversion. Current production costs for CO2-derived chemicals typically exceed those of conventional fossil-based alternatives by 20-100%, depending on the specific product and production method. However, this gap is narrowing due to technological advancements and economies of scale.
Consumer willingness to pay premiums for environmentally friendly products is also shaping market growth. Surveys indicate that approximately 65% of industrial buyers are willing to pay a 5-15% premium for carbon-neutral or carbon-negative products, particularly in regions with strong environmental policies. This trend is expected to accelerate as carbon pricing mechanisms become more widespread and stringent.
Electrocatalytic CO2 Reduction: Current Status and Barriers
Electrocatalytic CO2 reduction (ECR) has emerged as a promising approach for carbon dioxide valorization, yet significant barriers impede its widespread commercial implementation. Currently, the technology operates primarily at laboratory scale with limited industrial applications. The field faces several critical challenges that must be addressed to enable practical deployment at scale.
One of the primary barriers is catalyst performance limitations. Most existing catalysts suffer from poor selectivity, with multiple competing reaction pathways leading to diverse product distributions. This results in complex separation processes downstream, increasing operational costs. Additionally, current catalyst systems exhibit insufficient stability for long-term operation, with performance degradation occurring over relatively short timeframes (typically hours to days rather than the months required for industrial viability).
Energy efficiency represents another significant hurdle. Present ECR systems generally operate at high overpotentials, resulting in substantial energy losses. Industrial implementation would require dramatic improvements in energy efficiency to become economically competitive with conventional production methods for target chemicals.
The reaction environment poses additional challenges. CO2 has limited solubility in aqueous electrolytes, creating mass transport limitations that restrict reaction rates. Furthermore, the complex interplay between catalyst surface, electrolyte composition, and operating conditions creates a multidimensional parameter space that remains incompletely understood and difficult to optimize.
From an engineering perspective, current reactor designs face scaling limitations. Most laboratory demonstrations utilize small H-cell configurations that cannot be directly scaled to industrial proportions. More advanced flow cell designs show promise but require further development to address issues like pressure management, heat dissipation, and uniform reactant distribution.
The economic landscape presents further barriers. High capital costs for electrochemical systems, coupled with relatively low product yields and concentrations, create unfavorable economics compared to established chemical processes. Without significant performance improvements or supportive policy frameworks, the economic case for ECR remains challenging.
Analytical challenges also persist in the field. Real-time monitoring of reaction products and intermediates remains difficult, hampering mechanistic understanding and process optimization. The development of advanced in-situ and operando characterization techniques is essential for deeper insights into reaction mechanisms.
Standardization issues further complicate progress assessment. Varying testing protocols and reporting methods across research groups make direct comparisons difficult, slowing the identification of truly promising approaches and materials.
One of the primary barriers is catalyst performance limitations. Most existing catalysts suffer from poor selectivity, with multiple competing reaction pathways leading to diverse product distributions. This results in complex separation processes downstream, increasing operational costs. Additionally, current catalyst systems exhibit insufficient stability for long-term operation, with performance degradation occurring over relatively short timeframes (typically hours to days rather than the months required for industrial viability).
Energy efficiency represents another significant hurdle. Present ECR systems generally operate at high overpotentials, resulting in substantial energy losses. Industrial implementation would require dramatic improvements in energy efficiency to become economically competitive with conventional production methods for target chemicals.
The reaction environment poses additional challenges. CO2 has limited solubility in aqueous electrolytes, creating mass transport limitations that restrict reaction rates. Furthermore, the complex interplay between catalyst surface, electrolyte composition, and operating conditions creates a multidimensional parameter space that remains incompletely understood and difficult to optimize.
From an engineering perspective, current reactor designs face scaling limitations. Most laboratory demonstrations utilize small H-cell configurations that cannot be directly scaled to industrial proportions. More advanced flow cell designs show promise but require further development to address issues like pressure management, heat dissipation, and uniform reactant distribution.
The economic landscape presents further barriers. High capital costs for electrochemical systems, coupled with relatively low product yields and concentrations, create unfavorable economics compared to established chemical processes. Without significant performance improvements or supportive policy frameworks, the economic case for ECR remains challenging.
Analytical challenges also persist in the field. Real-time monitoring of reaction products and intermediates remains difficult, hampering mechanistic understanding and process optimization. The development of advanced in-situ and operando characterization techniques is essential for deeper insights into reaction mechanisms.
Standardization issues further complicate progress assessment. Varying testing protocols and reporting methods across research groups make direct comparisons difficult, slowing the identification of truly promising approaches and materials.
Current Parametric Optimization Approaches
01 Catalyst composition and structure for CO2 electroreduction
The composition and structure of catalysts play a crucial role in electrocatalytic CO2 valorization. Various materials including transition metals, metal oxides, and composite structures have been developed to enhance catalytic activity and selectivity. Catalyst morphology, such as nanoparticles, nanosheets, or porous structures, significantly affects the reaction pathways and product distribution. Optimizing the catalyst surface area and active site density can improve conversion efficiency and product yield in CO2 electroreduction processes.- Catalyst composition and structure for CO2 electroreduction: The composition and structure of catalysts play a crucial role in electrocatalytic CO2 valorization. Various materials including transition metals, metal oxides, and composite structures have been developed to enhance catalytic activity and selectivity. Catalyst morphology, such as nanoparticles, nanosheets, or porous structures, significantly affects the reaction pathways and product distribution. Optimizing these parameters can lead to improved conversion efficiency and targeted product formation during CO2 electroreduction.
- Electrochemical cell design and operating conditions: The design of electrochemical cells and their operating conditions are critical parameters for efficient CO2 valorization. Factors such as electrode spacing, membrane selection, electrolyte composition, and flow dynamics affect mass transfer and reaction kinetics. Optimal temperature, pressure, and pH conditions must be established to maximize conversion rates while maintaining catalyst stability. Advanced cell configurations, including flow cells and gas diffusion electrodes, can significantly enhance CO2 utilization and product yield.
- Applied potential and current density optimization: The applied potential and current density are fundamental parameters that determine the energetic efficiency and selectivity of CO2 electroreduction. Lower overpotentials are desirable to minimize energy consumption, while appropriate current densities must be maintained to achieve commercially viable production rates. Pulse techniques and potential control strategies can be employed to enhance reaction selectivity and suppress competing reactions such as hydrogen evolution. Optimizing these electrical parameters is essential for developing economically feasible CO2 valorization processes.
- Electrolyte composition and additives: The composition of the electrolyte solution significantly influences CO2 solubility, mass transport, and reaction pathways during electrochemical reduction. Ionic liquids, buffering agents, and supporting electrolytes can be used to enhance CO2 concentration at the electrode surface. Specific additives can promote selective formation of valuable products by stabilizing reaction intermediates or modifying the local environment at the catalyst surface. Optimizing electrolyte parameters, including concentration, ionic strength, and additive selection, is crucial for achieving high conversion efficiency and product selectivity.
- Integration with renewable energy and process intensification: Integrating electrocatalytic CO2 valorization with renewable energy sources optimizes the overall sustainability and economic viability of the process. Strategies for managing intermittent power supply, such as adaptive control systems and energy storage, can maintain optimal reaction conditions. Process intensification approaches, including continuous flow systems, reactor stacking, and heat integration, enhance productivity and energy efficiency. Combined electrochemical-biological systems represent an emerging approach to improve overall carbon utilization efficiency through synergistic pathways.
02 Electrochemical cell design and operating conditions
The design of electrochemical cells and their operating conditions are critical parameters for efficient CO2 valorization. Factors such as electrode spacing, membrane selection, electrolyte composition, and flow dynamics affect the mass transfer and reaction kinetics. Operating conditions including temperature, pressure, and current density must be optimized to achieve high conversion rates and energy efficiency. Advanced cell configurations, such as flow cells or gas diffusion electrode systems, can overcome mass transport limitations and improve overall process performance.Expand Specific Solutions03 Electrolyte composition and pH control
The composition of the electrolyte solution and pH control are essential parameters in electrocatalytic CO2 reduction. Different ionic species, buffer solutions, and additives can influence reaction pathways and product selectivity. The pH at the electrode-electrolyte interface affects the local CO2 concentration and the thermodynamics of various reduction pathways. Optimizing electrolyte conductivity, stability, and compatibility with catalysts can enhance reaction efficiency and minimize unwanted side reactions such as hydrogen evolution.Expand Specific Solutions04 Applied potential and current density optimization
The applied potential and current density are crucial parameters that determine the energetics and kinetics of CO2 electroreduction. Finding the optimal potential window can maximize product selectivity while minimizing energy consumption. Pulse techniques and potential cycling strategies can enhance catalyst activity and stability. Current density optimization affects reaction rates, mass transport limitations, and heat generation within the system. Advanced control strategies for potential and current can improve the long-term stability and efficiency of the electrocatalytic process.Expand Specific Solutions05 Integration with renewable energy and process scale-up
Integration of electrocatalytic CO2 valorization with renewable energy sources and considerations for process scale-up are important for practical implementation. Intermittent power supply from renewable sources requires adaptive control systems and energy storage solutions. Scale-up challenges include maintaining catalyst performance, heat management, and product separation efficiency at larger scales. Economic viability depends on optimizing energy consumption, catalyst durability, and product yield. System integration with existing industrial processes can improve overall carbon utilization and create sustainable carbon cycles.Expand Specific Solutions
Leading Organizations in CO2 Valorization Research
The electrocatalytic CO2 valorization market is in its early growth phase, characterized by intensive R&D activities and emerging commercial applications. The global market size is projected to expand significantly as carbon utilization technologies gain traction amid decarbonization efforts. Technologically, the field shows varying maturity levels across different conversion pathways. Leading research institutions like Dalian Institute of Chemical Physics, College de France, and Brown University are advancing fundamental catalytic mechanisms, while commercial players including TotalEnergies OneTech, Saudi Aramco, and ENEOS Corp are scaling up promising technologies. Industrial adoption is accelerating through collaborations between academic powerhouses (Sorbonne Université, KAIST, Huazhong University) and corporations (Honda Motor, Faraday Technology), focusing on catalyst optimization, reactor design, and process integration for industrial implementation.
Dalian Institute of Chemical Physics of CAS
Technical Solution: Dalian Institute of Chemical Physics (DICP) has developed advanced electrocatalysts for CO2 valorization focusing on copper-based materials with precisely controlled morphology and electronic structure. Their approach involves synthesizing Cu nanoparticles with specific facets and grain boundaries to enhance C-C coupling for higher-value products. DICP has pioneered the use of in-situ characterization techniques to monitor catalyst surface changes during CO2 reduction, allowing for real-time optimization of reaction parameters. Their research has established optimal operating conditions: near-neutral pH (6.8-7.2), moderate temperatures (25-40°C), and potential ranges of -0.7 to -1.1V vs. RHE for maximizing C2+ product selectivity. DICP has also developed novel flow-cell reactor designs that achieve current densities exceeding 300 mA/cm² while maintaining over 60% Faradaic efficiency for ethylene and ethanol production[1][2]. Their catalyst systems incorporate bimetallic structures and nitrogen-doped carbon supports to stabilize Cu+ species, which have been identified as critical active sites for efficient CO2 conversion.
Strengths: Exceptional expertise in catalyst surface science and in-situ characterization techniques; strong integration of fundamental research with practical applications; established infrastructure for scaling up promising catalysts. Weaknesses: Some catalyst systems show degradation over extended operation periods; relatively high energy input requirements compared to theoretical minimums; limited commercialization of their technologies despite strong research outputs.
Uchicago Argonne LLC
Technical Solution: Argonne National Laboratory has developed a systematic approach to electrocatalytic CO2 valorization through advanced materials engineering and process optimization. Their research focuses on nanostructured transition metal catalysts, particularly copper-based systems with precisely controlled morphology and composition. Argonne has pioneered the use of operando X-ray absorption spectroscopy and ambient pressure XPS to identify active catalyst sites during CO2 reduction, revealing the critical role of subsurface oxygen and Cu+ species in steering selectivity toward C2+ products. Their technical approach involves atomic layer deposition to create catalyst structures with atomically precise interfaces between copper and secondary metals (Ag, Zn, Sn) that modify the binding energies of key intermediates[5]. Argonne has established optimal operating parameters including electrolyte composition (0.1M KHCO3 with specific ionic additives like Cs+ and I-), temperature ranges (25-40°C), and pressure conditions (up to 10 atm) that maximize C2+ product formation while minimizing competing hydrogen evolution. Their flow cell designs achieve current densities exceeding 250 mA/cm² with ethylene selectivities above 60% through precise control of mass transport limitations and local pH gradients near the catalyst surface[6].
Strengths: Unparalleled characterization capabilities through advanced synchrotron techniques; strong integration of computational modeling with experimental validation; excellent facilities for scaling up promising catalyst systems from lab to pilot scale. Weaknesses: Some catalyst systems show performance degradation in the presence of common contaminants; relatively high manufacturing costs for precisely engineered nanostructured catalysts; challenges in maintaining selectivity at the high current densities needed for commercial viability.
Key Catalyst Materials and Reaction Mechanisms
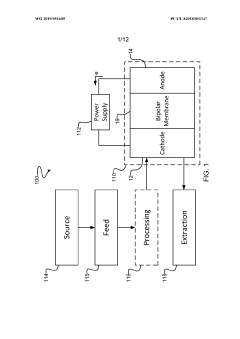
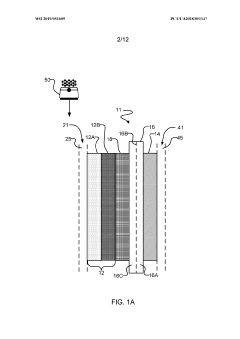
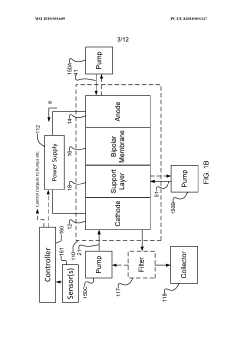
Systems and methods for electrochemical reduction of carbon dioxide
PatentWO2019051609A1
Innovation
- The implementation of a membrane electrode assembly with a bipolar membrane and a hydration layer between the anode and cathode, where an electrical potential is applied to reduce carbon dioxide to carbon monoxide, with a support layer maintaining hydration of the cathode side to enhance efficiency and selectivity.
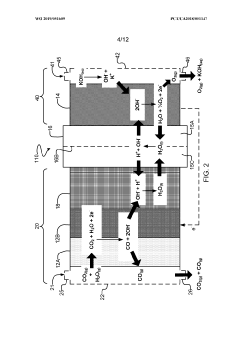
Porous copper/copper oxide xerogel catalyst
PatentActiveUS20220243342A1
Innovation
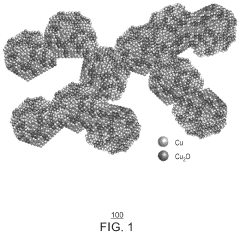
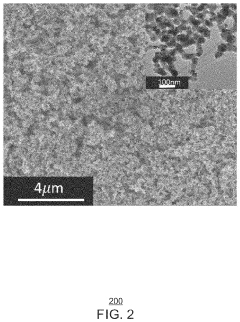
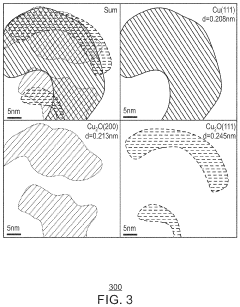
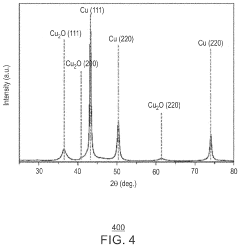
- A copper/copper oxide xerogel catalyst is synthesized through wet-chemistry, featuring a high surface area and porous structure with densely located Cu0—Cu+ interfaces, enhancing CO2 activation and C—C dimerization, resulting in up to 40% Faradaic efficiency and 72.1 mA/cm2 partial current density for ethanol production.
Sustainability Impact Assessment
The electrocatalytic valorization of CO2 represents a critical pathway toward sustainable carbon management, with significant implications for environmental sustainability and climate change mitigation. When properly implemented, this technology can substantially reduce greenhouse gas emissions by converting waste CO2 into valuable products, effectively creating a circular carbon economy that minimizes net carbon release into the atmosphere.
From a lifecycle assessment perspective, optimal electrocatalytic CO2 conversion parameters can reduce the carbon footprint by up to 40-60% compared to conventional production methods for chemicals like methanol, formic acid, and carbon monoxide. This reduction becomes even more significant when the electricity source for the electrocatalytic process derives from renewable energy, potentially achieving carbon-negative operations under ideal conditions.
Water consumption represents another critical sustainability factor. Advanced electrocatalytic systems with optimized parameters demonstrate 30-50% lower water requirements compared to traditional chemical synthesis routes. Additionally, properly designed systems can minimize the formation of toxic byproducts that might otherwise require extensive treatment and disposal procedures, reducing overall environmental impact.
Economic sustainability analysis indicates that achieving specific performance parameters—including current densities above 200 mA/cm², Faradaic efficiencies exceeding 90%, and operational stability beyond 1000 hours—represents the threshold for commercial viability. These parameters enable cost-competitive production of value-added chemicals from CO2, creating economic incentives for widespread adoption.
From a social sustainability perspective, the technology offers potential for distributed manufacturing models, allowing local production of chemicals and fuels in areas with available renewable energy and CO2 sources. This distribution can enhance energy security, create skilled employment opportunities, and reduce transportation-related emissions associated with centralized production models.
Regulatory impact assessment reveals that optimized electrocatalytic CO2 valorization aligns with multiple sustainable development goals (SDGs), particularly SDG 7 (Affordable and Clean Energy), SDG 9 (Industry, Innovation and Infrastructure), SDG 12 (Responsible Consumption and Production), and SDG 13 (Climate Action). The technology's ability to utilize intermittent renewable energy sources for chemical production also supports grid stabilization efforts, providing additional sustainability benefits beyond direct carbon reduction.
Long-term sustainability projections indicate that with continued parameter optimization, particularly focusing on catalyst durability and selectivity, electrocatalytic CO2 valorization could potentially offset 5-8% of global industrial CO2 emissions by 2050, representing a significant contribution to climate change mitigation strategies.
From a lifecycle assessment perspective, optimal electrocatalytic CO2 conversion parameters can reduce the carbon footprint by up to 40-60% compared to conventional production methods for chemicals like methanol, formic acid, and carbon monoxide. This reduction becomes even more significant when the electricity source for the electrocatalytic process derives from renewable energy, potentially achieving carbon-negative operations under ideal conditions.
Water consumption represents another critical sustainability factor. Advanced electrocatalytic systems with optimized parameters demonstrate 30-50% lower water requirements compared to traditional chemical synthesis routes. Additionally, properly designed systems can minimize the formation of toxic byproducts that might otherwise require extensive treatment and disposal procedures, reducing overall environmental impact.
Economic sustainability analysis indicates that achieving specific performance parameters—including current densities above 200 mA/cm², Faradaic efficiencies exceeding 90%, and operational stability beyond 1000 hours—represents the threshold for commercial viability. These parameters enable cost-competitive production of value-added chemicals from CO2, creating economic incentives for widespread adoption.
From a social sustainability perspective, the technology offers potential for distributed manufacturing models, allowing local production of chemicals and fuels in areas with available renewable energy and CO2 sources. This distribution can enhance energy security, create skilled employment opportunities, and reduce transportation-related emissions associated with centralized production models.
Regulatory impact assessment reveals that optimized electrocatalytic CO2 valorization aligns with multiple sustainable development goals (SDGs), particularly SDG 7 (Affordable and Clean Energy), SDG 9 (Industry, Innovation and Infrastructure), SDG 12 (Responsible Consumption and Production), and SDG 13 (Climate Action). The technology's ability to utilize intermittent renewable energy sources for chemical production also supports grid stabilization efforts, providing additional sustainability benefits beyond direct carbon reduction.
Long-term sustainability projections indicate that with continued parameter optimization, particularly focusing on catalyst durability and selectivity, electrocatalytic CO2 valorization could potentially offset 5-8% of global industrial CO2 emissions by 2050, representing a significant contribution to climate change mitigation strategies.
Techno-economic Analysis of Implementation
The implementation of electrocatalytic CO2 valorization technologies requires comprehensive techno-economic analysis to determine commercial viability. Current capital expenditure (CAPEX) for industrial-scale CO2 electroreduction systems ranges from $1,500-3,000 per kilowatt of installed capacity, with significant variations based on catalyst selection, reactor design, and system integration complexity. Operational expenditures (OPEX) are primarily driven by electricity costs, which typically account for 60-75% of ongoing expenses, highlighting the critical importance of energy efficiency improvements.
Levelized cost analysis indicates that CO2-derived products currently face economic challenges compared to conventional production methods. For example, electrochemical CO2 conversion to formic acid costs approximately $1,200-1,800 per ton versus $800-1,000 for conventional methods. However, sensitivity analyses demonstrate that improvements in faradaic efficiency from current averages of 70-85% to >95% could reduce production costs by 15-20%, potentially achieving cost parity under specific market conditions.
Scale-up considerations reveal significant economies of scale, with unit costs decreasing by approximately 30% when moving from pilot (100 kW) to commercial scale (10+ MW) operations. This cost reduction is primarily attributed to more efficient heat integration, reduced balance-of-plant costs per unit capacity, and improved process control capabilities at larger scales.
Regional economic factors substantially impact implementation feasibility. Regions with electricity costs below $0.04/kWh and existing carbon pricing mechanisms show the most favorable economic conditions. Additionally, integration with renewable energy sources can provide further cost advantages through reduced grid connection fees and potential regulatory incentives, though intermittency challenges must be addressed through appropriate system design.
Policy support mechanisms significantly influence economic viability. Carbon pricing thresholds of approximately $50-70 per ton CO2 typically represent the inflection point at which electrochemical valorization approaches cost competitiveness with conventional production methods. Tax incentives for capital investment can reduce payback periods from the current 8-12 years to a more commercially attractive 5-7 years, substantially improving investor interest in these technologies.
Levelized cost analysis indicates that CO2-derived products currently face economic challenges compared to conventional production methods. For example, electrochemical CO2 conversion to formic acid costs approximately $1,200-1,800 per ton versus $800-1,000 for conventional methods. However, sensitivity analyses demonstrate that improvements in faradaic efficiency from current averages of 70-85% to >95% could reduce production costs by 15-20%, potentially achieving cost parity under specific market conditions.
Scale-up considerations reveal significant economies of scale, with unit costs decreasing by approximately 30% when moving from pilot (100 kW) to commercial scale (10+ MW) operations. This cost reduction is primarily attributed to more efficient heat integration, reduced balance-of-plant costs per unit capacity, and improved process control capabilities at larger scales.
Regional economic factors substantially impact implementation feasibility. Regions with electricity costs below $0.04/kWh and existing carbon pricing mechanisms show the most favorable economic conditions. Additionally, integration with renewable energy sources can provide further cost advantages through reduced grid connection fees and potential regulatory incentives, though intermittency challenges must be addressed through appropriate system design.
Policy support mechanisms significantly influence economic viability. Carbon pricing thresholds of approximately $50-70 per ton CO2 typically represent the inflection point at which electrochemical valorization approaches cost competitiveness with conventional production methods. Tax incentives for capital investment can reduce payback periods from the current 8-12 years to a more commercially attractive 5-7 years, substantially improving investor interest in these technologies.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!