Electrocatalytic CO2 Valorization with Advanced Electrode Kinetics
OCT 23, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CO2 Valorization Background and Objectives
Carbon dioxide (CO2) valorization represents a transformative approach to addressing the dual challenges of climate change and sustainable resource utilization. The concept involves converting CO2, a primary greenhouse gas, into valuable chemicals and fuels through various technological pathways. This approach has gained significant momentum over the past decade as global carbon emissions continue to rise, with atmospheric CO2 concentrations reaching unprecedented levels of over 415 ppm in recent years.
Historically, CO2 has been viewed primarily as a waste product and environmental liability. However, the paradigm shift toward considering CO2 as a potential carbon resource began in earnest during the early 2000s, driven by increasing climate concerns and advances in catalytic chemistry. The evolution of this field has accelerated dramatically since 2010, with breakthrough developments in materials science, nanotechnology, and electrochemical engineering enabling more efficient conversion processes.
Electrocatalytic CO2 valorization specifically focuses on the use of electrical energy to drive the reduction of CO2 into value-added products. This approach offers distinct advantages over thermal or biological methods, including operation under ambient conditions, potential integration with renewable electricity sources, and precise reaction control through applied potential modulation. The field has progressed from early proof-of-concept demonstrations achieving limited product selectivity to current systems capable of generating specific products with faradaic efficiencies exceeding 90%.
The primary objective of advanced electrode kinetics research in this domain is to overcome the fundamental limitations that have historically constrained CO2 electroreduction. These include the significant overpotential requirements, limited product selectivity, catalyst deactivation issues, and low current densities that have prevented widespread commercial implementation. By enhancing electrode kinetics through novel catalyst design, interface engineering, and reaction environment optimization, researchers aim to develop economically viable processes for CO2 conversion.
Looking forward, the technical goals for this field include developing electrocatalytic systems capable of achieving energy conversion efficiencies above 60%, maintaining stable operation for thousands of hours, and producing complex carbon products (C2+ molecules) with high selectivity. Additionally, there is growing emphasis on designing integrated systems that can operate directly with flue gas or atmospheric CO2 sources, rather than requiring pre-concentrated CO2 streams.
The ultimate vision driving this research is the establishment of a circular carbon economy, where CO2 emissions are captured and recycled into the chemical value chain, reducing dependence on fossil resources while mitigating climate impacts. This aligns with global sustainability initiatives and presents opportunities for creating new economic value from what has traditionally been considered an environmental liability.
Historically, CO2 has been viewed primarily as a waste product and environmental liability. However, the paradigm shift toward considering CO2 as a potential carbon resource began in earnest during the early 2000s, driven by increasing climate concerns and advances in catalytic chemistry. The evolution of this field has accelerated dramatically since 2010, with breakthrough developments in materials science, nanotechnology, and electrochemical engineering enabling more efficient conversion processes.
Electrocatalytic CO2 valorization specifically focuses on the use of electrical energy to drive the reduction of CO2 into value-added products. This approach offers distinct advantages over thermal or biological methods, including operation under ambient conditions, potential integration with renewable electricity sources, and precise reaction control through applied potential modulation. The field has progressed from early proof-of-concept demonstrations achieving limited product selectivity to current systems capable of generating specific products with faradaic efficiencies exceeding 90%.
The primary objective of advanced electrode kinetics research in this domain is to overcome the fundamental limitations that have historically constrained CO2 electroreduction. These include the significant overpotential requirements, limited product selectivity, catalyst deactivation issues, and low current densities that have prevented widespread commercial implementation. By enhancing electrode kinetics through novel catalyst design, interface engineering, and reaction environment optimization, researchers aim to develop economically viable processes for CO2 conversion.
Looking forward, the technical goals for this field include developing electrocatalytic systems capable of achieving energy conversion efficiencies above 60%, maintaining stable operation for thousands of hours, and producing complex carbon products (C2+ molecules) with high selectivity. Additionally, there is growing emphasis on designing integrated systems that can operate directly with flue gas or atmospheric CO2 sources, rather than requiring pre-concentrated CO2 streams.
The ultimate vision driving this research is the establishment of a circular carbon economy, where CO2 emissions are captured and recycled into the chemical value chain, reducing dependence on fossil resources while mitigating climate impacts. This aligns with global sustainability initiatives and presents opportunities for creating new economic value from what has traditionally been considered an environmental liability.
Market Analysis for CO2 Conversion Technologies
The global market for CO2 conversion technologies is experiencing significant growth, driven by increasing environmental concerns and regulatory pressures to reduce carbon emissions. The market was valued at approximately $1.8 billion in 2022 and is projected to reach $4.2 billion by 2030, growing at a CAGR of 11.2% during the forecast period. This growth trajectory reflects the urgent need for sustainable solutions to address climate change challenges.
Electrocatalytic CO2 valorization represents a particularly promising segment within this market, with advanced electrode kinetics enabling more efficient conversion processes. The technology allows for the transformation of carbon dioxide into valuable products such as carbon monoxide, formic acid, methanol, ethylene, and other hydrocarbons, creating economic incentives for carbon capture and utilization.
Regional analysis indicates that North America currently holds the largest market share at 35%, followed by Europe at 30% and Asia-Pacific at 25%. However, the Asia-Pacific region is expected to witness the fastest growth rate due to rapid industrialization, increasing carbon emissions, and supportive government policies in countries like China, Japan, and South Korea.
Key market drivers include stringent carbon emission regulations, government incentives for green technologies, and corporate sustainability commitments. The European Union's Green Deal and carbon pricing mechanisms have created strong market pull for CO2 conversion technologies. Similarly, the Inflation Reduction Act in the United States provides significant tax credits for carbon capture and utilization projects.
Industry-wise, the chemical manufacturing sector represents the largest end-user segment (40%), followed by energy production (25%) and fuel synthesis (20%). The remaining 15% is distributed across various applications including pharmaceuticals, food and beverage, and materials production.
Market challenges include high capital costs, energy intensity of conversion processes, and competition from established carbon-neutral alternatives. The levelized cost of electrocatalytic CO2 conversion remains 2-3 times higher than conventional production methods for many target chemicals, highlighting the need for continued technological advancement and cost reduction.
Consumer awareness and demand for carbon-neutral products are growing, with 65% of global consumers expressing willingness to pay premium prices for products manufactured using carbon-neutral processes. This trend is particularly strong among younger demographics and in developed economies, creating market pull for CO2-derived products.
Electrocatalytic CO2 valorization represents a particularly promising segment within this market, with advanced electrode kinetics enabling more efficient conversion processes. The technology allows for the transformation of carbon dioxide into valuable products such as carbon monoxide, formic acid, methanol, ethylene, and other hydrocarbons, creating economic incentives for carbon capture and utilization.
Regional analysis indicates that North America currently holds the largest market share at 35%, followed by Europe at 30% and Asia-Pacific at 25%. However, the Asia-Pacific region is expected to witness the fastest growth rate due to rapid industrialization, increasing carbon emissions, and supportive government policies in countries like China, Japan, and South Korea.
Key market drivers include stringent carbon emission regulations, government incentives for green technologies, and corporate sustainability commitments. The European Union's Green Deal and carbon pricing mechanisms have created strong market pull for CO2 conversion technologies. Similarly, the Inflation Reduction Act in the United States provides significant tax credits for carbon capture and utilization projects.
Industry-wise, the chemical manufacturing sector represents the largest end-user segment (40%), followed by energy production (25%) and fuel synthesis (20%). The remaining 15% is distributed across various applications including pharmaceuticals, food and beverage, and materials production.
Market challenges include high capital costs, energy intensity of conversion processes, and competition from established carbon-neutral alternatives. The levelized cost of electrocatalytic CO2 conversion remains 2-3 times higher than conventional production methods for many target chemicals, highlighting the need for continued technological advancement and cost reduction.
Consumer awareness and demand for carbon-neutral products are growing, with 65% of global consumers expressing willingness to pay premium prices for products manufactured using carbon-neutral processes. This trend is particularly strong among younger demographics and in developed economies, creating market pull for CO2-derived products.
Electrocatalytic CO2 Reduction: Status and Barriers
Electrocatalytic CO2 reduction (ECR) has emerged as a promising approach for carbon dioxide utilization, offering pathways to convert this greenhouse gas into value-added chemicals and fuels. Currently, the technology has reached demonstration scale for certain products like carbon monoxide and formate, with laboratory-scale successes for more complex C2+ products. However, significant barriers remain before widespread commercial implementation becomes viable.
The primary technical challenge facing ECR is the limited energy efficiency, with most systems operating at 30-50% Faradaic efficiency for desired products. This inefficiency stems from competing hydrogen evolution reactions and the formation of multiple carbon products. Current densities in most laboratory systems remain below 200 mA/cm², whereas industrial viability typically requires >500 mA/cm².
Catalyst performance presents another critical barrier. State-of-the-art catalysts, primarily copper-based for multi-carbon products, suffer from rapid deactivation under industrial conditions. Most catalysts demonstrate stability for only 100-200 hours, whereas commercial applications require thousands of hours of consistent performance. Additionally, catalyst selectivity remains problematic, with product distributions often spanning 5-10 different compounds rather than targeting a single high-value product.
Scale-up challenges further complicate implementation. Laboratory systems typically operate at square-centimeter scales, while industrial applications require square-meter electrodes. This scale-up introduces mass transport limitations, uneven current distribution, and heat management issues that significantly reduce performance metrics observed in controlled laboratory environments.
Economic barriers also persist. Current ECR systems produce chemicals at costs 3-5 times higher than conventional fossil-based routes. The capital expenditure for electrochemical systems remains high at approximately $1,000-2,000 per kilowatt, compared to mature chemical processes at $200-500 per kilowatt equivalent.
Geographically, ECR technology development shows distinct patterns. North America leads in fundamental catalyst research, with significant contributions from institutions like Stanford University and University of Toronto. Europe dominates system integration and demonstration projects, particularly through initiatives in Germany and Denmark. East Asia, especially China and Japan, has made substantial progress in novel catalyst materials and scale-up methodologies.
Recent advances in operando characterization techniques and computational modeling have begun addressing these barriers, providing deeper insights into reaction mechanisms and catalyst degradation pathways. However, the field requires breakthrough innovations in catalyst design, electrode architecture, and system engineering before ECR can achieve the performance metrics necessary for industrial adoption.
The primary technical challenge facing ECR is the limited energy efficiency, with most systems operating at 30-50% Faradaic efficiency for desired products. This inefficiency stems from competing hydrogen evolution reactions and the formation of multiple carbon products. Current densities in most laboratory systems remain below 200 mA/cm², whereas industrial viability typically requires >500 mA/cm².
Catalyst performance presents another critical barrier. State-of-the-art catalysts, primarily copper-based for multi-carbon products, suffer from rapid deactivation under industrial conditions. Most catalysts demonstrate stability for only 100-200 hours, whereas commercial applications require thousands of hours of consistent performance. Additionally, catalyst selectivity remains problematic, with product distributions often spanning 5-10 different compounds rather than targeting a single high-value product.
Scale-up challenges further complicate implementation. Laboratory systems typically operate at square-centimeter scales, while industrial applications require square-meter electrodes. This scale-up introduces mass transport limitations, uneven current distribution, and heat management issues that significantly reduce performance metrics observed in controlled laboratory environments.
Economic barriers also persist. Current ECR systems produce chemicals at costs 3-5 times higher than conventional fossil-based routes. The capital expenditure for electrochemical systems remains high at approximately $1,000-2,000 per kilowatt, compared to mature chemical processes at $200-500 per kilowatt equivalent.
Geographically, ECR technology development shows distinct patterns. North America leads in fundamental catalyst research, with significant contributions from institutions like Stanford University and University of Toronto. Europe dominates system integration and demonstration projects, particularly through initiatives in Germany and Denmark. East Asia, especially China and Japan, has made substantial progress in novel catalyst materials and scale-up methodologies.
Recent advances in operando characterization techniques and computational modeling have begun addressing these barriers, providing deeper insights into reaction mechanisms and catalyst degradation pathways. However, the field requires breakthrough innovations in catalyst design, electrode architecture, and system engineering before ECR can achieve the performance metrics necessary for industrial adoption.
Current Electrode Design and Catalyst Solutions
01 Catalyst materials for CO2 electroreduction
Various catalyst materials can enhance the efficiency of CO2 electroreduction processes. These include metal-based catalysts, metal oxides, and composite materials that provide active sites for CO2 adsorption and conversion. The selection of appropriate catalyst materials is crucial for controlling reaction pathways, improving selectivity toward desired products, and reducing overpotentials required for the electrochemical conversion of CO2.- Catalyst materials for CO2 electroreduction: Various catalyst materials can be used to enhance the electrochemical reduction of CO2. These include metal-based catalysts, metal oxides, and composite materials that improve reaction efficiency and selectivity. The catalysts are designed to lower activation energy barriers and facilitate electron transfer during the CO2 reduction process, resulting in higher conversion rates and better product selectivity. Optimization of catalyst composition and structure is crucial for achieving desired electrode kinetics in CO2 valorization.
- Electrode design and structure for CO2 electroreduction: The physical structure and design of electrodes significantly impact the efficiency of CO2 electroreduction. Factors such as porosity, surface area, and morphology affect mass transport, electron transfer, and reaction kinetics. Advanced electrode designs incorporate hierarchical structures, 3D architectures, and controlled surface features to enhance catalytic activity and stability. Proper electrode engineering can mitigate transport limitations and improve the overall performance of electrocatalytic CO2 conversion systems.
- Reaction mechanisms and kinetic analysis: Understanding the reaction mechanisms and kinetics of CO2 electroreduction is essential for process optimization. This includes studying electron transfer steps, intermediate formation, rate-determining steps, and reaction pathways. Techniques such as electrochemical impedance spectroscopy, cyclic voltammetry, and in-situ spectroscopic methods are employed to elucidate reaction mechanisms and quantify kinetic parameters. Insights into reaction kinetics guide the development of more efficient catalysts and electrode systems for CO2 valorization.
- Electrolyte composition and effects: The composition of the electrolyte plays a crucial role in CO2 electroreduction kinetics. Factors such as pH, ionic strength, buffer capacity, and specific ion effects influence reaction rates, selectivity, and stability. Tailored electrolytes can enhance CO2 solubility, facilitate mass transport, and stabilize reaction intermediates. Innovations in electrolyte design include ionic liquids, deep eutectic solvents, and additives that promote desired reaction pathways while suppressing competing reactions like hydrogen evolution.
- System integration and process parameters: Successful CO2 electroreduction requires optimization of overall system design and operating parameters. This includes factors such as cell configuration, temperature, pressure, current density, and potential control. Advanced reactor designs address challenges related to mass transport limitations, bubble formation, and heat management. Continuous flow systems, gas diffusion electrodes, and membrane electrode assemblies represent innovations that enhance process efficiency. Optimization of these parameters is essential for scaling up CO2 electroreduction technologies for practical applications.
02 Electrode design and structure optimization
The design and structure of electrodes significantly impact the kinetics of CO2 electroreduction. Optimizing parameters such as porosity, surface area, and morphology can enhance mass transport, increase active site accessibility, and improve overall electrode performance. Advanced electrode architectures, including hierarchical structures and 3D configurations, can facilitate efficient CO2 conversion by providing more reaction sites and better reactant diffusion pathways.Expand Specific Solutions03 Reaction mechanisms and kinetic studies
Understanding the reaction mechanisms and kinetics of CO2 electroreduction is essential for process optimization. Studies focus on identifying rate-determining steps, intermediate species formation, and electron transfer processes. Advanced analytical techniques are employed to monitor reaction pathways in real-time, providing insights into the complex multi-electron transfer processes involved in CO2 valorization and helping to overcome kinetic limitations.Expand Specific Solutions04 Electrolyte composition and interface engineering
The composition of electrolytes and the electrode-electrolyte interface play crucial roles in CO2 electroreduction kinetics. Tailoring electrolyte properties such as pH, ionic strength, and buffer capacity can significantly influence reaction rates and product selectivity. Interface engineering approaches, including the use of ionic liquids, polymer coatings, or molecular additives, can modify the local environment near the electrode surface to enhance CO2 activation and conversion efficiency.Expand Specific Solutions05 System integration and process intensification
Integrating CO2 electroreduction systems with other processes can enhance overall efficiency and economic viability. This includes coupling with renewable energy sources, developing continuous flow reactors, or combining with thermal catalytic processes. Process intensification strategies focus on optimizing operating conditions such as temperature, pressure, and current density to improve reaction kinetics while maintaining energy efficiency and product selectivity.Expand Specific Solutions
Leading Institutions and Companies in CO2 Electrocatalysis
Electrocatalytic CO2 valorization is currently in an early growth phase, with the market expanding rapidly due to increasing focus on carbon neutrality technologies. The global market size is projected to reach significant scale as industries seek sustainable carbon utilization solutions. Technologically, the field shows varying maturity levels across different approaches. Leading academic institutions like Zhejiang University, Brown University, and National University of Singapore are advancing fundamental research, while commercial players including TotalEnergies, Saudi Aramco, and Siemens Energy are scaling up applications. Research centers such as Dalian Institute of Chemical Physics and Centre National de la Recherche Scientifique are bridging the gap between laboratory discoveries and industrial implementation, focusing on electrode kinetics optimization to improve efficiency and selectivity in CO2 conversion processes.
Dalian Institute of Chemical Physics of CAS
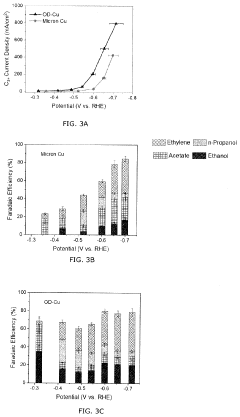
Technical Solution: Dalian Institute has developed advanced copper-based catalysts with precisely controlled morphology and electronic structure for CO2 electroreduction. Their approach involves synthesizing Cu nanomaterials with specific facets and defects to enhance C-C coupling for multi-carbon product formation. They've pioneered the use of in-situ characterization techniques to understand reaction mechanisms at the electrode-electrolyte interface, allowing for rational catalyst design. Recent breakthroughs include copper-zinc bimetallic catalysts that achieve over 80% Faradaic efficiency for ethylene production at industrially relevant current densities (>200 mA/cm²)[1]. Their work also extends to developing flow-cell reactors with gas diffusion electrodes to overcome mass transport limitations, enabling stable operation at high current densities for hundreds of hours[2].
Strengths: World-leading expertise in catalyst design with atomic-level precision; strong capabilities in operando characterization techniques; demonstrated high selectivity toward valuable C2+ products. Weaknesses: Scale-up challenges remain for industrial implementation; catalyst stability under high current densities needs further improvement; relatively high overpotentials still required for C2+ product formation.
Uchicago Argonne LLC
Technical Solution: Argonne National Laboratory has developed a comprehensive approach to CO2 electroreduction focusing on advanced electrode architectures and reaction environment control. Their technology employs hierarchically structured electrodes with tailored porosity to optimize reactant transport and product selectivity. A key innovation is their development of ionic liquid-based electrolytes that significantly enhance CO2 solubility while suppressing the competing hydrogen evolution reaction. Their catalytic systems incorporate bimetallic and oxide-derived catalysts with engineered interfaces that promote C-C coupling through optimized binding of key reaction intermediates. Recent work demonstrates sustained conversion of CO2 to ethanol with Faradaic efficiencies exceeding 60% at moderate overpotentials using copper-based catalysts modified with cerium oxide[3]. They've also pioneered the use of pulsed electrochemical techniques to control reaction pathways and improve energy efficiency by up to 25% compared to conventional methods[4].
Strengths: Exceptional capabilities in advanced materials characterization; strong integration of computational modeling with experimental design; demonstrated ability to control reaction selectivity. Weaknesses: Current systems still require significant energy input; catalyst deactivation remains a challenge for long-term operation; limited demonstration at scales beyond laboratory testing.
Key Patents and Breakthroughs in Advanced Electrode Materials
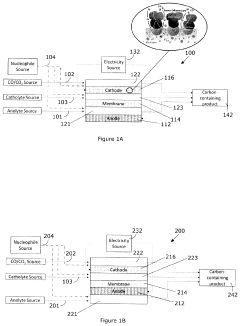
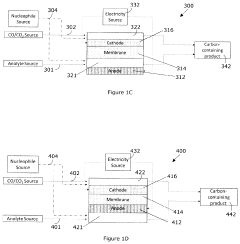
Systems and methods for electrochemical reduction of carbon dioxide
PatentWO2019051609A1
Innovation
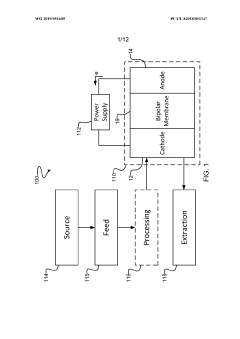
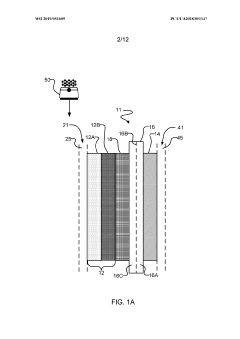
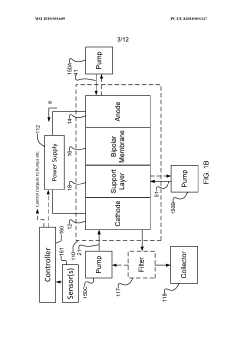
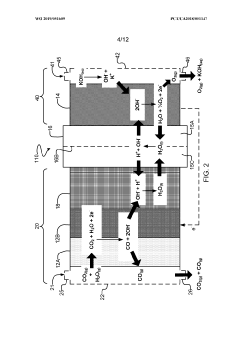
- The implementation of a membrane electrode assembly with a bipolar membrane and a hydration layer between the anode and cathode, where an electrical potential is applied to reduce carbon dioxide to carbon monoxide, with a support layer maintaining hydration of the cathode side to enhance efficiency and selectivity.
Electrochemical generation of carbon-containing products from carbon dioxide and carbon monoxide
PatentActiveUS20210140056A1
Innovation
- A three-compartment and two-compartment CO flow electrolyzer design is employed, where a hydrophobic porous carbon support loaded with a copper catalyst is used to enhance CO conversion, allowing for high reaction rates and improved selectivity by maintaining an efficient electrode-electrolyte interface for CO reduction, which surpasses the performance of CO2 reduction.
Techno-economic Assessment of CO2 Valorization Processes
The techno-economic assessment of CO2 valorization processes reveals significant economic challenges despite technological advancements. Current capital expenditure (CAPEX) for electrochemical CO2 reduction plants ranges from $1,500-3,000 per kW of installed capacity, with operating expenses (OPEX) between $0.05-0.12 per kWh. These costs remain substantially higher than conventional carbon-based production methods, creating a barrier to widespread commercial adoption.
Energy efficiency presents a critical economic factor, with most systems operating at 30-60% faradaic efficiency. Advanced electrode kinetics have improved this metric, but the energy input still represents 40-65% of total operational costs. The levelized cost of carbon conversion (LCCC) currently stands at $100-180 per ton of CO2 processed, significantly above the carbon pricing in most markets.
Product selectivity directly impacts economic viability, as multi-product streams require costly separation processes. Recent catalyst innovations have achieved up to 95% selectivity for specific products like CO and formate, reducing downstream processing costs by approximately 30%. However, higher-value products such as ethylene and ethanol still face selectivity challenges that impact overall economics.
Scale-up considerations reveal that laboratory performance rarely translates directly to industrial implementation. Pilot plants typically experience a 15-25% efficiency decrease compared to laboratory conditions. The economic assessment indicates that facilities processing at least 10,000 tons of CO2 annually are necessary to approach economic viability under current market conditions.
Renewable energy integration offers a pathway to improved economics. Systems coupled with dedicated renewable sources can reduce operational costs by 20-35% compared to grid electricity, though this requires higher initial capital investment. Sensitivity analysis shows that electricity prices below $0.04/kWh are critical for competitive production costs.
Market analysis for CO2-derived products indicates variable competitiveness. While formic acid and carbon monoxide can achieve price parity with fossil-based alternatives at scale, more complex molecules like ethylene require either carbon pricing above $75/ton or premium market positioning to compete effectively. The projected payback period for commercial installations ranges from 7-12 years under current conditions, with potential reduction to 4-6 years with optimized electrode kinetics and supportive policy frameworks.
Energy efficiency presents a critical economic factor, with most systems operating at 30-60% faradaic efficiency. Advanced electrode kinetics have improved this metric, but the energy input still represents 40-65% of total operational costs. The levelized cost of carbon conversion (LCCC) currently stands at $100-180 per ton of CO2 processed, significantly above the carbon pricing in most markets.
Product selectivity directly impacts economic viability, as multi-product streams require costly separation processes. Recent catalyst innovations have achieved up to 95% selectivity for specific products like CO and formate, reducing downstream processing costs by approximately 30%. However, higher-value products such as ethylene and ethanol still face selectivity challenges that impact overall economics.
Scale-up considerations reveal that laboratory performance rarely translates directly to industrial implementation. Pilot plants typically experience a 15-25% efficiency decrease compared to laboratory conditions. The economic assessment indicates that facilities processing at least 10,000 tons of CO2 annually are necessary to approach economic viability under current market conditions.
Renewable energy integration offers a pathway to improved economics. Systems coupled with dedicated renewable sources can reduce operational costs by 20-35% compared to grid electricity, though this requires higher initial capital investment. Sensitivity analysis shows that electricity prices below $0.04/kWh are critical for competitive production costs.
Market analysis for CO2-derived products indicates variable competitiveness. While formic acid and carbon monoxide can achieve price parity with fossil-based alternatives at scale, more complex molecules like ethylene require either carbon pricing above $75/ton or premium market positioning to compete effectively. The projected payback period for commercial installations ranges from 7-12 years under current conditions, with potential reduction to 4-6 years with optimized electrode kinetics and supportive policy frameworks.
Environmental Impact and Carbon Neutrality Implications
Electrocatalytic CO2 valorization represents a pivotal technology in addressing global climate change challenges. The environmental impact of this technology extends far beyond laboratory applications, offering tangible pathways toward carbon neutrality goals established under international frameworks such as the Paris Agreement.
The implementation of CO2 electroreduction processes at industrial scale could significantly reduce atmospheric carbon dioxide concentrations by converting this greenhouse gas into valuable chemicals and fuels. Life cycle assessments indicate that when powered by renewable energy sources, these electrocatalytic systems can achieve negative carbon footprints, effectively removing more CO2 than they generate during operation and manufacturing.
Current estimates suggest that widespread adoption of advanced electrode kinetics in CO2 valorization could contribute to a 5-8% reduction in global industrial carbon emissions by 2040. This potential impact is particularly significant in hard-to-abate sectors such as chemical manufacturing, where traditional decarbonization approaches face substantial technical and economic barriers.
The carbon neutrality implications are further enhanced by the circular economy aspects of this technology. By utilizing waste CO2 streams from industrial processes as feedstock, electrocatalytic valorization creates a closed-loop system that minimizes resource extraction while maximizing value creation. This alignment with circular economy principles strengthens the technology's contribution to sustainable development goals.
Water consumption represents an important environmental consideration, as most CO2 electroreduction processes require water as a hydrogen source. Advanced electrode designs with improved kinetics have demonstrated up to 40% reduction in water requirements compared to first-generation systems, addressing potential concerns about resource competition in water-stressed regions.
Land use impacts must also be considered when evaluating the environmental footprint of this technology. The integration with renewable energy infrastructure necessitates careful planning to minimize habitat disruption and biodiversity impacts. Modular system designs with high energy efficiency can help reduce the physical footprint of implementation.
From a policy perspective, electrocatalytic CO2 valorization aligns with emerging carbon pricing mechanisms and regulatory frameworks designed to incentivize negative emission technologies. The quantifiable carbon reduction potential of these systems positions them favorably within evolving carbon markets, potentially creating additional revenue streams through carbon credits that improve economic viability.
The implementation of CO2 electroreduction processes at industrial scale could significantly reduce atmospheric carbon dioxide concentrations by converting this greenhouse gas into valuable chemicals and fuels. Life cycle assessments indicate that when powered by renewable energy sources, these electrocatalytic systems can achieve negative carbon footprints, effectively removing more CO2 than they generate during operation and manufacturing.
Current estimates suggest that widespread adoption of advanced electrode kinetics in CO2 valorization could contribute to a 5-8% reduction in global industrial carbon emissions by 2040. This potential impact is particularly significant in hard-to-abate sectors such as chemical manufacturing, where traditional decarbonization approaches face substantial technical and economic barriers.
The carbon neutrality implications are further enhanced by the circular economy aspects of this technology. By utilizing waste CO2 streams from industrial processes as feedstock, electrocatalytic valorization creates a closed-loop system that minimizes resource extraction while maximizing value creation. This alignment with circular economy principles strengthens the technology's contribution to sustainable development goals.
Water consumption represents an important environmental consideration, as most CO2 electroreduction processes require water as a hydrogen source. Advanced electrode designs with improved kinetics have demonstrated up to 40% reduction in water requirements compared to first-generation systems, addressing potential concerns about resource competition in water-stressed regions.
Land use impacts must also be considered when evaluating the environmental footprint of this technology. The integration with renewable energy infrastructure necessitates careful planning to minimize habitat disruption and biodiversity impacts. Modular system designs with high energy efficiency can help reduce the physical footprint of implementation.
From a policy perspective, electrocatalytic CO2 valorization aligns with emerging carbon pricing mechanisms and regulatory frameworks designed to incentivize negative emission technologies. The quantifiable carbon reduction potential of these systems positions them favorably within evolving carbon markets, potentially creating additional revenue streams through carbon credits that improve economic viability.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!