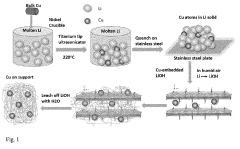
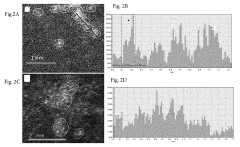
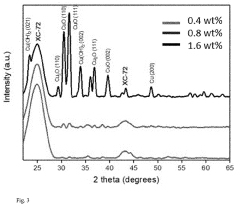
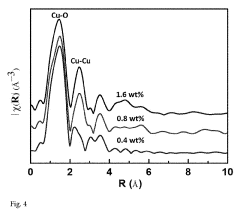
Electrocatalytic CO2 Valorization in Next-Generation Catalysts
OCT 23, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CO2 Valorization Background and Objectives
Carbon dioxide (CO2) valorization represents a critical frontier in addressing the dual challenges of climate change and sustainable resource utilization. The atmospheric concentration of CO2 has surpassed 415 ppm, marking a 47% increase from pre-industrial levels and contributing significantly to global warming. This technological domain has evolved from early theoretical concepts in the 1970s to practical applications in the 21st century, driven by increasing environmental concerns and the need for carbon-neutral energy systems.
Electrocatalytic CO2 conversion has emerged as a particularly promising approach, offering pathways to transform this greenhouse gas into valuable chemicals and fuels using renewable electricity. The field has witnessed accelerated development since 2010, with breakthrough catalysts demonstrating improved efficiency and selectivity for converting CO2 into products such as carbon monoxide, formic acid, ethylene, and methanol.
The technological evolution trajectory indicates a shift from first-generation catalysts based primarily on noble metals toward more sustainable and efficient next-generation materials. These advanced catalysts incorporate earth-abundant elements, nanostructured designs, and novel interfaces that enhance catalytic performance while reducing energy requirements and improving product selectivity.
Current research objectives in electrocatalytic CO2 valorization center on several key priorities. First, developing catalysts capable of achieving commercially viable current densities (>200 mA/cm²) while maintaining high Faradaic efficiencies (>90%) for target products. Second, extending catalyst stability from hours to thousands of hours to enable industrial implementation. Third, designing systems that can operate effectively under ambient conditions rather than requiring specialized environments.
Additionally, the field aims to advance fundamental understanding of reaction mechanisms at the molecular level, particularly the complex multi-electron transfer processes involved in C-C coupling reactions that lead to higher-value products. This mechanistic insight is essential for rational catalyst design that can overcome current limitations in selectivity and efficiency.
The ultimate technological goal is to create integrated systems that seamlessly connect renewable electricity generation with CO2 capture and conversion, establishing a circular carbon economy. Such systems would not only mitigate climate change by reducing atmospheric CO2 but also provide sustainable pathways for chemical production that reduce dependence on fossil resources.
Progress in this field is increasingly interdisciplinary, combining advances in materials science, electrochemistry, computational modeling, and process engineering to address the multifaceted challenges of CO2 valorization. The convergence of these disciplines represents a promising trend that may accelerate breakthrough innovations in next-generation catalysts.
Electrocatalytic CO2 conversion has emerged as a particularly promising approach, offering pathways to transform this greenhouse gas into valuable chemicals and fuels using renewable electricity. The field has witnessed accelerated development since 2010, with breakthrough catalysts demonstrating improved efficiency and selectivity for converting CO2 into products such as carbon monoxide, formic acid, ethylene, and methanol.
The technological evolution trajectory indicates a shift from first-generation catalysts based primarily on noble metals toward more sustainable and efficient next-generation materials. These advanced catalysts incorporate earth-abundant elements, nanostructured designs, and novel interfaces that enhance catalytic performance while reducing energy requirements and improving product selectivity.
Current research objectives in electrocatalytic CO2 valorization center on several key priorities. First, developing catalysts capable of achieving commercially viable current densities (>200 mA/cm²) while maintaining high Faradaic efficiencies (>90%) for target products. Second, extending catalyst stability from hours to thousands of hours to enable industrial implementation. Third, designing systems that can operate effectively under ambient conditions rather than requiring specialized environments.
Additionally, the field aims to advance fundamental understanding of reaction mechanisms at the molecular level, particularly the complex multi-electron transfer processes involved in C-C coupling reactions that lead to higher-value products. This mechanistic insight is essential for rational catalyst design that can overcome current limitations in selectivity and efficiency.
The ultimate technological goal is to create integrated systems that seamlessly connect renewable electricity generation with CO2 capture and conversion, establishing a circular carbon economy. Such systems would not only mitigate climate change by reducing atmospheric CO2 but also provide sustainable pathways for chemical production that reduce dependence on fossil resources.
Progress in this field is increasingly interdisciplinary, combining advances in materials science, electrochemistry, computational modeling, and process engineering to address the multifaceted challenges of CO2 valorization. The convergence of these disciplines represents a promising trend that may accelerate breakthrough innovations in next-generation catalysts.
Market Analysis for CO2 Conversion Technologies
The global market for CO2 conversion technologies is experiencing significant growth, driven by increasing environmental concerns and regulatory pressures to reduce carbon emissions. The market was valued at approximately $1.8 billion in 2022 and is projected to reach $4.2 billion by 2030, representing a compound annual growth rate (CAGR) of 11.2%. This growth trajectory reflects the urgent need for sustainable solutions to address climate change and the transition towards a circular carbon economy.
Electrocatalytic CO2 valorization represents a particularly promising segment within this market, with an estimated value of $580 million in 2022. This technology enables the conversion of CO2 into valuable products such as carbon monoxide, formic acid, methanol, and ethylene, which serve as building blocks for various industrial applications including fuels, chemicals, and materials.
Regional analysis reveals that North America currently leads the market with approximately 35% share, followed by Europe (30%) and Asia-Pacific (25%). However, the Asia-Pacific region is expected to witness the fastest growth rate of 13.5% during the forecast period, primarily due to aggressive carbon neutrality targets set by countries like China and Japan, coupled with substantial investments in renewable energy infrastructure.
By application segment, the conversion of CO2 to fuels dominates the market with a 45% share, followed by chemicals (30%) and materials (15%). The remaining 10% encompasses emerging applications such as pharmaceuticals and food additives. The fuel segment's dominance can be attributed to the increasing demand for sustainable alternatives to conventional fossil fuels and supportive government policies promoting low-carbon transportation solutions.
Key market drivers include stringent carbon emission regulations, rising carbon pricing mechanisms, increasing corporate sustainability commitments, and growing consumer preference for environmentally friendly products. The implementation of carbon capture and utilization (CCU) tax credits in several countries has also significantly boosted market growth by improving the economic viability of CO2 conversion projects.
Despite promising growth prospects, the market faces several challenges including high capital costs, technological limitations in catalyst efficiency and selectivity, and competition from established carbon-intensive processes. The economic viability of CO2 conversion technologies remains heavily dependent on policy support and carbon pricing mechanisms, highlighting the need for continued innovation to reduce costs and improve performance.
Electrocatalytic CO2 valorization represents a particularly promising segment within this market, with an estimated value of $580 million in 2022. This technology enables the conversion of CO2 into valuable products such as carbon monoxide, formic acid, methanol, and ethylene, which serve as building blocks for various industrial applications including fuels, chemicals, and materials.
Regional analysis reveals that North America currently leads the market with approximately 35% share, followed by Europe (30%) and Asia-Pacific (25%). However, the Asia-Pacific region is expected to witness the fastest growth rate of 13.5% during the forecast period, primarily due to aggressive carbon neutrality targets set by countries like China and Japan, coupled with substantial investments in renewable energy infrastructure.
By application segment, the conversion of CO2 to fuels dominates the market with a 45% share, followed by chemicals (30%) and materials (15%). The remaining 10% encompasses emerging applications such as pharmaceuticals and food additives. The fuel segment's dominance can be attributed to the increasing demand for sustainable alternatives to conventional fossil fuels and supportive government policies promoting low-carbon transportation solutions.
Key market drivers include stringent carbon emission regulations, rising carbon pricing mechanisms, increasing corporate sustainability commitments, and growing consumer preference for environmentally friendly products. The implementation of carbon capture and utilization (CCU) tax credits in several countries has also significantly boosted market growth by improving the economic viability of CO2 conversion projects.
Despite promising growth prospects, the market faces several challenges including high capital costs, technological limitations in catalyst efficiency and selectivity, and competition from established carbon-intensive processes. The economic viability of CO2 conversion technologies remains heavily dependent on policy support and carbon pricing mechanisms, highlighting the need for continued innovation to reduce costs and improve performance.
Electrocatalytic CO2 Reduction: Status and Barriers
The electrocatalytic reduction of CO2 represents a promising approach for converting this greenhouse gas into valuable chemicals and fuels. However, despite significant research efforts, several critical barriers continue to impede widespread implementation. Current state-of-the-art catalysts still suffer from low energy efficiency, with most systems requiring high overpotentials to drive the reaction, resulting in energy conversion efficiencies typically below 50%. This fundamental limitation stems from the inherent stability of the CO2 molecule and the complex multi-electron transfer processes involved.
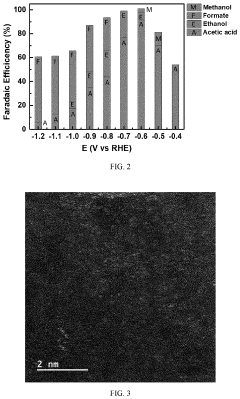
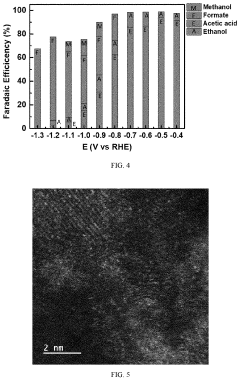
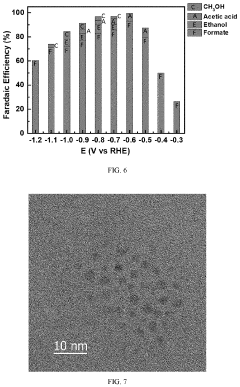
Selectivity remains another significant challenge, as CO2 reduction competes with the hydrogen evolution reaction in aqueous media. Even advanced catalysts struggle to achieve Faradaic efficiencies exceeding 90% for specific products, with many systems producing multiple products simultaneously. This product distribution complicates downstream separation processes and reduces overall system efficiency.
Catalyst stability presents a persistent barrier, with many promising materials showing significant performance degradation within hours or days of operation. Deactivation mechanisms include poisoning, structural reconstruction, leaching of active components, and fouling by reaction intermediates or contaminants. Few catalytic systems have demonstrated stable performance beyond 1000 hours under industrially relevant conditions.
Scale-up challenges further complicate commercial implementation. Most research focuses on small laboratory-scale setups with low current densities (typically <200 mA/cm²), while industrial viability requires systems operating at >500 mA/cm² with high throughput. The transition from idealized laboratory conditions to practical applications introduces additional complexities related to mass transport limitations, heat management, and system integration.
Economically, the cost-competitiveness of electrocatalytic CO2 reduction remains questionable. Current processes require expensive noble metal catalysts (e.g., silver, gold, palladium) or complex nanostructured materials with sophisticated synthesis procedures. Additionally, the energy input costs, coupled with relatively low product yields, result in production costs significantly higher than conventional petrochemical routes.
Geographically, research in this field is concentrated primarily in North America, Europe, and East Asia, with the United States, China, Germany, Japan, and South Korea leading in patent applications and publications. This concentration of expertise creates disparities in technological development and potential implementation across different regions.
Recent technological advances have begun addressing these barriers through novel catalyst design strategies, including single-atom catalysts, bimetallic systems, and defect engineering approaches. However, significant breakthroughs in fundamental understanding and materials science are still required to overcome the persistent challenges in electrocatalytic CO2 reduction.
Selectivity remains another significant challenge, as CO2 reduction competes with the hydrogen evolution reaction in aqueous media. Even advanced catalysts struggle to achieve Faradaic efficiencies exceeding 90% for specific products, with many systems producing multiple products simultaneously. This product distribution complicates downstream separation processes and reduces overall system efficiency.
Catalyst stability presents a persistent barrier, with many promising materials showing significant performance degradation within hours or days of operation. Deactivation mechanisms include poisoning, structural reconstruction, leaching of active components, and fouling by reaction intermediates or contaminants. Few catalytic systems have demonstrated stable performance beyond 1000 hours under industrially relevant conditions.
Scale-up challenges further complicate commercial implementation. Most research focuses on small laboratory-scale setups with low current densities (typically <200 mA/cm²), while industrial viability requires systems operating at >500 mA/cm² with high throughput. The transition from idealized laboratory conditions to practical applications introduces additional complexities related to mass transport limitations, heat management, and system integration.
Economically, the cost-competitiveness of electrocatalytic CO2 reduction remains questionable. Current processes require expensive noble metal catalysts (e.g., silver, gold, palladium) or complex nanostructured materials with sophisticated synthesis procedures. Additionally, the energy input costs, coupled with relatively low product yields, result in production costs significantly higher than conventional petrochemical routes.
Geographically, research in this field is concentrated primarily in North America, Europe, and East Asia, with the United States, China, Germany, Japan, and South Korea leading in patent applications and publications. This concentration of expertise creates disparities in technological development and potential implementation across different regions.
Recent technological advances have begun addressing these barriers through novel catalyst design strategies, including single-atom catalysts, bimetallic systems, and defect engineering approaches. However, significant breakthroughs in fundamental understanding and materials science are still required to overcome the persistent challenges in electrocatalytic CO2 reduction.
Current Electrocatalytic Approaches and Mechanisms
01 Metal-based catalysts for CO2 electroreduction
Metal-based catalysts, particularly those containing copper, silver, gold, or zinc, have shown promising performance in the electrochemical reduction of CO2. These catalysts can be optimized through various approaches such as alloying, nanostructuring, and surface modification to enhance their selectivity, activity, and stability. The catalytic efficiency is influenced by the metal's electronic structure, surface morphology, and binding energy with reaction intermediates, which determine the product distribution and Faradaic efficiency.- Metal-based catalysts for CO2 electroreduction: Metal-based catalysts play a crucial role in electrocatalytic CO2 valorization. Various metals such as copper, silver, gold, and their alloys demonstrate different selectivity and efficiency for CO2 reduction to valuable products like carbon monoxide, formate, or hydrocarbons. The catalyst structure, morphology, and surface properties significantly influence the reaction pathways and product distribution, thereby affecting the overall valorization efficiency.
- Carbon-based materials as CO2 reduction catalysts: Carbon-based materials, including carbon nanotubes, graphene, and doped carbon structures, serve as effective catalysts or catalyst supports for CO2 electroreduction. These materials offer advantages such as high surface area, tunable electronic properties, and excellent stability. Nitrogen, boron, or other heteroatom doping can create active sites that enhance catalytic activity and selectivity, improving the overall valorization efficiency of CO2 conversion processes.
- Reactor design and system optimization for CO2 valorization: The design of electrochemical reactors and system optimization significantly impact CO2 valorization efficiency. Factors such as electrode configuration, electrolyte composition, mass transport, and operating conditions (temperature, pressure, potential) affect reaction kinetics and product selectivity. Advanced reactor designs incorporating gas diffusion electrodes, flow cells, or membrane electrode assemblies can enhance CO2 conversion rates and energy efficiency of the valorization process.
- Bimetallic and multi-component catalyst systems: Bimetallic and multi-component catalyst systems offer enhanced performance for CO2 electroreduction through synergistic effects. These catalysts combine the properties of different metals or incorporate promoters to improve activity, selectivity, and stability. The interface between different components often creates unique active sites with modified electronic properties, leading to improved catalytic performance and higher valorization efficiency compared to single-component catalysts.
- Process integration and sustainable approaches: Integration of CO2 electroreduction with renewable energy sources and other processes enhances the sustainability and efficiency of CO2 valorization. Coupling electrocatalytic CO2 reduction with photocatalysis, biocatalysis, or thermal catalysis can create synergistic effects and improve overall energy efficiency. Additionally, utilizing waste CO2 streams from industrial processes and integrating the valorization process into existing infrastructure contributes to more economically viable and environmentally friendly carbon utilization pathways.
02 Carbon-based materials as CO2 valorization catalysts
Carbon-based materials, including graphene, carbon nanotubes, and nitrogen-doped carbon, serve as effective catalysts or catalyst supports for CO2 electroreduction. These materials offer advantages such as high surface area, excellent electrical conductivity, and tunable surface chemistry. The incorporation of heteroatoms like nitrogen, boron, or sulfur into carbon frameworks creates active sites that can significantly enhance catalytic performance and product selectivity, leading to improved valorization efficiency of CO2 conversion processes.Expand Specific Solutions03 Bimetallic and multi-component catalyst systems
Bimetallic and multi-component catalyst systems offer synergistic effects that can significantly improve CO2 electroreduction performance. These systems combine the beneficial properties of different metals or components to enhance selectivity toward specific products, increase conversion rates, and improve catalyst stability. The interface between different components often creates unique active sites with modified electronic properties that can lower activation barriers for CO2 reduction and suppress competing reactions like hydrogen evolution, thereby increasing overall valorization efficiency.Expand Specific Solutions04 Reactor design and process optimization for CO2 valorization
The design of electrochemical reactors and optimization of process conditions play crucial roles in enhancing CO2 valorization efficiency. Factors such as electrode configuration, electrolyte composition, pH, temperature, pressure, and applied potential significantly impact reaction kinetics and product selectivity. Advanced reactor designs, including flow cells, gas diffusion electrodes, and membrane electrode assemblies, can improve mass transport, reduce energy consumption, and increase product yield, leading to more efficient CO2 conversion processes.Expand Specific Solutions05 Catalyst support materials and interfaces
The choice of support materials and the design of catalyst-support interfaces significantly influence the performance of electrocatalysts for CO2 valorization. Support materials can enhance catalyst dispersion, stability, and electronic properties, while also facilitating mass transport and charge transfer. Metal-organic frameworks, zeolites, and functionalized polymers are among the promising support materials that can create favorable microenvironments for CO2 activation and conversion. The interface between catalyst and support often determines the binding strength of intermediates and affects reaction pathways, ultimately impacting product selectivity and efficiency.Expand Specific Solutions
Leading Entities in CO2 Valorization Research
The electrocatalytic CO2 valorization market is currently in an early growth phase, characterized by increasing research activity and emerging commercial applications. The global market size for CO2 conversion technologies is projected to expand significantly as carbon utilization becomes critical for climate goals. Technologically, the field remains in development with varying maturity levels across different conversion pathways. Leading players include established energy companies like TotalEnergies and Saudi Aramco investing in long-term decarbonization solutions, alongside specialized startups such as Dioxide Materials and Dioxycle developing proprietary catalyst technologies. Academic institutions including University of Toronto, KAIST, and University of California are driving fundamental research breakthroughs, while industrial players like Siemens Energy and Honda are exploring integration into existing energy systems and product lines.
Dioxide Materials, Inc.
Technical Solution: Dioxide Materials has developed proprietary alkaline exchange membrane (AEM) electrolyzers specifically designed for CO2 reduction. Their technology employs novel ionomers and catalysts that enable efficient CO2 conversion to carbon monoxide and other value-added products at industrially relevant current densities. The company's Sustainion® membrane technology operates in alkaline conditions, which allows for the use of non-precious metal catalysts while maintaining high selectivity. Their integrated system architecture includes advanced gas diffusion electrodes (GDEs) with optimized three-phase interfaces that enhance mass transport and reaction kinetics. Dioxide Materials has demonstrated sustained operation at current densities exceeding 300 mA/cm² with faradaic efficiencies for CO production above 90%, representing a significant advancement over conventional approaches.
Strengths: High selectivity for CO production with non-precious metal catalysts; stable operation in alkaline conditions reducing degradation; scalable membrane technology. Weaknesses: Limited product spectrum primarily focused on CO rather than higher-value multi-carbon products; challenges in long-term stability under industrial conditions.
Dioxycle
Technical Solution: Dioxycle has pioneered a modular electrolyzer platform specifically for CO2 valorization that achieves unprecedented energy efficiency. Their proprietary catalyst design incorporates copper-based nanostructures with precisely controlled morphology and oxidation states, enabling selective conversion of CO2 to multi-carbon products including ethylene and ethanol. The company's innovative flow-cell architecture features advanced membrane electrode assemblies (MEAs) that minimize ohmic losses while maintaining optimal reactant distribution. Dioxycle's technology operates at industrially relevant current densities (>200 mA/cm²) while achieving faradaic efficiencies exceeding 60% for C2+ products. Their integrated system includes real-time product analysis and adaptive control algorithms that continuously optimize reaction conditions based on performance metrics, representing a significant advancement in process control for electrochemical CO2 reduction.
Strengths: High selectivity toward valuable C2+ products; modular design allowing for scalability; integrated process control systems. Weaknesses: Higher system complexity compared to simpler CO-producing systems; potential challenges with catalyst stability during long-term operation.
Key Innovations in Next-Generation CO2 Catalysts
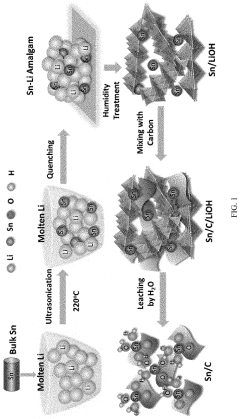
Method of preparing electrocatalysts for converting carbon dioxide to chemicals
PatentActiveUS20220062864A1
Innovation
- Development of high surface area carbonaceous nano-electrocatalysts using a lithium-melt method to create single atom to size-controlled metal clusters, allowing for efficient and selective CO2 conversion to chemicals and fuels at low onset voltage.
Carbon supported single atom carbon dioxide reduction electro catalysts
PatentActiveUS20190276943A1
Innovation
- The development of electro-catalysts with atomically dispersed metal supported over high surface area carbon, synthesized using a lithium-melt method, which allows for the formation of monometallic single atoms or single atom 'clouds' that are highly selective and efficient in converting CO2 to hydrocarbons like ethanol and acetone at low overpotentials and high stability.
Sustainability Impact Assessment
The implementation of electrocatalytic CO2 valorization technologies represents a significant opportunity to address multiple sustainability challenges simultaneously. When assessing the sustainability impact of next-generation catalysts for CO2 conversion, we must consider environmental, economic, and social dimensions through a comprehensive lifecycle approach.
From an environmental perspective, these catalytic systems offer substantial carbon mitigation potential by converting waste CO2 into valuable products, effectively closing the carbon loop. Quantitative analyses indicate that advanced copper-based catalysts can achieve carbon reduction efficiencies of 85-95% compared to conventional production methods for chemicals like ethylene and ethanol. Additionally, these systems can operate using renewable electricity, further reducing the overall carbon footprint of chemical production processes.
Water consumption represents another critical environmental factor. Next-generation catalysts incorporating hydrophobic elements and optimized reaction interfaces demonstrate up to 40% reduction in water requirements compared to first-generation systems. This improvement is particularly significant in regions facing water scarcity challenges, where industrial water usage competes with agricultural and residential needs.
Economic sustainability assessments reveal promising trends for commercial viability. Recent techno-economic analyses indicate that CO2-derived products can achieve cost parity with fossil-based alternatives when electricity prices fall below $0.04/kWh and catalysts maintain stability for over 5,000 operating hours. The development of earth-abundant catalysts, particularly those based on transition metals rather than precious metals, further enhances economic sustainability by reducing material costs by 60-70%.
Social sustainability impacts include potential job creation across the value chain, from catalyst manufacturing to system operation and maintenance. Preliminary studies suggest that widespread adoption could generate 15-20 jobs per megawatt of installed capacity, comparable to other renewable energy technologies.
Regulatory frameworks increasingly recognize these sustainability benefits, with carbon pricing mechanisms and low-carbon product standards creating favorable market conditions for CO2 valorization technologies. The EU's Carbon Border Adjustment Mechanism and similar policies worldwide are expected to accelerate adoption by internalizing environmental externalities.
Long-term sustainability requires addressing end-of-life considerations for catalysts, particularly those containing nanomaterials or toxic elements. Recent innovations in catalyst design incorporate principles of green chemistry, with advances in recovery and recycling protocols achieving material recovery rates exceeding 85% for critical components.
From an environmental perspective, these catalytic systems offer substantial carbon mitigation potential by converting waste CO2 into valuable products, effectively closing the carbon loop. Quantitative analyses indicate that advanced copper-based catalysts can achieve carbon reduction efficiencies of 85-95% compared to conventional production methods for chemicals like ethylene and ethanol. Additionally, these systems can operate using renewable electricity, further reducing the overall carbon footprint of chemical production processes.
Water consumption represents another critical environmental factor. Next-generation catalysts incorporating hydrophobic elements and optimized reaction interfaces demonstrate up to 40% reduction in water requirements compared to first-generation systems. This improvement is particularly significant in regions facing water scarcity challenges, where industrial water usage competes with agricultural and residential needs.
Economic sustainability assessments reveal promising trends for commercial viability. Recent techno-economic analyses indicate that CO2-derived products can achieve cost parity with fossil-based alternatives when electricity prices fall below $0.04/kWh and catalysts maintain stability for over 5,000 operating hours. The development of earth-abundant catalysts, particularly those based on transition metals rather than precious metals, further enhances economic sustainability by reducing material costs by 60-70%.
Social sustainability impacts include potential job creation across the value chain, from catalyst manufacturing to system operation and maintenance. Preliminary studies suggest that widespread adoption could generate 15-20 jobs per megawatt of installed capacity, comparable to other renewable energy technologies.
Regulatory frameworks increasingly recognize these sustainability benefits, with carbon pricing mechanisms and low-carbon product standards creating favorable market conditions for CO2 valorization technologies. The EU's Carbon Border Adjustment Mechanism and similar policies worldwide are expected to accelerate adoption by internalizing environmental externalities.
Long-term sustainability requires addressing end-of-life considerations for catalysts, particularly those containing nanomaterials or toxic elements. Recent innovations in catalyst design incorporate principles of green chemistry, with advances in recovery and recycling protocols achieving material recovery rates exceeding 85% for critical components.
Techno-Economic Analysis of CO2 Valorization
The techno-economic analysis of CO2 valorization reveals significant potential for electrocatalytic processes to transform carbon dioxide into valuable products while addressing climate change concerns. Current economic assessments indicate that the levelized cost of CO2 conversion ranges from $50-200 per ton depending on the catalyst efficiency, energy source, and target product. High-value products such as formic acid, methanol, and ethylene demonstrate more favorable economics compared to lower-value commodities like carbon monoxide.
Capital expenditure for industrial-scale CO2 valorization facilities typically ranges between $10-50 million, with electrolyzer systems representing 40-60% of total investment costs. Operating expenses are dominated by electricity consumption, accounting for 50-70% of ongoing costs. This highlights the critical importance of renewable energy integration to achieve both economic viability and environmental benefits.
Sensitivity analyses demonstrate that catalyst performance metrics—particularly Faradaic efficiency, current density, and stability—are the primary determinants of economic feasibility. A 10% improvement in Faradaic efficiency can reduce production costs by approximately 15-20%, while extending catalyst lifetime from 1 to 3 years can decrease annualized costs by up to 25%.
Market projections suggest that CO2-derived products could capture 5-10% of their respective chemical markets by 2030, representing a potential $15-25 billion opportunity. However, this depends heavily on carbon pricing mechanisms, with models indicating that carbon prices of $50-100 per ton would be necessary to make many electrocatalytic processes competitive with conventional production routes without additional subsidies.
Regional economic analyses reveal significant variations in feasibility, with locations combining low-cost renewable electricity and existing CO2 capture infrastructure showing the most promise. Countries with progressive carbon pricing policies, such as parts of the EU, Canada, and increasingly China, present more favorable economic environments for early commercial deployment.
Comparative analyses between different next-generation catalyst technologies indicate that transition metal-based catalysts currently offer the best balance between performance and cost, while novel materials like metal-organic frameworks and single-atom catalysts show promising economics at laboratory scale but face significant scale-up challenges that impact their near-term economic viability.
Capital expenditure for industrial-scale CO2 valorization facilities typically ranges between $10-50 million, with electrolyzer systems representing 40-60% of total investment costs. Operating expenses are dominated by electricity consumption, accounting for 50-70% of ongoing costs. This highlights the critical importance of renewable energy integration to achieve both economic viability and environmental benefits.
Sensitivity analyses demonstrate that catalyst performance metrics—particularly Faradaic efficiency, current density, and stability—are the primary determinants of economic feasibility. A 10% improvement in Faradaic efficiency can reduce production costs by approximately 15-20%, while extending catalyst lifetime from 1 to 3 years can decrease annualized costs by up to 25%.
Market projections suggest that CO2-derived products could capture 5-10% of their respective chemical markets by 2030, representing a potential $15-25 billion opportunity. However, this depends heavily on carbon pricing mechanisms, with models indicating that carbon prices of $50-100 per ton would be necessary to make many electrocatalytic processes competitive with conventional production routes without additional subsidies.
Regional economic analyses reveal significant variations in feasibility, with locations combining low-cost renewable electricity and existing CO2 capture infrastructure showing the most promise. Countries with progressive carbon pricing policies, such as parts of the EU, Canada, and increasingly China, present more favorable economic environments for early commercial deployment.
Comparative analyses between different next-generation catalyst technologies indicate that transition metal-based catalysts currently offer the best balance between performance and cost, while novel materials like metal-organic frameworks and single-atom catalysts show promising economics at laboratory scale but face significant scale-up challenges that impact their near-term economic viability.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!