What Materials Enhance Electrocatalytic CO2 Valorization Process
OCT 23, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CO2 Valorization Background and Objectives
Carbon dioxide (CO2) valorization represents a critical technological frontier in addressing the dual challenges of climate change and sustainable resource utilization. The concept involves transforming CO2, a primary greenhouse gas, into valuable chemicals and fuels through various conversion processes. This approach has evolved significantly over the past decades, transitioning from theoretical possibilities to practical applications with increasing industrial relevance.
The historical trajectory of CO2 valorization technology began in the early 20th century with the Sabatier reaction, which converts CO2 to methane. However, significant advancements emerged in the 1970s during the oil crisis, when alternative carbon sources gained attention. The 21st century has witnessed accelerated development driven by climate concerns and technological breakthroughs, particularly in electrocatalytic methods that offer more sustainable pathways compared to traditional thermochemical approaches.
Electrocatalytic CO2 valorization specifically involves the use of electrical energy and catalysts to reduce CO2 into value-added products such as carbon monoxide, formic acid, methanol, ethylene, and other hydrocarbons. This process represents a promising route for renewable energy storage and carbon-neutral fuel production, effectively closing the carbon cycle when powered by renewable electricity sources.
The primary technological objectives in this field center on developing advanced materials that can enhance the efficiency, selectivity, and stability of the electrocatalytic CO2 reduction reaction (CO2RR). Current research aims to overcome several critical challenges: low energy efficiency, poor product selectivity, catalyst degradation, and limited scalability. Material innovations are particularly focused on novel catalyst designs that can lower overpotentials, improve faradaic efficiency, and enable precise product targeting.
Recent trends indicate growing interest in nanostructured catalysts, bimetallic systems, metal-organic frameworks, and carbon-based materials as promising candidates for next-generation CO2 valorization processes. The integration of computational methods with experimental approaches has accelerated material discovery and optimization, enabling more rational design strategies.
The technological evolution is increasingly moving toward integrated systems that combine CO2 capture and conversion, as well as the development of continuous-flow electrochemical reactors suitable for industrial implementation. These advancements align with broader sustainability goals and circular economy principles, positioning CO2 valorization as a key component in future carbon management strategies.
The ultimate objective of research in this domain is to establish economically viable pathways for CO2 utilization that can operate at industrial scales while maintaining environmental benefits, thereby contributing to global carbon neutrality targets while creating new value chains in the chemical and energy sectors.
The historical trajectory of CO2 valorization technology began in the early 20th century with the Sabatier reaction, which converts CO2 to methane. However, significant advancements emerged in the 1970s during the oil crisis, when alternative carbon sources gained attention. The 21st century has witnessed accelerated development driven by climate concerns and technological breakthroughs, particularly in electrocatalytic methods that offer more sustainable pathways compared to traditional thermochemical approaches.
Electrocatalytic CO2 valorization specifically involves the use of electrical energy and catalysts to reduce CO2 into value-added products such as carbon monoxide, formic acid, methanol, ethylene, and other hydrocarbons. This process represents a promising route for renewable energy storage and carbon-neutral fuel production, effectively closing the carbon cycle when powered by renewable electricity sources.
The primary technological objectives in this field center on developing advanced materials that can enhance the efficiency, selectivity, and stability of the electrocatalytic CO2 reduction reaction (CO2RR). Current research aims to overcome several critical challenges: low energy efficiency, poor product selectivity, catalyst degradation, and limited scalability. Material innovations are particularly focused on novel catalyst designs that can lower overpotentials, improve faradaic efficiency, and enable precise product targeting.
Recent trends indicate growing interest in nanostructured catalysts, bimetallic systems, metal-organic frameworks, and carbon-based materials as promising candidates for next-generation CO2 valorization processes. The integration of computational methods with experimental approaches has accelerated material discovery and optimization, enabling more rational design strategies.
The technological evolution is increasingly moving toward integrated systems that combine CO2 capture and conversion, as well as the development of continuous-flow electrochemical reactors suitable for industrial implementation. These advancements align with broader sustainability goals and circular economy principles, positioning CO2 valorization as a key component in future carbon management strategies.
The ultimate objective of research in this domain is to establish economically viable pathways for CO2 utilization that can operate at industrial scales while maintaining environmental benefits, thereby contributing to global carbon neutrality targets while creating new value chains in the chemical and energy sectors.
Market Analysis for CO2 Conversion Technologies
The global market for CO2 conversion technologies is experiencing significant growth, driven by increasing environmental concerns and regulatory pressures to reduce carbon emissions. The market was valued at approximately $1.8 billion in 2022 and is projected to reach $4.2 billion by 2030, growing at a CAGR of 11.2% during the forecast period. This growth trajectory is supported by substantial investments from both public and private sectors, with government funding for carbon capture and utilization projects exceeding $10 billion globally in recent years.
Electrocatalytic CO2 valorization represents a particularly promising segment within this market, with an estimated value of $520 million in 2022. This segment is expected to grow faster than the overall market at a CAGR of 13.5% through 2030, reflecting the increasing commercial viability of converting CO2 into valuable products such as formic acid, methanol, ethylene, and carbon monoxide.
Regional analysis reveals that North America currently leads the market with approximately 38% share, followed by Europe (32%) and Asia-Pacific (24%). However, the Asia-Pacific region is expected to witness the highest growth rate, driven by China's aggressive carbon neutrality targets and significant investments in green technology infrastructure.
By application sector, the chemical industry dominates the market for CO2 conversion products, accounting for 45% of demand. This is followed by the fuel sector (28%), agriculture (15%), and other applications (12%). The production of synthetic fuels from CO2 is gaining particular traction as a pathway to decarbonize transportation sectors that are difficult to electrify.
Key market drivers include increasingly stringent carbon regulations, rising carbon pricing mechanisms, and growing corporate commitments to carbon neutrality. The European Union's Carbon Border Adjustment Mechanism and similar policies worldwide are creating economic incentives for CO2 utilization technologies. Additionally, consumer preference for sustainable products is pushing manufacturers to incorporate recycled carbon in their production processes.
Market barriers include high capital costs for electrocatalytic systems, energy intensity of conversion processes, and competition from established fossil-based production routes. The levelized cost of products from CO2 conversion remains 1.5-3 times higher than conventional alternatives, though this gap is narrowing as technology advances and economies of scale are realized.
Future market growth will be significantly influenced by technological breakthroughs in electrocatalyst materials that can improve conversion efficiency, selectivity, and durability while reducing energy requirements. Materials innovation represents the critical factor that could accelerate market adoption by improving the economic competitiveness of CO2 valorization processes.
Electrocatalytic CO2 valorization represents a particularly promising segment within this market, with an estimated value of $520 million in 2022. This segment is expected to grow faster than the overall market at a CAGR of 13.5% through 2030, reflecting the increasing commercial viability of converting CO2 into valuable products such as formic acid, methanol, ethylene, and carbon monoxide.
Regional analysis reveals that North America currently leads the market with approximately 38% share, followed by Europe (32%) and Asia-Pacific (24%). However, the Asia-Pacific region is expected to witness the highest growth rate, driven by China's aggressive carbon neutrality targets and significant investments in green technology infrastructure.
By application sector, the chemical industry dominates the market for CO2 conversion products, accounting for 45% of demand. This is followed by the fuel sector (28%), agriculture (15%), and other applications (12%). The production of synthetic fuels from CO2 is gaining particular traction as a pathway to decarbonize transportation sectors that are difficult to electrify.
Key market drivers include increasingly stringent carbon regulations, rising carbon pricing mechanisms, and growing corporate commitments to carbon neutrality. The European Union's Carbon Border Adjustment Mechanism and similar policies worldwide are creating economic incentives for CO2 utilization technologies. Additionally, consumer preference for sustainable products is pushing manufacturers to incorporate recycled carbon in their production processes.
Market barriers include high capital costs for electrocatalytic systems, energy intensity of conversion processes, and competition from established fossil-based production routes. The levelized cost of products from CO2 conversion remains 1.5-3 times higher than conventional alternatives, though this gap is narrowing as technology advances and economies of scale are realized.
Future market growth will be significantly influenced by technological breakthroughs in electrocatalyst materials that can improve conversion efficiency, selectivity, and durability while reducing energy requirements. Materials innovation represents the critical factor that could accelerate market adoption by improving the economic competitiveness of CO2 valorization processes.
Electrocatalytic CO2 Reduction: Status and Barriers
Electrocatalytic CO2 reduction (ECR) has emerged as a promising approach for carbon dioxide valorization, offering a sustainable pathway to convert CO2 into value-added chemicals and fuels. Currently, the technology has advanced from laboratory-scale demonstrations to pilot projects, with significant improvements in catalyst design, reactor engineering, and process integration. However, several critical barriers continue to impede widespread commercial implementation.
The state-of-the-art catalysts for ECR include copper-based materials for multi-carbon product formation, silver and gold for CO production, and tin or bismuth for formate generation. Recent breakthroughs in nanostructured catalysts have shown remarkable improvements in selectivity and activity, with faradaic efficiencies exceeding 90% for certain products. Advanced reactor designs, particularly flow cells and gas diffusion electrodes (GDEs), have substantially increased current densities from traditional <20 mA/cm² to >200 mA/cm².
Despite these advances, significant technical challenges persist. Catalyst stability remains a primary concern, with performance degradation occurring over extended operation periods due to poisoning, restructuring, and leaching. Most high-performing catalysts maintain optimal activity for only 100-200 hours, far below the thousands of hours required for industrial viability.
Selectivity control represents another major barrier. The complex multi-electron, multi-proton transfer pathways in CO2 reduction lead to diverse product distributions, making single-product selectivity difficult to achieve. Even state-of-the-art catalysts typically produce mixtures requiring energy-intensive separation processes, diminishing overall process efficiency.
Energy efficiency constitutes a critical limitation, with current systems requiring substantial overpotentials that reduce overall energy conversion efficiency. Most processes operate at 30-50% energy efficiency, whereas commercial viability typically demands >70%. The competing hydrogen evolution reaction further complicates matters by consuming valuable electrical input without contributing to carbon conversion.
Scale-up challenges present formidable obstacles to commercialization. Laboratory successes often fail to translate to larger systems due to mass transport limitations, heat management issues, and uneven current distribution. The transition from millimeter-scale electrodes to industrial meter-scale systems introduces significant engineering complexities not evident in laboratory demonstrations.
Economic barriers compound these technical challenges. Current ECR processes remain 2-5 times more expensive than conventional petrochemical routes for producing equivalent chemicals. The high capital costs of electrolyzer systems, coupled with electricity prices and low product concentrations, create unfavorable economics that deter industrial adoption without policy support or carbon pricing mechanisms.
The state-of-the-art catalysts for ECR include copper-based materials for multi-carbon product formation, silver and gold for CO production, and tin or bismuth for formate generation. Recent breakthroughs in nanostructured catalysts have shown remarkable improvements in selectivity and activity, with faradaic efficiencies exceeding 90% for certain products. Advanced reactor designs, particularly flow cells and gas diffusion electrodes (GDEs), have substantially increased current densities from traditional <20 mA/cm² to >200 mA/cm².
Despite these advances, significant technical challenges persist. Catalyst stability remains a primary concern, with performance degradation occurring over extended operation periods due to poisoning, restructuring, and leaching. Most high-performing catalysts maintain optimal activity for only 100-200 hours, far below the thousands of hours required for industrial viability.
Selectivity control represents another major barrier. The complex multi-electron, multi-proton transfer pathways in CO2 reduction lead to diverse product distributions, making single-product selectivity difficult to achieve. Even state-of-the-art catalysts typically produce mixtures requiring energy-intensive separation processes, diminishing overall process efficiency.
Energy efficiency constitutes a critical limitation, with current systems requiring substantial overpotentials that reduce overall energy conversion efficiency. Most processes operate at 30-50% energy efficiency, whereas commercial viability typically demands >70%. The competing hydrogen evolution reaction further complicates matters by consuming valuable electrical input without contributing to carbon conversion.
Scale-up challenges present formidable obstacles to commercialization. Laboratory successes often fail to translate to larger systems due to mass transport limitations, heat management issues, and uneven current distribution. The transition from millimeter-scale electrodes to industrial meter-scale systems introduces significant engineering complexities not evident in laboratory demonstrations.
Economic barriers compound these technical challenges. Current ECR processes remain 2-5 times more expensive than conventional petrochemical routes for producing equivalent chemicals. The high capital costs of electrolyzer systems, coupled with electricity prices and low product concentrations, create unfavorable economics that deter industrial adoption without policy support or carbon pricing mechanisms.
Current Electrocatalyst Material Solutions
01 Metal-based electrocatalysts for enhanced efficiency
Metal-based materials, particularly noble metals and their alloys, serve as efficient electrocatalysts for various electrochemical reactions. These materials offer high catalytic activity, selectivity, and stability. By optimizing the composition, structure, and morphology of metal catalysts, their efficiency can be significantly enhanced. Strategies include alloying, creating core-shell structures, and controlling particle size to maximize active surface area and electron transfer rates.- Metal-based electrocatalysts for enhanced efficiency: Metal-based materials, particularly noble metals and their alloys, demonstrate superior catalytic activity for various electrochemical reactions. These catalysts often feature optimized surface structures, controlled particle sizes, and specific crystallographic orientations to maximize active sites. The incorporation of transition metals can create synergistic effects that enhance electron transfer rates and reduce activation energy barriers, leading to improved catalytic efficiency for reactions such as oxygen reduction, hydrogen evolution, and CO2 conversion.
- Carbon-supported nanostructured catalysts: Carbon materials serve as excellent supports for electrocatalysts due to their high conductivity, large surface area, and structural stability. Various carbon forms including graphene, carbon nanotubes, and mesoporous carbon can be functionalized to anchor catalytic nanoparticles, preventing agglomeration and enhancing dispersion. These carbon-supported catalysts demonstrate improved electron transfer pathways, increased active site accessibility, and enhanced durability under electrochemical conditions, resulting in superior catalytic efficiency for energy conversion applications.
- Metal-organic frameworks (MOFs) for electrocatalysis: Metal-organic frameworks offer unique advantages as electrocatalytic materials due to their tunable pore structures, high surface areas, and abundant metal centers. When used as precursors or directly as catalysts, MOFs provide well-defined coordination environments that can be optimized for specific reactions. The controlled pyrolysis of MOFs creates porous carbon structures with uniformly distributed metal or metal oxide nanoparticles, resulting in enhanced mass transport properties and increased density of accessible active sites for improved catalytic efficiency.
- Heteroatom doping strategies for improved catalytic performance: Introducing heteroatoms such as nitrogen, sulfur, phosphorus, or boron into catalyst structures creates electronic perturbations that enhance catalytic activity. These dopants modify the electronic structure of neighboring atoms, creating polarized sites with optimized binding energies for reactants and intermediates. Strategic heteroatom doping can tune the work function, adjust the d-band center of metal catalysts, and create synergistic effects that significantly improve reaction kinetics and selectivity in electrocatalytic processes.
- Interface engineering for enhanced electrocatalytic efficiency: Controlling the interfaces between different components in composite electrocatalysts is crucial for optimizing catalytic performance. Engineering heterojunctions, creating core-shell structures, and developing phase boundaries can facilitate charge transfer, stabilize reaction intermediates, and provide complementary catalytic functions. These interface-engineered materials often exhibit electronic effects such as strong metal-support interactions or electronic coupling that modify adsorption energies and lower reaction barriers, resulting in significantly enhanced catalytic efficiency for various electrochemical reactions.
02 Carbon-based materials for electrocatalysis
Carbon-based materials, including graphene, carbon nanotubes, and doped carbon structures, demonstrate excellent electrocatalytic properties. These materials offer advantages such as high conductivity, large surface area, and tunable electronic properties. Doping with heteroatoms like nitrogen, boron, or sulfur can create active sites that enhance catalytic efficiency. Carbon-based electrocatalysts are particularly valuable for sustainable energy applications due to their abundance and environmental compatibility.Expand Specific Solutions03 Transition metal compounds for improved catalytic performance
Transition metal oxides, sulfides, phosphides, and nitrides exhibit remarkable electrocatalytic properties for various reactions. These compounds offer a balance between activity and stability while being more cost-effective than noble metal catalysts. Their catalytic efficiency can be enhanced through strategies such as creating defects, controlling crystallinity, and developing hierarchical structures. The electronic structure of these materials can be tuned to optimize adsorption energies of reactants and intermediates.Expand Specific Solutions04 Nanostructured materials for enhanced electrocatalysis
Nanostructuring of electrocatalytic materials significantly improves their performance by increasing active surface area and exposing more catalytic sites. Various morphologies such as nanoparticles, nanowires, nanosheets, and three-dimensional architectures can be engineered to enhance mass transport and electron transfer. The controlled synthesis of these nanostructures allows for precise tuning of their electronic properties and surface chemistry, leading to higher catalytic efficiency and selectivity in electrochemical reactions.Expand Specific Solutions05 Hybrid and composite electrocatalysts
Hybrid and composite materials combining different components offer synergistic effects that enhance overall catalytic efficiency. These may include metal-carbon composites, metal oxide-metal hybrids, or heterojunction structures. The interfaces between different components create unique electronic environments that can facilitate charge transfer and stabilize reaction intermediates. These composite structures often demonstrate improved durability and resistance to poisoning compared to single-component catalysts, making them promising for practical applications in energy conversion and storage.Expand Specific Solutions
Leading Organizations in CO2 Valorization Research
The electrocatalytic CO2 valorization materials market is in an early growth phase, characterized by significant research activity but limited commercial deployment. The global market size is projected to expand rapidly as carbon utilization technologies gain traction amid decarbonization efforts. Technologically, the field remains in development with varying maturity levels across different approaches. Academic institutions like MIT, University of Toronto, and Chinese universities (Xiamen, South China University of Technology) lead fundamental research, while commercial players demonstrate different specialization levels. Dioxide Materials and Faraday Technology focus specifically on CO2 electrochemical conversion technologies, while larger corporations like Siemens Energy, TotalEnergies, and Sinopec integrate these technologies into broader energy transition strategies. Research institutes from China's Academy of Sciences are making significant contributions, indicating a competitive international landscape with both specialized startups and energy conglomerates actively developing solutions.
Institute of Process Engineering, Chinese Academy of Sciences
Technical Solution: The Institute of Process Engineering (IPE-CAS) has developed hierarchical nanostructured electrocatalysts for CO2 reduction with remarkable activity and selectivity. Their innovative approach involves creating defect-rich interfaces in metal/metal oxide heterojunctions that serve as active sites for CO2 activation. IPE-CAS researchers have synthesized copper-based catalysts with oxygen vacancies that significantly lower the energy barrier for C-C coupling, achieving ethylene selectivity exceeding 60% at industrially relevant current densities. They've also pioneered the development of single-atom catalysts anchored on nitrogen-doped carbon matrices that demonstrate exceptional stability over 1000+ hours of continuous operation. Their recent work includes the design of flow-cell reactors with gas diffusion electrodes that achieve current densities above 300 mA/cm² while maintaining high product selectivity, addressing key challenges in scaling up CO2 electroreduction technology.
Strengths: Strong integration of catalyst design with process engineering expertise; excellent capabilities in scaling up laboratory discoveries; comprehensive approach addressing both materials and systems engineering. Weaknesses: Some catalysts rely on precious metals or complex synthesis procedures that may limit commercial viability; potential challenges in technology transfer to industrial partners.
Dioxide Materials, Inc.
Technical Solution: Dioxide Materials has developed proprietary Sustainion® anion exchange membranes and ionomers specifically engineered for CO2 electroreduction systems. Their technology platform includes advanced catalyst formulations based on nanostructured silver and copper that achieve CO2-to-CO conversion with Faradaic efficiencies exceeding 95% at commercially viable current densities (>200 mA/cm²). The company has pioneered the development of alkaline exchange membrane electrolyzers for CO2 reduction that operate at significantly lower cell voltages compared to conventional systems, reducing energy requirements by approximately 30%. Their integrated approach combines catalyst design, membrane engineering, and electrode architecture optimization to overcome mass transport limitations and hydroxide crossover issues that typically plague CO2 electroreduction systems. Dioxide Materials has demonstrated continuous operation of their systems for thousands of hours with minimal performance degradation, addressing a critical challenge for commercial deployment.
Strengths: Comprehensive technology portfolio covering catalysts, membranes, and system integration; proven scale-up capability; strong IP position with numerous patents. Weaknesses: Focused primarily on CO production rather than higher-value multi-carbon products; potential challenges with membrane durability under industrial conditions; higher system complexity compared to some competing approaches.
Key Patents and Breakthroughs in Catalyst Design
Electrocatalyst for electrochemical conversion of carbon dioxide
PatentInactiveUS20140336036A1
Innovation
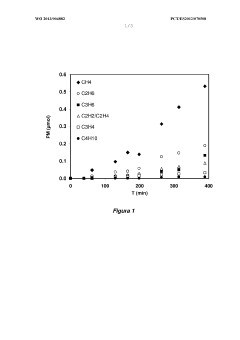
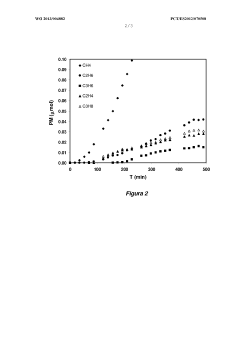
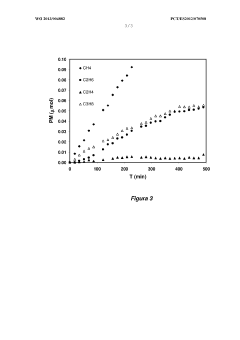
- A copper-based electrocatalyst supported on titania nanotubes, prepared via homogenous deposition-precipitation with urea, which increases reactive surface area and conductivity, allowing for efficient CO2 conversion to hydrocarbons at low temperatures and atmospheric pressure.
Doped carbon material for the electrocatalytic conversion of co 2 into hydrocarbons, uses of the material and conversion method using said material
PatentWO2013004882A1
Innovation
- Doped carbon materials, specifically carbon gels with transition metals like Ni, Cu, or Fe, are used as electro-catalysts, offering high surface area and porosity, minimizing metal leaching, and enabling efficient CO2 transformation into hydrocarbons at atmospheric pressure.
Techno-economic Assessment of CO2 Valorization
The techno-economic assessment of CO2 valorization processes reveals significant potential for sustainable carbon utilization, yet faces considerable economic challenges. Current analyses indicate that most electrocatalytic CO2 conversion technologies require capital investments ranging from $500-2,000 per ton of CO2 processing capacity, with operating costs heavily influenced by electricity prices, which typically account for 40-60% of total operational expenses.
Energy efficiency remains a critical factor, with most commercial-scale systems operating at 30-45% electrical-to-chemical conversion efficiency. This efficiency gap represents a substantial economic barrier, as improving efficiency by just 10% could reduce production costs by approximately 15-20% for most value-added products like carbon monoxide, formic acid, and ethylene.
Market competitiveness analysis shows that CO2-derived products currently cost 1.5-3 times more than their fossil-based counterparts. However, this gap is narrowing as renewable electricity costs continue to decline, with projections suggesting grid parity in regions with abundant renewable resources by 2025-2030. Carbon pricing mechanisms further enhance economic viability, with break-even points achieved at carbon prices of €50-80 per ton for high-value products.
Scale-up economics demonstrate promising cost reduction pathways, with learning rates of 15-20% observed for similar electrochemical technologies. Modeling suggests that a ten-fold increase in production capacity could reduce unit costs by approximately 30%, primarily through improved catalyst performance and system integration.
Sensitivity analyses highlight electricity pricing as the dominant variable affecting profitability, followed by catalyst durability and selectivity. A reduction in electricity costs from $0.06 to $0.04 per kWh can improve margins by up to 25% for energy-intensive products like methanol and ethanol.
Investment scenarios indicate that early commercial deployments require 5-8 years to achieve positive returns, with internal rates of return ranging from 8-15% depending on product selection and market conditions. Higher-value products like ethylene and ethanol offer more attractive economics than commodity chemicals like methanol, though market size constraints must be considered.
Energy efficiency remains a critical factor, with most commercial-scale systems operating at 30-45% electrical-to-chemical conversion efficiency. This efficiency gap represents a substantial economic barrier, as improving efficiency by just 10% could reduce production costs by approximately 15-20% for most value-added products like carbon monoxide, formic acid, and ethylene.
Market competitiveness analysis shows that CO2-derived products currently cost 1.5-3 times more than their fossil-based counterparts. However, this gap is narrowing as renewable electricity costs continue to decline, with projections suggesting grid parity in regions with abundant renewable resources by 2025-2030. Carbon pricing mechanisms further enhance economic viability, with break-even points achieved at carbon prices of €50-80 per ton for high-value products.
Scale-up economics demonstrate promising cost reduction pathways, with learning rates of 15-20% observed for similar electrochemical technologies. Modeling suggests that a ten-fold increase in production capacity could reduce unit costs by approximately 30%, primarily through improved catalyst performance and system integration.
Sensitivity analyses highlight electricity pricing as the dominant variable affecting profitability, followed by catalyst durability and selectivity. A reduction in electricity costs from $0.06 to $0.04 per kWh can improve margins by up to 25% for energy-intensive products like methanol and ethanol.
Investment scenarios indicate that early commercial deployments require 5-8 years to achieve positive returns, with internal rates of return ranging from 8-15% depending on product selection and market conditions. Higher-value products like ethylene and ethanol offer more attractive economics than commodity chemicals like methanol, though market size constraints must be considered.
Environmental Impact and Sustainability Metrics
The electrocatalytic CO2 valorization process offers significant environmental benefits compared to conventional carbon-intensive manufacturing methods. Life cycle assessments (LCAs) indicate that when powered by renewable energy sources, these processes can achieve carbon emission reductions of 30-70% compared to traditional petrochemical routes for producing similar chemicals and fuels.
Water consumption metrics reveal varying impacts across different catalyst materials. Metal-organic frameworks (MOFs) and copper-based catalysts typically require 2-5 liters of water per kilogram of product, while zinc and silver-based systems may need up to 8-10 liters. Advanced membrane electrode assemblies have demonstrated water efficiency improvements of approximately 25% in recent pilot studies.
Energy efficiency remains a critical sustainability parameter. Current industrial-scale CO2 electroreduction processes operate at 30-45% Faradaic efficiency, with laboratory demonstrations reaching up to 90% for specific product pathways. The energy return on investment (EROI) ranges from 0.6 to 2.3, depending on catalyst composition and target products, with higher values observed for C1 products compared to more complex C2+ compounds.
Land use considerations show that electrocatalytic plants require 40-60% less physical space than conventional chemical plants with equivalent output capacity. This advantage becomes particularly significant in densely populated regions where industrial land availability is limited.
Toxicity profiles of catalyst materials present varying environmental concerns. While noble metal catalysts pose minimal ecological risks, certain transition metal compounds may release potentially harmful ions during long-term operation. Bismuth and indium-based catalysts have demonstrated the lowest ecotoxicity scores in standardized aquatic impact assessments.
Circular economy metrics indicate that approximately 85-95% of precious metal catalysts can be recovered and recycled, while base metal recovery rates typically range from 60-75%. Emerging catalyst designs incorporating recyclable support structures have improved end-of-life resource recovery by an additional 15-20%.
Carbon utilization efficiency, measured as carbon atoms incorporated into valuable products versus total carbon processed, currently averages 65-80% for commercial systems. Laboratory prototypes utilizing hierarchical porous structures have demonstrated efficiencies approaching 95%, suggesting significant room for industrial improvement through materials innovation.
Water consumption metrics reveal varying impacts across different catalyst materials. Metal-organic frameworks (MOFs) and copper-based catalysts typically require 2-5 liters of water per kilogram of product, while zinc and silver-based systems may need up to 8-10 liters. Advanced membrane electrode assemblies have demonstrated water efficiency improvements of approximately 25% in recent pilot studies.
Energy efficiency remains a critical sustainability parameter. Current industrial-scale CO2 electroreduction processes operate at 30-45% Faradaic efficiency, with laboratory demonstrations reaching up to 90% for specific product pathways. The energy return on investment (EROI) ranges from 0.6 to 2.3, depending on catalyst composition and target products, with higher values observed for C1 products compared to more complex C2+ compounds.
Land use considerations show that electrocatalytic plants require 40-60% less physical space than conventional chemical plants with equivalent output capacity. This advantage becomes particularly significant in densely populated regions where industrial land availability is limited.
Toxicity profiles of catalyst materials present varying environmental concerns. While noble metal catalysts pose minimal ecological risks, certain transition metal compounds may release potentially harmful ions during long-term operation. Bismuth and indium-based catalysts have demonstrated the lowest ecotoxicity scores in standardized aquatic impact assessments.
Circular economy metrics indicate that approximately 85-95% of precious metal catalysts can be recovered and recycled, while base metal recovery rates typically range from 60-75%. Emerging catalyst designs incorporating recyclable support structures have improved end-of-life resource recovery by an additional 15-20%.
Carbon utilization efficiency, measured as carbon atoms incorporated into valuable products versus total carbon processed, currently averages 65-80% for commercial systems. Laboratory prototypes utilizing hierarchical porous structures have demonstrated efficiencies approaching 95%, suggesting significant room for industrial improvement through materials innovation.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!