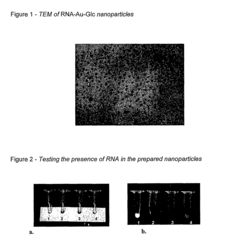
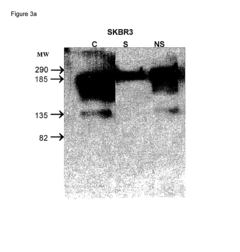
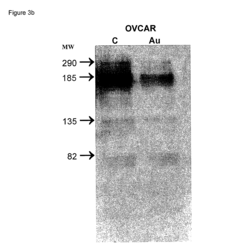
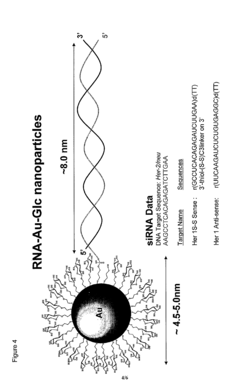
Analysis of mRNA Nanoparticle and Regulatory Interactions
OCT 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
mRNA Nanoparticle Technology Background and Objectives
Messenger RNA (mRNA) technology has emerged as a revolutionary platform in the field of therapeutics and vaccines, with its roots dating back to the 1990s when researchers first demonstrated the potential of mRNA for protein expression in vivo. The trajectory of mRNA technology development accelerated significantly in the early 2000s with breakthroughs in RNA stability and delivery mechanisms. The COVID-19 pandemic marked a watershed moment for mRNA technology, catapulting it from experimental status to mainstream application with unprecedented speed and scale.
The evolution of mRNA technology has been closely intertwined with advances in nanoparticle delivery systems. Traditional challenges of RNA instability and inefficient cellular uptake have been progressively addressed through sophisticated nanoparticle formulations, particularly lipid nanoparticles (LNPs). These delivery vehicles have evolved from simple liposomal structures to complex, engineered particles with specific targeting capabilities and controlled release properties.
Current technological trends indicate a shift towards precision-engineered mRNA-nanoparticle complexes with enhanced tissue specificity, reduced immunogenicity, and improved translational efficiency. The integration of computational modeling and high-throughput screening methodologies has accelerated the optimization of these nanoparticle formulations, enabling rapid iteration and refinement of delivery systems tailored to specific therapeutic applications.
The regulatory landscape surrounding mRNA nanoparticle technology has also evolved significantly, with frameworks developing to address the unique characteristics and risk profiles of these novel therapeutics. Understanding the complex interplay between mRNA design, nanoparticle composition, and regulatory requirements has become essential for successful technology development and commercialization.
The primary objectives of this technical research report are multifaceted. First, to comprehensively map the current state of mRNA nanoparticle technology across different therapeutic domains. Second, to analyze the critical regulatory interactions that govern the development, approval, and deployment of mRNA-based therapeutics. Third, to identify emerging trends and potential breakthrough areas that could reshape the technology landscape in the coming years.
Additionally, this report aims to elucidate the technical challenges that continue to limit the broader application of mRNA nanoparticle technology, including issues related to stability, manufacturing scalability, cold chain requirements, and tissue-specific targeting. By examining these challenges through the lens of recent innovations and regulatory developments, we seek to provide a roadmap for future research and development efforts in this rapidly evolving field.
The evolution of mRNA technology has been closely intertwined with advances in nanoparticle delivery systems. Traditional challenges of RNA instability and inefficient cellular uptake have been progressively addressed through sophisticated nanoparticle formulations, particularly lipid nanoparticles (LNPs). These delivery vehicles have evolved from simple liposomal structures to complex, engineered particles with specific targeting capabilities and controlled release properties.
Current technological trends indicate a shift towards precision-engineered mRNA-nanoparticle complexes with enhanced tissue specificity, reduced immunogenicity, and improved translational efficiency. The integration of computational modeling and high-throughput screening methodologies has accelerated the optimization of these nanoparticle formulations, enabling rapid iteration and refinement of delivery systems tailored to specific therapeutic applications.
The regulatory landscape surrounding mRNA nanoparticle technology has also evolved significantly, with frameworks developing to address the unique characteristics and risk profiles of these novel therapeutics. Understanding the complex interplay between mRNA design, nanoparticle composition, and regulatory requirements has become essential for successful technology development and commercialization.
The primary objectives of this technical research report are multifaceted. First, to comprehensively map the current state of mRNA nanoparticle technology across different therapeutic domains. Second, to analyze the critical regulatory interactions that govern the development, approval, and deployment of mRNA-based therapeutics. Third, to identify emerging trends and potential breakthrough areas that could reshape the technology landscape in the coming years.
Additionally, this report aims to elucidate the technical challenges that continue to limit the broader application of mRNA nanoparticle technology, including issues related to stability, manufacturing scalability, cold chain requirements, and tissue-specific targeting. By examining these challenges through the lens of recent innovations and regulatory developments, we seek to provide a roadmap for future research and development efforts in this rapidly evolving field.
Market Analysis for mRNA Nanoparticle Therapeutics
The global mRNA therapeutics market has experienced unprecedented growth following the successful deployment of mRNA-based COVID-19 vaccines. Current market valuations indicate that the mRNA therapeutics sector reached approximately $40 billion in 2022, with projections suggesting a compound annual growth rate (CAGR) of 12.5% through 2030. This remarkable expansion is driven by both established pharmaceutical giants and emerging biotech companies investing heavily in mRNA nanoparticle delivery technologies.
The market segmentation reveals distinct therapeutic application areas beyond vaccines, including oncology, rare genetic disorders, and protein replacement therapies. Oncology represents the fastest-growing segment, with over 30% of mRNA clinical trials focusing on cancer immunotherapies. The ability of mRNA nanoparticles to deliver instructions for producing tumor-specific antigens has created significant commercial opportunities in personalized cancer treatments.
Regional analysis shows North America dominating the market with approximately 45% share, followed by Europe at 30% and Asia-Pacific at 20%. However, the Asia-Pacific region is expected to witness the highest growth rate over the next five years due to increasing healthcare expenditure and expanding research infrastructure in countries like China, Japan, and South Korea.
Key market drivers include technological advancements in lipid nanoparticle (LNP) formulations, which have dramatically improved mRNA stability and cellular uptake efficiency. The regulatory landscape has also evolved favorably, with accelerated approval pathways being established for mRNA therapeutics in major markets, reducing time-to-market by an estimated 30% compared to traditional drug development timelines.
Consumer and healthcare provider acceptance has significantly improved following the widespread use of mRNA COVID-19 vaccines, creating a more receptive market environment for future mRNA-based treatments. This shift in perception has translated to increased investment, with venture capital funding for mRNA startups exceeding $6 billion in 2022 alone.
Market challenges persist, primarily centered around manufacturing scalability, cold chain requirements, and pricing strategies. The cost of goods for mRNA therapeutics remains high, with production expenses approximately 2-3 times greater than traditional biologics. However, technological innovations in continuous manufacturing and room-temperature stable formulations are expected to address these limitations within the next 3-5 years.
The competitive landscape features established players like Moderna, BioNTech, and CureVac alongside pharmaceutical giants including Pfizer, Merck, and Sanofi who have made strategic acquisitions or partnerships to secure mRNA technology platforms. This market consolidation trend is expected to continue as companies position themselves to capitalize on the expanding therapeutic applications of mRNA nanoparticles.
The market segmentation reveals distinct therapeutic application areas beyond vaccines, including oncology, rare genetic disorders, and protein replacement therapies. Oncology represents the fastest-growing segment, with over 30% of mRNA clinical trials focusing on cancer immunotherapies. The ability of mRNA nanoparticles to deliver instructions for producing tumor-specific antigens has created significant commercial opportunities in personalized cancer treatments.
Regional analysis shows North America dominating the market with approximately 45% share, followed by Europe at 30% and Asia-Pacific at 20%. However, the Asia-Pacific region is expected to witness the highest growth rate over the next five years due to increasing healthcare expenditure and expanding research infrastructure in countries like China, Japan, and South Korea.
Key market drivers include technological advancements in lipid nanoparticle (LNP) formulations, which have dramatically improved mRNA stability and cellular uptake efficiency. The regulatory landscape has also evolved favorably, with accelerated approval pathways being established for mRNA therapeutics in major markets, reducing time-to-market by an estimated 30% compared to traditional drug development timelines.
Consumer and healthcare provider acceptance has significantly improved following the widespread use of mRNA COVID-19 vaccines, creating a more receptive market environment for future mRNA-based treatments. This shift in perception has translated to increased investment, with venture capital funding for mRNA startups exceeding $6 billion in 2022 alone.
Market challenges persist, primarily centered around manufacturing scalability, cold chain requirements, and pricing strategies. The cost of goods for mRNA therapeutics remains high, with production expenses approximately 2-3 times greater than traditional biologics. However, technological innovations in continuous manufacturing and room-temperature stable formulations are expected to address these limitations within the next 3-5 years.
The competitive landscape features established players like Moderna, BioNTech, and CureVac alongside pharmaceutical giants including Pfizer, Merck, and Sanofi who have made strategic acquisitions or partnerships to secure mRNA technology platforms. This market consolidation trend is expected to continue as companies position themselves to capitalize on the expanding therapeutic applications of mRNA nanoparticles.
Current Technical Challenges in mRNA Delivery Systems
Despite significant advancements in mRNA technology, particularly highlighted by COVID-19 vaccine development, several critical technical challenges persist in mRNA delivery systems that impede broader therapeutic applications. The foremost challenge remains the inherent instability of mRNA molecules, which are highly susceptible to enzymatic degradation by ubiquitous ribonucleases. This instability necessitates sophisticated delivery vehicles and storage conditions, significantly complicating manufacturing and distribution logistics.
Lipid nanoparticle (LNP) formulations, while currently the gold standard for mRNA delivery, face substantial hurdles in targeting specificity. Most LNPs predominantly accumulate in the liver, limiting their utility for treating conditions affecting other tissues and organs. Engineering LNPs with tissue-specific targeting capabilities without compromising their delivery efficiency represents a significant technical bottleneck.
Immunogenicity remains another persistent challenge, as both the mRNA molecule itself and delivery components can trigger innate immune responses. These responses not only reduce therapeutic efficacy but also potentially cause adverse effects. While chemical modifications to mRNA have partially addressed this issue, balancing immunogenicity reduction with maintained translational efficiency requires further optimization.
The endosomal escape mechanism presents a critical barrier to efficient cytosolic delivery. Following cellular uptake, a substantial portion of mRNA-containing nanoparticles become trapped in endosomes and are subsequently degraded in lysosomes. Current endosomal escape strategies demonstrate limited efficiency, with estimates suggesting less than 2% of internalized mRNA successfully reaches the cytosol.
Manufacturing scalability and reproducibility pose significant challenges for clinical translation. The production of GMP-grade mRNA and consistent nanoparticle formulation at commercial scale involves complex processes that are difficult to standardize. Batch-to-batch variability in physicochemical properties can significantly impact biological performance.
Cold chain requirements for mRNA formulations (often -70°C for unmodified mRNA products) create substantial logistical barriers, particularly in resource-limited settings. Developing thermostable formulations that maintain integrity at higher temperatures remains an unresolved technical challenge.
Regulatory frameworks for mRNA therapeutics are still evolving, creating uncertainty in development pathways. The complex nature of these products, combining biological and nanotechnology elements, necessitates novel analytical methods for characterization and quality control that meet regulatory standards while accurately predicting in vivo performance.
Lipid nanoparticle (LNP) formulations, while currently the gold standard for mRNA delivery, face substantial hurdles in targeting specificity. Most LNPs predominantly accumulate in the liver, limiting their utility for treating conditions affecting other tissues and organs. Engineering LNPs with tissue-specific targeting capabilities without compromising their delivery efficiency represents a significant technical bottleneck.
Immunogenicity remains another persistent challenge, as both the mRNA molecule itself and delivery components can trigger innate immune responses. These responses not only reduce therapeutic efficacy but also potentially cause adverse effects. While chemical modifications to mRNA have partially addressed this issue, balancing immunogenicity reduction with maintained translational efficiency requires further optimization.
The endosomal escape mechanism presents a critical barrier to efficient cytosolic delivery. Following cellular uptake, a substantial portion of mRNA-containing nanoparticles become trapped in endosomes and are subsequently degraded in lysosomes. Current endosomal escape strategies demonstrate limited efficiency, with estimates suggesting less than 2% of internalized mRNA successfully reaches the cytosol.
Manufacturing scalability and reproducibility pose significant challenges for clinical translation. The production of GMP-grade mRNA and consistent nanoparticle formulation at commercial scale involves complex processes that are difficult to standardize. Batch-to-batch variability in physicochemical properties can significantly impact biological performance.
Cold chain requirements for mRNA formulations (often -70°C for unmodified mRNA products) create substantial logistical barriers, particularly in resource-limited settings. Developing thermostable formulations that maintain integrity at higher temperatures remains an unresolved technical challenge.
Regulatory frameworks for mRNA therapeutics are still evolving, creating uncertainty in development pathways. The complex nature of these products, combining biological and nanotechnology elements, necessitates novel analytical methods for characterization and quality control that meet regulatory standards while accurately predicting in vivo performance.
Current mRNA Nanoparticle Delivery Platforms
01 Lipid nanoparticle formulations for mRNA delivery
Lipid nanoparticles (LNPs) serve as effective delivery vehicles for mRNA therapeutics. These formulations typically consist of ionizable lipids, helper lipids, cholesterol, and PEG-lipids that encapsulate and protect mRNA molecules. The composition and structure of these lipid components can be optimized to enhance cellular uptake, endosomal escape, and overall transfection efficiency. Advanced LNP formulations enable targeted delivery to specific tissues and improved stability of the encapsulated mRNA cargo.- Lipid nanoparticle formulations for mRNA delivery: Lipid nanoparticles (LNPs) serve as effective delivery vehicles for mRNA therapeutics. These formulations typically consist of ionizable lipids, helper lipids, cholesterol, and PEG-lipids that encapsulate and protect mRNA molecules. The composition and structure of these lipid components can be optimized to enhance cellular uptake, endosomal escape, and overall transfection efficiency. Advanced LNP formulations enable targeted delivery to specific tissues while minimizing off-target effects and immunogenicity.
- Polymer-based nanoparticles for mRNA delivery: Polymer-based nanoparticles represent an alternative approach to lipid systems for mRNA delivery. These formulations utilize biodegradable polymers such as PLGA, PEI, or chitosan derivatives that can complex with mRNA through electrostatic interactions. The polymer composition can be engineered to control release kinetics, improve stability, and enhance cellular uptake. These systems often incorporate additional functional groups to facilitate endosomal escape and targeted delivery to specific cell types or tissues.
- Hybrid and composite nanoparticle systems: Hybrid nanoparticle systems combine multiple delivery technologies to overcome limitations of single-component systems. These formulations may integrate lipids with polymers, incorporate inorganic components, or utilize protein-based elements to create multifunctional delivery vehicles. Such hybrid approaches can enhance mRNA stability, improve cellular uptake mechanisms, and provide precise control over release kinetics. These composite systems often demonstrate superior performance in overcoming biological barriers compared to conventional delivery systems.
- Surface modification strategies for targeted delivery: Surface modification of mRNA nanoparticles with targeting ligands enables cell-specific delivery and reduces off-target effects. These modifications include conjugation with antibodies, aptamers, peptides, or small molecules that recognize specific receptors on target cells. Additionally, surface engineering can incorporate stealth properties through PEGylation or other hydrophilic coatings to extend circulation time and reduce immunogenicity. These targeting strategies significantly improve therapeutic efficacy while minimizing potential side effects.
- Manufacturing and stability enhancement techniques: Advanced manufacturing processes and formulation techniques are critical for producing consistent, stable mRNA nanoparticles. These include microfluidic mixing, controlled precipitation methods, and freeze-drying technologies that preserve mRNA integrity. Formulation additives such as cryoprotectants, antioxidants, and pH stabilizers can significantly extend shelf-life and maintain activity during storage. Novel encapsulation methods and process controls ensure batch-to-batch consistency and scalability for commercial production of mRNA therapeutics.
02 Polymer-based nanoparticles for mRNA delivery
Polymer-based nanoparticles represent an alternative approach to lipid systems for mRNA delivery. These formulations utilize biodegradable polymers such as PLGA, PEI, or chitosan derivatives that can complex with mRNA through electrostatic interactions. The polymer composition can be modified to control release kinetics, improve cellular uptake, and reduce cytotoxicity. These systems offer advantages including enhanced stability, controlled degradation profiles, and versatility in surface modification for targeted delivery applications.Expand Specific Solutions03 Hybrid and composite nanoparticle systems for mRNA delivery
Hybrid nanoparticle systems combine multiple materials to leverage the advantages of different carrier types. These formulations may integrate lipids with polymers, incorporate inorganic components, or utilize protein-based elements to create composite structures with enhanced functionality. Such hybrid approaches can improve stability, cellular uptake, endosomal escape, and targeting specificity of mRNA therapeutics while potentially reducing toxicity concerns associated with individual carrier types.Expand Specific Solutions04 Surface modification strategies for mRNA nanoparticles
Surface engineering of mRNA nanoparticles enables targeted delivery to specific tissues or cell types. These modifications include conjugation with targeting ligands, antibodies, peptides, or aptamers that recognize specific cellular receptors. Additionally, surface modifications can enhance circulation time, improve stability in biological fluids, reduce immunogenicity, and facilitate crossing of biological barriers. PEGylation and other stealth coating approaches are commonly employed to prevent rapid clearance and improve the pharmacokinetic profile of mRNA nanoparticles.Expand Specific Solutions05 Manufacturing and characterization techniques for mRNA nanoparticles
Advanced manufacturing processes for mRNA nanoparticles include microfluidic mixing, nanoprecipitation, and extrusion techniques that enable precise control over particle size, polydispersity, and encapsulation efficiency. Quality control methods involve comprehensive characterization of physical properties (size, zeta potential, morphology), chemical composition, and biological functionality. Analytical techniques such as dynamic light scattering, electron microscopy, and various spectroscopic methods are employed to ensure batch-to-batch consistency and stability during storage and administration.Expand Specific Solutions
Key Industry Players in mRNA Nanoparticle Development
The mRNA nanoparticle field is currently in a growth phase, with the global market expanding rapidly following COVID-19 vaccine breakthroughs. The competitive landscape features established research institutions (CNRS, Max Planck Society) alongside pharmaceutical companies developing commercial applications. Leading companies like CureVac, Ionis Pharmaceuticals, and Novartis are advancing mRNA delivery technologies, while academic players (Duke University, Zhejiang University) contribute fundamental research. The technology maturity varies across applications - therapeutic delivery systems are advancing toward clinical implementation, while regulatory RNA interactions remain in earlier research stages. Companies like Avidity Biosciences and Regulus Therapeutics are specifically focusing on RNA-regulatory mechanisms, indicating growing commercial interest in this specialized segment of the broader mRNA field.
Ionis Pharmaceuticals, Inc.
Technical Solution: Ionis Pharmaceuticals has developed a comprehensive platform for analyzing and manipulating mRNA regulatory interactions through their antisense oligonucleotide (ASO) technology. While not focused on traditional nanoparticle delivery, their approach represents a sophisticated alternative for modulating mRNA function. Ionis' proprietary antisense technology uses chemically modified oligonucleotides that bind to target mRNAs with high specificity, allowing precise control over gene expression. Their Generation 2.5 and Generation 3 ASOs incorporate advanced chemical modifications including constrained ethyl (cEt) and locked nucleic acid (LNA) designs that enhance target binding affinity while improving nuclease resistance. Ionis has developed sophisticated computational tools to predict RNA secondary structures and identify optimal binding sites for their ASOs, enabling precise targeting of specific regulatory regions within mRNAs. Their platform includes conjugation strategies with various moieties (including GalNAc for liver targeting) that enhance cellular uptake without requiring complex nanoparticle formulations. Ionis has extensively characterized the interactions between their ASOs and various RNA-binding proteins, providing insights into the regulatory networks that control mRNA stability and translation.
Strengths: Highly specific targeting of individual mRNAs; well-established safety profile with multiple FDA-approved drugs; simplified manufacturing compared to nanoparticle formulations. Weaknesses: Limited delivery to certain tissues without additional targeting strategies; inability to replace or supplement proteins (unlike mRNA therapeutics); potential for off-target binding effects.
Regulus Therapeutics, Inc.
Technical Solution: Regulus Therapeutics has developed a specialized platform focused on microRNA (miRNA) therapeutics that directly addresses the regulatory interactions between miRNAs and their target mRNAs. Their technology centers on chemically modified oligonucleotides designed to either inhibit miRNAs (anti-miRs) or mimic miRNA function, thereby modulating entire networks of gene expression. Regulus has pioneered advanced chemical modifications including 2'-O-methoxyethyl (2'-MOE), 2'-fluoro, and phosphorothioate backbones that enhance stability and pharmacokinetic properties of their compounds. Their platform includes sophisticated bioinformatic tools for identifying miRNA-mRNA interaction networks and predicting the consequences of miRNA modulation across multiple pathways. Regulus has developed proprietary delivery approaches including conjugation strategies and targeted nanoparticle formulations that enhance delivery to specific tissues. Their research has characterized complex regulatory interactions between miRNAs and their target mRNAs, providing insights into how these interactions influence mRNA stability, localization, and translation efficiency. Regulus has demonstrated that modulating a single miRNA can affect hundreds of target mRNAs, offering a unique approach to addressing complex diseases with dysregulated gene expression networks.
Strengths: Ability to modulate entire networks of gene expression with a single agent; extensive understanding of miRNA-mRNA regulatory interactions; potential for addressing complex diseases with multifactorial causes. Weaknesses: Challenges with predicting all consequences of miRNA modulation; potential for off-target effects due to the broad regulatory role of miRNAs; delivery challenges to certain tissues.
Critical Patents and Innovations in mRNA Encapsulation
Nanoparticles comprising RNA ligands
PatentInactiveEP2330208A1
Innovation
- Nanoparticles with cores made of metal and/or semiconductor atoms covalently linked to RNA ligands, such as siRNA and miRNA, which are designed to mimic short interfering RNA sequences, enabling targeted gene silencing, mRNA degradation, and imaging, with optional additional ligands for enhanced targeting and stability.
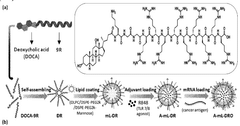
Nanoparticle comprising cell-penetrating peptides conjugated with deoxycholic acid and use thereof
PatentWO2025159411A1
Innovation
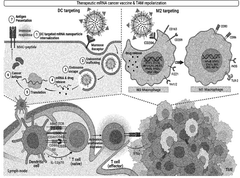
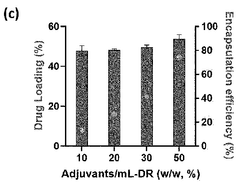
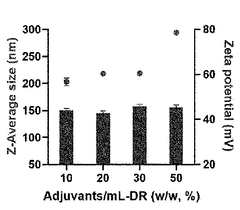
- A nanoparticle comprising a deoxycholic acid-linked cell-penetrating peptide assembly coated with mannose-linked lipids, loaded with mRNA and an adjuvant, specifically designed to target dendritic cells and tumor-associated macrophages, enhancing antigen-specific immune responses and repolarizing macrophages.
Regulatory Framework for mRNA-Based Therapeutics
The regulatory landscape for mRNA-based therapeutics has evolved significantly in response to the rapid advancement of this technology, particularly accelerated by the COVID-19 pandemic. Regulatory bodies worldwide, including the FDA, EMA, and NMPA, have established frameworks that address the unique characteristics of mRNA therapeutics while ensuring safety and efficacy standards are maintained.
Current regulatory approaches for mRNA nanoparticle therapeutics focus on several key areas: quality control of the mRNA sequence and structure, characterization of lipid nanoparticle delivery systems, stability assessment, and comprehensive safety evaluation. These frameworks typically require manufacturers to demonstrate consistent production processes, with particular attention to potential contaminants such as residual DNA templates or aberrant protein products that could trigger immunogenicity concerns.
The FDA's approach employs a risk-based assessment methodology, evaluating mRNA therapeutics through both the traditional IND (Investigational New Drug) pathway and expedited programs when applicable. Similarly, the EMA has developed specific guidelines addressing advanced therapy medicinal products (ATMPs), which include considerations for mRNA-based interventions. These guidelines emphasize characterization of the delivery system and the need for specialized stability studies reflecting the unique degradation pathways of mRNA products.
Regulatory challenges specific to mRNA nanoparticle interactions include standardization of analytical methods for characterizing lipid-mRNA complexes, establishing appropriate reference standards, and developing predictive models for biodistribution and pharmacokinetics. The dynamic nature of these nanoparticle systems presents unique challenges for traditional regulatory paradigms designed for small molecules or conventional biologics.
International harmonization efforts are underway through initiatives like the International Council for Harmonisation (ICH) to develop consistent global standards for mRNA therapeutics. These efforts aim to address variations in regulatory requirements across different jurisdictions that currently create challenges for global development programs.
Emerging regulatory considerations include the development of frameworks for personalized mRNA therapeutics, requirements for comparative studies when multiple similar products enter the market, and evolving standards for manufacturing changes as technology advances. Regulatory agencies are increasingly adopting adaptive licensing approaches that allow for iterative assessment and approval as additional data becomes available, particularly relevant for novel therapeutic modalities like mRNA.
Current regulatory approaches for mRNA nanoparticle therapeutics focus on several key areas: quality control of the mRNA sequence and structure, characterization of lipid nanoparticle delivery systems, stability assessment, and comprehensive safety evaluation. These frameworks typically require manufacturers to demonstrate consistent production processes, with particular attention to potential contaminants such as residual DNA templates or aberrant protein products that could trigger immunogenicity concerns.
The FDA's approach employs a risk-based assessment methodology, evaluating mRNA therapeutics through both the traditional IND (Investigational New Drug) pathway and expedited programs when applicable. Similarly, the EMA has developed specific guidelines addressing advanced therapy medicinal products (ATMPs), which include considerations for mRNA-based interventions. These guidelines emphasize characterization of the delivery system and the need for specialized stability studies reflecting the unique degradation pathways of mRNA products.
Regulatory challenges specific to mRNA nanoparticle interactions include standardization of analytical methods for characterizing lipid-mRNA complexes, establishing appropriate reference standards, and developing predictive models for biodistribution and pharmacokinetics. The dynamic nature of these nanoparticle systems presents unique challenges for traditional regulatory paradigms designed for small molecules or conventional biologics.
International harmonization efforts are underway through initiatives like the International Council for Harmonisation (ICH) to develop consistent global standards for mRNA therapeutics. These efforts aim to address variations in regulatory requirements across different jurisdictions that currently create challenges for global development programs.
Emerging regulatory considerations include the development of frameworks for personalized mRNA therapeutics, requirements for comparative studies when multiple similar products enter the market, and evolving standards for manufacturing changes as technology advances. Regulatory agencies are increasingly adopting adaptive licensing approaches that allow for iterative assessment and approval as additional data becomes available, particularly relevant for novel therapeutic modalities like mRNA.
Safety and Immunogenicity Considerations
The safety profile and immunogenicity of mRNA nanoparticles represent critical considerations in their development and regulatory approval pathway. Current evidence indicates that lipid nanoparticles (LNPs), the primary delivery vehicle for mRNA therapeutics, can trigger innate immune responses through pattern recognition receptors, particularly Toll-like receptors (TLRs) and retinoic acid-inducible gene I (RIG-I)-like receptors. These interactions may lead to the production of type I interferons and proinflammatory cytokines, which while beneficial for vaccine applications, could pose challenges for non-immunogenic therapeutic applications.
Recent advancements in LNP formulation have focused on modifying the lipid composition to mitigate unwanted immunostimulatory effects. Specifically, the incorporation of ionizable lipids with optimized pKa values has demonstrated reduced immunogenicity while maintaining efficient cellular uptake and endosomal escape. Additionally, PEGylation strategies have been employed to extend circulation time and reduce recognition by the mononuclear phagocyte system, though PEG-specific antibodies have been reported in some cases.
The biodistribution and clearance kinetics of mRNA nanoparticles significantly impact their safety profile. Studies indicate that LNPs predominantly accumulate in the liver, spleen, and to a lesser extent, other organs following systemic administration. The lipid components typically undergo metabolism through endogenous pathways, while the mRNA payload is degraded by cellular nucleases. However, the long-term effects of repeated administration require further investigation, particularly regarding potential cumulative toxicity.
Hypersensitivity reactions represent another safety concern, with reports of anaphylactic responses to certain LNP components, notably PEGylated lipids. Regulatory agencies now recommend careful monitoring and risk mitigation strategies during clinical development. Furthermore, the potential for off-target effects resulting from unintended protein expression in non-target tissues necessitates comprehensive biodistribution studies and tissue-specific delivery approaches.
From a regulatory perspective, the FDA, EMA, and other global authorities have established frameworks for evaluating the safety of mRNA therapeutics, emphasizing the need for thorough characterization of both the mRNA construct and delivery system. These include assessments of genotoxicity, reproductive toxicity, and carcinogenicity potential, though modified risk assessment approaches may apply given the unique properties of mRNA compared to traditional small molecule drugs or biologics.
The COVID-19 pandemic has accelerated the development of regulatory guidance specific to mRNA therapeutics, with emerging consensus on appropriate preclinical safety evaluation packages. However, harmonization of international standards remains an ongoing challenge, particularly regarding novel analytical methods for characterizing mRNA-LNP complexes and their immunological properties.
Recent advancements in LNP formulation have focused on modifying the lipid composition to mitigate unwanted immunostimulatory effects. Specifically, the incorporation of ionizable lipids with optimized pKa values has demonstrated reduced immunogenicity while maintaining efficient cellular uptake and endosomal escape. Additionally, PEGylation strategies have been employed to extend circulation time and reduce recognition by the mononuclear phagocyte system, though PEG-specific antibodies have been reported in some cases.
The biodistribution and clearance kinetics of mRNA nanoparticles significantly impact their safety profile. Studies indicate that LNPs predominantly accumulate in the liver, spleen, and to a lesser extent, other organs following systemic administration. The lipid components typically undergo metabolism through endogenous pathways, while the mRNA payload is degraded by cellular nucleases. However, the long-term effects of repeated administration require further investigation, particularly regarding potential cumulative toxicity.
Hypersensitivity reactions represent another safety concern, with reports of anaphylactic responses to certain LNP components, notably PEGylated lipids. Regulatory agencies now recommend careful monitoring and risk mitigation strategies during clinical development. Furthermore, the potential for off-target effects resulting from unintended protein expression in non-target tissues necessitates comprehensive biodistribution studies and tissue-specific delivery approaches.
From a regulatory perspective, the FDA, EMA, and other global authorities have established frameworks for evaluating the safety of mRNA therapeutics, emphasizing the need for thorough characterization of both the mRNA construct and delivery system. These include assessments of genotoxicity, reproductive toxicity, and carcinogenicity potential, though modified risk assessment approaches may apply given the unique properties of mRNA compared to traditional small molecule drugs or biologics.
The COVID-19 pandemic has accelerated the development of regulatory guidance specific to mRNA therapeutics, with emerging consensus on appropriate preclinical safety evaluation packages. However, harmonization of international standards remains an ongoing challenge, particularly regarding novel analytical methods for characterizing mRNA-LNP complexes and their immunological properties.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!