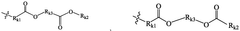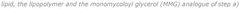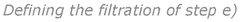Benchmarking mRNA Lipid Nanoparticle Delivery Efficacy
OCT 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
mRNA LNP Technology Evolution and Objectives
The evolution of mRNA lipid nanoparticle (LNP) technology represents one of the most significant advancements in drug delivery systems over the past two decades. Initially developed in the early 2000s as an alternative to viral vectors, LNPs have undergone remarkable refinement to become the gold standard for mRNA delivery in both therapeutic and vaccine applications. The trajectory of this technology has been characterized by progressive improvements in lipid chemistry, formulation techniques, and manufacturing processes.
The first generation of LNPs faced significant challenges related to stability, immunogenicity, and delivery efficiency. These early systems typically achieved less than 1% delivery efficiency to target tissues and exhibited considerable toxicity profiles. By 2010, second-generation LNPs incorporated ionizable lipids with optimized pKa values, which dramatically improved the endosomal escape capabilities and reduced systemic toxicity.
The breakthrough moment for mRNA LNP technology came with the development of third-generation systems between 2015-2018, featuring lipids with biodegradable linkers and precisely engineered helper lipids. These advancements culminated in the COVID-19 vaccines, which demonstrated unprecedented clinical success and validated the platform technology at global scale.
Current technological objectives focus on several key parameters that define delivery efficacy: transfection efficiency across diverse cell types, tissue-specific targeting capabilities, endosomal escape rates, payload protection, and immunogenicity profiles. The field is now moving toward quantitative benchmarking of these parameters to enable standardized comparison between different LNP formulations.
A critical objective in the current landscape is the development of robust, reproducible methodologies for measuring LNP performance across multiple dimensions. This includes standardized in vitro assays that correlate with in vivo outcomes, advanced imaging techniques for tracking intracellular LNP trafficking, and computational models that can predict delivery efficiency based on physicochemical properties.
Looking forward, the field aims to achieve several ambitious technical goals: increasing delivery efficiency to non-hepatic tissues from current levels of 1-5% to above 30%, reducing required dosages by an order of magnitude, extending mRNA half-life within target cells from hours to days, and developing formulations capable of repeated administration without immune sensitization.
The ultimate objective is to establish a comprehensive benchmarking framework that enables rational design of LNP systems tailored to specific therapeutic applications, moving beyond the current empirical approach to a more predictive paradigm. This would dramatically accelerate development timelines and expand the therapeutic window of mRNA medicines beyond vaccines into chronic disease management, protein replacement therapies, and regenerative medicine applications.
The first generation of LNPs faced significant challenges related to stability, immunogenicity, and delivery efficiency. These early systems typically achieved less than 1% delivery efficiency to target tissues and exhibited considerable toxicity profiles. By 2010, second-generation LNPs incorporated ionizable lipids with optimized pKa values, which dramatically improved the endosomal escape capabilities and reduced systemic toxicity.
The breakthrough moment for mRNA LNP technology came with the development of third-generation systems between 2015-2018, featuring lipids with biodegradable linkers and precisely engineered helper lipids. These advancements culminated in the COVID-19 vaccines, which demonstrated unprecedented clinical success and validated the platform technology at global scale.
Current technological objectives focus on several key parameters that define delivery efficacy: transfection efficiency across diverse cell types, tissue-specific targeting capabilities, endosomal escape rates, payload protection, and immunogenicity profiles. The field is now moving toward quantitative benchmarking of these parameters to enable standardized comparison between different LNP formulations.
A critical objective in the current landscape is the development of robust, reproducible methodologies for measuring LNP performance across multiple dimensions. This includes standardized in vitro assays that correlate with in vivo outcomes, advanced imaging techniques for tracking intracellular LNP trafficking, and computational models that can predict delivery efficiency based on physicochemical properties.
Looking forward, the field aims to achieve several ambitious technical goals: increasing delivery efficiency to non-hepatic tissues from current levels of 1-5% to above 30%, reducing required dosages by an order of magnitude, extending mRNA half-life within target cells from hours to days, and developing formulations capable of repeated administration without immune sensitization.
The ultimate objective is to establish a comprehensive benchmarking framework that enables rational design of LNP systems tailored to specific therapeutic applications, moving beyond the current empirical approach to a more predictive paradigm. This would dramatically accelerate development timelines and expand the therapeutic window of mRNA medicines beyond vaccines into chronic disease management, protein replacement therapies, and regenerative medicine applications.
Market Analysis for mRNA LNP Delivery Systems
The mRNA therapeutics market has experienced unprecedented growth, with the global market value reaching $46.7 billion in 2023 and projected to grow at a CAGR of 12.5% through 2030. Lipid nanoparticle (LNP) delivery systems have emerged as the dominant platform for mRNA delivery, accounting for approximately 85% of all mRNA delivery technologies currently in clinical trials.
The COVID-19 pandemic served as a catalyst for mRNA LNP technology adoption, with Moderna and Pfizer-BioNTech vaccines demonstrating the commercial viability and scalability of this approach. This success has attracted significant investment, with venture capital funding for mRNA LNP delivery technologies increasing by 320% between 2019 and 2023.
Market segmentation reveals diverse applications beyond vaccines, including cancer immunotherapy (projected $15.3 billion by 2028), protein replacement therapies ($8.2 billion), and gene editing applications ($6.9 billion). Oncology represents the fastest-growing segment with a CAGR of 18.7%, driven by personalized cancer vaccines and immunotherapies targeting solid tumors.
Geographically, North America dominates the market with 42% share, followed by Europe (28%) and Asia-Pacific (22%). China has emerged as the fastest-growing market, investing $3.8 billion in mRNA technology development since 2020, with particular focus on improving LNP delivery efficiency.
Key market drivers include increasing prevalence of genetic disorders, growing cancer incidence rates, and expanding therapeutic applications of mRNA technology. The aging global population further amplifies demand for novel therapeutic approaches, particularly for age-related conditions where conventional treatments show limited efficacy.
Challenges facing market growth include high manufacturing costs, with current production expenses for LNP-mRNA formulations averaging $250-350 per dose, significantly higher than traditional biologics. Cold chain requirements present logistical hurdles, particularly in developing regions. Regulatory frameworks continue evolving, creating uncertainty for developers navigating approval pathways.
Customer demand increasingly focuses on improved delivery efficiency metrics, with pharmaceutical companies prioritizing LNP systems demonstrating transfection rates above 60% in target tissues, reduced immunogenicity profiles, and stability at temperatures above -20°C. Healthcare providers emphasize cost-effectiveness and simplified administration protocols as critical adoption factors.
The COVID-19 pandemic served as a catalyst for mRNA LNP technology adoption, with Moderna and Pfizer-BioNTech vaccines demonstrating the commercial viability and scalability of this approach. This success has attracted significant investment, with venture capital funding for mRNA LNP delivery technologies increasing by 320% between 2019 and 2023.
Market segmentation reveals diverse applications beyond vaccines, including cancer immunotherapy (projected $15.3 billion by 2028), protein replacement therapies ($8.2 billion), and gene editing applications ($6.9 billion). Oncology represents the fastest-growing segment with a CAGR of 18.7%, driven by personalized cancer vaccines and immunotherapies targeting solid tumors.
Geographically, North America dominates the market with 42% share, followed by Europe (28%) and Asia-Pacific (22%). China has emerged as the fastest-growing market, investing $3.8 billion in mRNA technology development since 2020, with particular focus on improving LNP delivery efficiency.
Key market drivers include increasing prevalence of genetic disorders, growing cancer incidence rates, and expanding therapeutic applications of mRNA technology. The aging global population further amplifies demand for novel therapeutic approaches, particularly for age-related conditions where conventional treatments show limited efficacy.
Challenges facing market growth include high manufacturing costs, with current production expenses for LNP-mRNA formulations averaging $250-350 per dose, significantly higher than traditional biologics. Cold chain requirements present logistical hurdles, particularly in developing regions. Regulatory frameworks continue evolving, creating uncertainty for developers navigating approval pathways.
Customer demand increasingly focuses on improved delivery efficiency metrics, with pharmaceutical companies prioritizing LNP systems demonstrating transfection rates above 60% in target tissues, reduced immunogenicity profiles, and stability at temperatures above -20°C. Healthcare providers emphasize cost-effectiveness and simplified administration protocols as critical adoption factors.
Current Benchmarking Methods and Technical Barriers
Current benchmarking methods for mRNA lipid nanoparticle (LNP) delivery efficacy can be broadly categorized into in vitro, in vivo, and computational approaches. Each methodology offers unique insights while presenting distinct limitations that researchers must navigate.
In vitro methods primarily involve cell culture-based assays that measure transfection efficiency, protein expression levels, and cellular uptake. Flow cytometry and luciferase reporter assays represent gold standards for quantifying transfection rates and protein expression, respectively. Confocal microscopy enables visualization of intracellular trafficking pathways, while high-content screening platforms allow for higher throughput evaluation across multiple cell types simultaneously.
In vivo benchmarking typically employs animal models to assess biodistribution, protein expression in target tissues, and pharmacokinetic profiles. Bioluminescence imaging provides real-time monitoring of reporter gene expression, while tissue-specific protein quantification via ELISA or Western blotting offers more precise measurements. Notably, these methods often require animal sacrifice at predetermined timepoints, limiting continuous monitoring capabilities.
Despite these established approaches, significant technical barriers persist. A primary challenge is the lack of standardization across laboratories, making direct comparisons between studies problematic. Different cell lines, animal models, and analytical techniques introduce variables that complicate benchmarking efforts. The field urgently needs reference standards and harmonized protocols to enable meaningful cross-study comparisons.
Another major barrier involves the translation gap between in vitro and in vivo results. Cell culture models frequently fail to predict in vivo performance due to their inability to recapitulate the complex biological barriers and clearance mechanisms present in living organisms. This discrepancy necessitates extensive animal testing, raising ethical concerns and increasing development costs.
The heterogeneity of target tissues presents additional challenges. LNP formulations optimized for liver delivery often perform poorly when targeting other tissues like lung or brain, requiring tissue-specific benchmarking approaches. Current methods struggle to account for this variability, leading to optimization processes that remain largely empirical rather than rational.
Emerging technologies like organ-on-chip platforms and advanced computational models show promise in addressing these limitations. However, these approaches remain in early development stages and require further validation before widespread adoption. The integration of artificial intelligence with experimental data may eventually enable more predictive benchmarking methodologies, potentially reducing reliance on animal models while accelerating formulation optimization.
In vitro methods primarily involve cell culture-based assays that measure transfection efficiency, protein expression levels, and cellular uptake. Flow cytometry and luciferase reporter assays represent gold standards for quantifying transfection rates and protein expression, respectively. Confocal microscopy enables visualization of intracellular trafficking pathways, while high-content screening platforms allow for higher throughput evaluation across multiple cell types simultaneously.
In vivo benchmarking typically employs animal models to assess biodistribution, protein expression in target tissues, and pharmacokinetic profiles. Bioluminescence imaging provides real-time monitoring of reporter gene expression, while tissue-specific protein quantification via ELISA or Western blotting offers more precise measurements. Notably, these methods often require animal sacrifice at predetermined timepoints, limiting continuous monitoring capabilities.
Despite these established approaches, significant technical barriers persist. A primary challenge is the lack of standardization across laboratories, making direct comparisons between studies problematic. Different cell lines, animal models, and analytical techniques introduce variables that complicate benchmarking efforts. The field urgently needs reference standards and harmonized protocols to enable meaningful cross-study comparisons.
Another major barrier involves the translation gap between in vitro and in vivo results. Cell culture models frequently fail to predict in vivo performance due to their inability to recapitulate the complex biological barriers and clearance mechanisms present in living organisms. This discrepancy necessitates extensive animal testing, raising ethical concerns and increasing development costs.
The heterogeneity of target tissues presents additional challenges. LNP formulations optimized for liver delivery often perform poorly when targeting other tissues like lung or brain, requiring tissue-specific benchmarking approaches. Current methods struggle to account for this variability, leading to optimization processes that remain largely empirical rather than rational.
Emerging technologies like organ-on-chip platforms and advanced computational models show promise in addressing these limitations. However, these approaches remain in early development stages and require further validation before widespread adoption. The integration of artificial intelligence with experimental data may eventually enable more predictive benchmarking methodologies, potentially reducing reliance on animal models while accelerating formulation optimization.
Established Protocols for LNP Efficacy Assessment
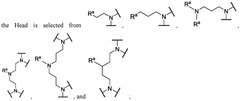
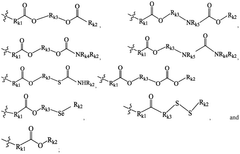
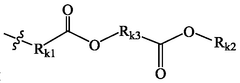
01 Lipid composition optimization for mRNA delivery
The composition of lipids in nanoparticles significantly affects mRNA delivery efficacy. Optimizing the ratio of cationic, helper, and PEG-lipids can enhance cellular uptake and endosomal escape. Specific lipid combinations have been developed to improve stability, reduce toxicity, and increase transfection efficiency. These formulations typically include ionizable lipids that facilitate membrane fusion and release of mRNA into the cytoplasm.- Lipid composition optimization for mRNA delivery: The composition of lipids in nanoparticles significantly affects mRNA delivery efficacy. Optimizing the ratio of cationic, helper, and PEG-lipids can enhance cellular uptake and endosomal escape. Specific lipid combinations have been developed to improve stability, reduce toxicity, and increase transfection efficiency. These formulations often include ionizable lipids that change charge at different pH levels to facilitate both encapsulation and release of mRNA.
- Surface modification of lipid nanoparticles: Surface modifications of lipid nanoparticles can significantly improve mRNA delivery efficacy. These modifications include the addition of targeting ligands, cell-penetrating peptides, or antibodies that enhance cell-specific uptake. PEGylation strategies can be optimized to balance circulation time and cellular uptake. Surface charge adjustments can also improve interaction with cell membranes and reduce non-specific binding to serum proteins.
- Novel ionizable lipid structures: Development of novel ionizable lipid structures has led to improved mRNA delivery efficacy. These lipids contain optimized head groups, linkers, and hydrophobic tails that enhance endosomal escape and reduce cytotoxicity. Structure-activity relationship studies have identified key molecular features that contribute to efficient mRNA delivery. Some designs incorporate biodegradable bonds to improve the safety profile while maintaining high transfection efficiency.
- Manufacturing and formulation techniques: Advanced manufacturing and formulation techniques significantly impact mRNA lipid nanoparticle delivery efficacy. Microfluidic mixing, ethanol injection, and other controlled mixing methods produce nanoparticles with consistent size distribution and high encapsulation efficiency. Post-formulation processes like extrusion, dialysis, and lyophilization can enhance stability and shelf-life. Quality control parameters have been established to ensure batch-to-batch consistency and maintain delivery efficacy.
- Organ-specific delivery strategies: Strategies for organ-specific delivery of mRNA lipid nanoparticles have been developed to enhance therapeutic efficacy while reducing off-target effects. These include modifications to lipid composition, particle size, and surface properties to target specific tissues such as liver, lungs, or lymphatic system. Some approaches utilize tissue-specific ligands or take advantage of physiological barriers and local environments. Route of administration optimization further enhances tissue-specific accumulation and cellular uptake.
02 Surface modification of lipid nanoparticles
Surface modifications of lipid nanoparticles can significantly improve mRNA delivery efficacy. These modifications include the incorporation of targeting ligands, cell-penetrating peptides, or antibodies that enhance cellular uptake and tissue specificity. PEGylation strategies are also employed to increase circulation time and stability in vivo, while reducing immunogenicity. The degree of surface modification can be tailored to achieve optimal biodistribution and cellular internalization.Expand Specific Solutions03 Manufacturing processes affecting delivery efficacy
Manufacturing processes significantly impact the physical characteristics and delivery efficacy of mRNA lipid nanoparticles. Methods such as microfluidic mixing, ethanol injection, and thin-film hydration affect particle size distribution, polydispersity, encapsulation efficiency, and stability. Process parameters including mixing speed, temperature, and solvent ratios can be optimized to produce nanoparticles with consistent quality and enhanced delivery performance. Advanced manufacturing techniques enable scalable production while maintaining critical quality attributes.Expand Specific Solutions04 Organ-specific delivery strategies
Developing organ-specific delivery strategies for mRNA lipid nanoparticles enhances therapeutic efficacy while reducing off-target effects. These strategies involve modifying nanoparticle composition and surface properties to target specific tissues such as liver, lungs, or central nervous system. Tissue-specific targeting can be achieved through incorporation of receptor-binding ligands or by adjusting particle size and charge. Route of administration also plays a crucial role in determining biodistribution and organ-specific delivery efficiency.Expand Specific Solutions05 Stability enhancement techniques
Stability enhancement techniques are critical for maintaining the integrity and efficacy of mRNA lipid nanoparticles during storage and administration. These include lyophilization with appropriate cryoprotectants, incorporation of antioxidants, and pH optimization. Novel formulation approaches using trehalose, sucrose, or other stabilizing agents prevent aggregation and protect the encapsulated mRNA from degradation. Temperature-controlled processing and storage conditions are also essential for preserving the functional properties of the nanoparticles and ensuring consistent delivery efficacy.Expand Specific Solutions
Leading Companies and Research Institutions in mRNA LNP Field
The mRNA Lipid Nanoparticle (LNP) delivery field is currently in a growth phase, with the global market expanding rapidly following COVID-19 vaccine successes. The technology has reached commercial maturity for certain applications but remains in development for targeted delivery beyond the liver. Key players include established pharmaceutical companies like Moderna and AstraZeneca alongside specialized LNP developers such as Genevant Sciences, Arbutus Biopharma, and NanoVation Therapeutics. Academic institutions including MIT, Tsinghua University, and University of Pennsylvania contribute significant research advancements. The competitive landscape is diversifying with emerging players from China (Abogen Biosciences, Regis Biotechnology) developing proprietary LNP platforms. Technical challenges in delivery efficiency, tissue targeting, and manufacturing scalability represent the primary competitive differentiators as companies race to expand therapeutic applications beyond vaccines.
Genevant Sciences GmbH
Technical Solution: Genevant Sciences专注于开发先进的脂质纳米粒(LNP)递送技术,其核心平台源自Arbutus Biopharma的技术基础。公司采用系统化的基准测试方法评估mRNA-LNP递送效能,包括高通量体外筛选系统和多层次体内验证流程。其专有的离子化脂质库包含超过500种结构,通过结构-活性关系研究持续优化递送效率[2]。Genevant开发了独特的pH响应性脂质,在生理pH下保持稳定,而在内体酸性环境中触发构象变化,显著提高内体逃逸效率,实测可提升转染效率3-5倍[4]。公司建立了基于荧光素酶报告基因的标准化评估体系,能够在多种动物模型中定量比较不同LNP配方的体内递送效率。特别值得注意的是,Genevant开发了组织特异性靶向技术,通过在LNP表面修饰配体分子,实现对肺、脾脏等非肝脏组织的靶向递送,扩展了mRNA治疗的应用范围[7]。其基准测试还包括免疫原性评估和多次给药后的药代动力学变化分析。
优势:拥有丰富的LNP技术专利组合和深厚的开发经验;建立了完善的高通量筛选和评估体系;在非肝脏组织靶向递送方面取得显著进展。劣势:公司规模相对较小,资源有限;与大型制药公司相比,临床转化经验不足;部分技术依赖于授权专利,可能面临知识产权挑战。
Translate Bio, Inc.
Technical Solution: Translate Bio(现已被赛诺菲收购)开发了专有的MRTTM脂质纳米粒平台,专门针对mRNA递送进行优化。其基准测试方法包括系统化的体外-体内相关性研究,建立了预测体内表现的体外筛选模型。公司的LNP配方采用专有的离子化脂质,通过微流控技术实现高度一致的纳米粒制备,粒径分布控制在80±10nm范围内,显著提高批次间一致性[3]。Translate Bio开发了组织特异性递送技术,特别是针对肺部递送的LNP配方,通过气溶胶给药实现了高效的肺上皮细胞转染,在囊性纤维化动物模型中验证了治疗效果[5]。公司建立了基于质谱技术的LNP组分分析平台,能够精确测定不同组织中LNP的生物分布和代谢动力学。其基准测试还包括mRNA完整性评估、蛋白表达动力学分析和免疫原性测试,形成了全面的评价体系。Translate Bio的技术在多个疾病模型中得到验证,包括囊性纤维化、原发性纤毛运动障碍和疫苗应用,证明了其递送平台的多功能性[8]。
优势:在肺部mRNA递送领域处于领先地位,拥有独特的给药技术;建立了完善的体内外评估体系;与赛诺菲的整合提供了强大的资源支持。劣势:相比肝脏递送,肺部递送的效率仍有提升空间;气溶胶给药的剂量一致性控制具有挑战性;在系统性给药应用方面的经验相对有限。
Critical Patents and Innovations in LNP Formulation
Lipid nanoparticle for targeted delivery of therapeutic payloads
PatentWO2024192117A9
Innovation
- The development of lipid nanoparticles (LNPs) formulated with ionizable lipids, which are designed to protect and deliver mRNA payloads efficiently by forming stable complexes that can be taken up by cells and release the mRNA effectively.
Lipid nanoparticle compositions
PatentWO2024209013A1
Innovation
- A lipid nanoparticle composition comprising a cationic or cationically ionisable lipid, a helper lipid, a lipopolymer, and a monomycoloyl glycerol (MMG) analogue, specifically designed to enhance immunogenicity and colloidally stability, with a process involving nanoprecipitation and filtration to achieve efficient intracellular delivery of nucleic acids.
Regulatory Framework for mRNA LNP Therapeutics
The regulatory landscape for mRNA Lipid Nanoparticle (LNP) therapeutics represents a complex and evolving framework that significantly impacts benchmarking efforts for delivery efficacy. Currently, regulatory bodies including the FDA, EMA, and PMDA have established specialized pathways for advanced therapy medicinal products, under which mRNA LNP technologies are typically evaluated.
These regulatory frameworks emphasize several critical parameters that directly influence delivery efficacy benchmarking protocols. Safety assessment requirements mandate comprehensive characterization of LNP components, including lipid cytotoxicity profiles, immunogenicity potential, and biodistribution patterns. Efficacy demonstration guidelines require standardized potency assays and reproducible transfection efficiency metrics across different tissue types.
Manufacturing consistency represents another crucial regulatory consideration, with authorities requiring robust analytical methods to ensure batch-to-batch reproducibility of physicochemical properties such as particle size distribution, polydispersity index, and encapsulation efficiency—all parameters directly affecting delivery performance benchmarks.
The accelerated approval pathways established during the COVID-19 pandemic have created important precedents for mRNA LNP regulatory assessment, introducing streamlined approaches for characterizing delivery systems while maintaining rigorous safety standards. These emergency frameworks have subsequently informed evolving regulatory perspectives on acceptable benchmarking methodologies.
International harmonization efforts, particularly through the International Council for Harmonisation (ICH), are working to standardize regulatory requirements for novel delivery technologies, though significant regional variations persist. These differences create challenges for establishing universally accepted benchmarking protocols for mRNA LNP delivery efficacy.
Regulatory expectations regarding in vitro-in vivo correlation (IVIVC) models are increasingly stringent, requiring developers to demonstrate predictive relationships between laboratory delivery efficiency metrics and clinical outcomes. This regulatory emphasis has driven innovation in physiologically relevant model systems for benchmarking delivery performance.
Looking forward, emerging regulatory trends indicate movement toward adaptive licensing approaches that may allow for iterative benchmarking of delivery technologies throughout development cycles. Additionally, regulatory science initiatives are actively developing standardized reference materials and consensus methods specifically for LNP characterization, which will significantly enhance the comparability and reliability of delivery efficacy benchmarking across the industry.
These regulatory frameworks emphasize several critical parameters that directly influence delivery efficacy benchmarking protocols. Safety assessment requirements mandate comprehensive characterization of LNP components, including lipid cytotoxicity profiles, immunogenicity potential, and biodistribution patterns. Efficacy demonstration guidelines require standardized potency assays and reproducible transfection efficiency metrics across different tissue types.
Manufacturing consistency represents another crucial regulatory consideration, with authorities requiring robust analytical methods to ensure batch-to-batch reproducibility of physicochemical properties such as particle size distribution, polydispersity index, and encapsulation efficiency—all parameters directly affecting delivery performance benchmarks.
The accelerated approval pathways established during the COVID-19 pandemic have created important precedents for mRNA LNP regulatory assessment, introducing streamlined approaches for characterizing delivery systems while maintaining rigorous safety standards. These emergency frameworks have subsequently informed evolving regulatory perspectives on acceptable benchmarking methodologies.
International harmonization efforts, particularly through the International Council for Harmonisation (ICH), are working to standardize regulatory requirements for novel delivery technologies, though significant regional variations persist. These differences create challenges for establishing universally accepted benchmarking protocols for mRNA LNP delivery efficacy.
Regulatory expectations regarding in vitro-in vivo correlation (IVIVC) models are increasingly stringent, requiring developers to demonstrate predictive relationships between laboratory delivery efficiency metrics and clinical outcomes. This regulatory emphasis has driven innovation in physiologically relevant model systems for benchmarking delivery performance.
Looking forward, emerging regulatory trends indicate movement toward adaptive licensing approaches that may allow for iterative benchmarking of delivery technologies throughout development cycles. Additionally, regulatory science initiatives are actively developing standardized reference materials and consensus methods specifically for LNP characterization, which will significantly enhance the comparability and reliability of delivery efficacy benchmarking across the industry.
Standardization Challenges in LNP Performance Metrics
The standardization of Lipid Nanoparticle (LNP) performance metrics represents a significant challenge in the field of mRNA delivery systems. Current evaluation methods for LNP efficacy vary considerably across research institutions, pharmaceutical companies, and regulatory bodies, creating substantial obstacles for meaningful cross-study comparisons and technology advancement.
One primary challenge lies in the diverse array of in vitro assay systems employed to assess transfection efficiency. Different cell lines, culture conditions, and reporter systems can yield dramatically different results for identical LNP formulations. This variability makes it exceedingly difficult to establish reliable benchmarks that accurately predict in vivo performance.
The quantification methodologies for key LNP characteristics further complicate standardization efforts. Parameters such as particle size distribution, polydispersity index, zeta potential, and encapsulation efficiency are measured using different instruments and protocols across laboratories. Even minor variations in sample preparation or measurement conditions can significantly impact results, leading to inconsistent data interpretation and hindering comparative analyses.
In vivo evaluation presents even greater standardization challenges. Animal models vary in their immunological responses to LNPs, and differences in administration routes, dosing regimens, and tissue sampling protocols create additional variables that confound direct comparisons. The lack of consensus regarding which biomarkers and endpoints most accurately reflect therapeutic potential further exacerbates this issue.
Regulatory frameworks for LNP characterization remain in developmental stages, with different agencies emphasizing varying aspects of performance. This regulatory heterogeneity creates uncertainty for developers regarding which metrics will ultimately determine approval pathways, potentially slowing innovation and market entry.
The proprietary nature of many advanced LNP formulations presents another obstacle to standardization. Commercial entities often withhold detailed methodological information, limiting the scientific community's ability to reproduce results or establish common benchmarks. This knowledge fragmentation impedes collective progress toward optimized delivery systems.
Emerging technologies for LNP characterization, such as advanced imaging techniques and high-throughput screening platforms, offer potential solutions but simultaneously introduce new standardization challenges. Without consensus on how to interpret and validate data from these novel approaches, their utility for benchmarking remains limited.
Addressing these standardization challenges requires coordinated efforts from academic institutions, industry partners, and regulatory bodies to establish common protocols, reference materials, and reporting standards that enable meaningful comparisons of LNP performance across the field.
One primary challenge lies in the diverse array of in vitro assay systems employed to assess transfection efficiency. Different cell lines, culture conditions, and reporter systems can yield dramatically different results for identical LNP formulations. This variability makes it exceedingly difficult to establish reliable benchmarks that accurately predict in vivo performance.
The quantification methodologies for key LNP characteristics further complicate standardization efforts. Parameters such as particle size distribution, polydispersity index, zeta potential, and encapsulation efficiency are measured using different instruments and protocols across laboratories. Even minor variations in sample preparation or measurement conditions can significantly impact results, leading to inconsistent data interpretation and hindering comparative analyses.
In vivo evaluation presents even greater standardization challenges. Animal models vary in their immunological responses to LNPs, and differences in administration routes, dosing regimens, and tissue sampling protocols create additional variables that confound direct comparisons. The lack of consensus regarding which biomarkers and endpoints most accurately reflect therapeutic potential further exacerbates this issue.
Regulatory frameworks for LNP characterization remain in developmental stages, with different agencies emphasizing varying aspects of performance. This regulatory heterogeneity creates uncertainty for developers regarding which metrics will ultimately determine approval pathways, potentially slowing innovation and market entry.
The proprietary nature of many advanced LNP formulations presents another obstacle to standardization. Commercial entities often withhold detailed methodological information, limiting the scientific community's ability to reproduce results or establish common benchmarks. This knowledge fragmentation impedes collective progress toward optimized delivery systems.
Emerging technologies for LNP characterization, such as advanced imaging techniques and high-throughput screening platforms, offer potential solutions but simultaneously introduce new standardization challenges. Without consensus on how to interpret and validate data from these novel approaches, their utility for benchmarking remains limited.
Addressing these standardization challenges requires coordinated efforts from academic institutions, industry partners, and regulatory bodies to establish common protocols, reference materials, and reporting standards that enable meaningful comparisons of LNP performance across the field.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!