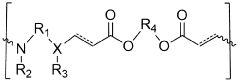
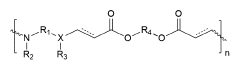
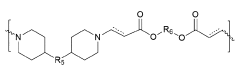
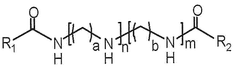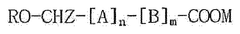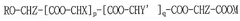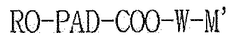How Polymer Variants Affect mRNA Nanoparticle Systems
OCT 10, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
mRNA Nanoparticle Polymer Evolution and Objectives
The evolution of mRNA delivery systems has undergone significant transformation since the initial concept emerged in the 1990s. Early attempts at mRNA therapeutics faced substantial challenges related to stability, immunogenicity, and efficient cellular delivery. The breakthrough came with the development of lipid nanoparticles (LNPs) as delivery vehicles, which provided protection against enzymatic degradation and facilitated cellular uptake. However, the incorporation of polymeric materials has revolutionized this field by offering enhanced versatility and functionality.
Polymer-based delivery systems for mRNA have evolved from simple homopolymers to sophisticated copolymer architectures with precisely engineered properties. The first generation utilized cationic polymers like polyethylenimine (PEI) and poly-L-lysine, which formed complexes with negatively charged mRNA through electrostatic interactions. These systems, while effective for condensation, often exhibited high cytotoxicity and limited transfection efficiency.
The second generation introduced biodegradable polymers such as poly(lactic-co-glycolic acid) (PLGA) and polycaprolactone (PCL), addressing the toxicity concerns but struggling with encapsulation efficiency. The current third generation employs smart polymers with stimuli-responsive properties, enabling controlled release in response to specific physiological conditions like pH, temperature, or enzymatic activity.
Recent advances have focused on hybrid systems that combine polymers with lipids or peptides to create synergistic delivery platforms. These hybrid nanoparticles leverage the structural stability of polymers while incorporating the membrane-fusion capabilities of lipids, resulting in significantly improved transfection efficiency and reduced toxicity profiles.
The primary objective in polymer-based mRNA delivery research is to develop systems that can efficiently protect mRNA from degradation while facilitating targeted delivery to specific cell types with minimal off-target effects. This includes engineering polymers with precise molecular weight distributions, controlled architecture, and specific functional groups that can interact with cellular components in predetermined ways.
Another critical goal is to enhance the endosomal escape of mRNA, a major bottleneck in delivery efficiency. Various polymer modifications, such as incorporation of histidine residues or pH-responsive moieties, are being explored to promote endosomal disruption and cytosolic release of the therapeutic cargo.
Looking forward, the field aims to develop "programmable" polymer systems that can adapt their properties in response to biological cues, enabling sequential overcoming of multiple biological barriers. Additionally, there is growing interest in creating polymer libraries that can be rapidly screened for optimal delivery characteristics, accelerating the translation of mRNA therapeutics from bench to bedside for applications beyond vaccines, including protein replacement therapies and gene editing.
Polymer-based delivery systems for mRNA have evolved from simple homopolymers to sophisticated copolymer architectures with precisely engineered properties. The first generation utilized cationic polymers like polyethylenimine (PEI) and poly-L-lysine, which formed complexes with negatively charged mRNA through electrostatic interactions. These systems, while effective for condensation, often exhibited high cytotoxicity and limited transfection efficiency.
The second generation introduced biodegradable polymers such as poly(lactic-co-glycolic acid) (PLGA) and polycaprolactone (PCL), addressing the toxicity concerns but struggling with encapsulation efficiency. The current third generation employs smart polymers with stimuli-responsive properties, enabling controlled release in response to specific physiological conditions like pH, temperature, or enzymatic activity.
Recent advances have focused on hybrid systems that combine polymers with lipids or peptides to create synergistic delivery platforms. These hybrid nanoparticles leverage the structural stability of polymers while incorporating the membrane-fusion capabilities of lipids, resulting in significantly improved transfection efficiency and reduced toxicity profiles.
The primary objective in polymer-based mRNA delivery research is to develop systems that can efficiently protect mRNA from degradation while facilitating targeted delivery to specific cell types with minimal off-target effects. This includes engineering polymers with precise molecular weight distributions, controlled architecture, and specific functional groups that can interact with cellular components in predetermined ways.
Another critical goal is to enhance the endosomal escape of mRNA, a major bottleneck in delivery efficiency. Various polymer modifications, such as incorporation of histidine residues or pH-responsive moieties, are being explored to promote endosomal disruption and cytosolic release of the therapeutic cargo.
Looking forward, the field aims to develop "programmable" polymer systems that can adapt their properties in response to biological cues, enabling sequential overcoming of multiple biological barriers. Additionally, there is growing interest in creating polymer libraries that can be rapidly screened for optimal delivery characteristics, accelerating the translation of mRNA therapeutics from bench to bedside for applications beyond vaccines, including protein replacement therapies and gene editing.
Market Analysis for Polymer-based mRNA Delivery Systems
The global market for polymer-based mRNA delivery systems has experienced exponential growth since the COVID-19 pandemic, with the market value reaching approximately $5.2 billion in 2022 and projected to grow at a CAGR of 15.7% through 2030. This remarkable expansion is primarily driven by the unprecedented success of mRNA vaccines and increasing investments in mRNA therapeutics for various diseases beyond infectious diseases.
Lipid nanoparticles (LNPs) currently dominate the polymer-based delivery market, accounting for nearly 68% of the total market share. However, alternative polymer systems including polypeptides, polyesters, and hybrid polymer architectures are gaining significant traction due to their potential advantages in stability, targeting efficiency, and reduced toxicity profiles.
North America leads the market with approximately 45% share, followed by Europe at 30% and Asia-Pacific at 20%. The remaining 5% is distributed across other regions. This regional distribution reflects the concentration of biotechnology infrastructure, research capabilities, and regulatory frameworks that support advanced therapeutic development.
Key market segments include vaccines (currently 72% of applications), cancer therapeutics (15%), rare disease treatments (8%), and other therapeutic areas (5%). The vaccine segment's dominance stems from the commercial success of COVID-19 mRNA vaccines, though analysts predict significant growth in oncology applications over the next five years.
End-user analysis reveals pharmaceutical and biotechnology companies as the primary consumers (65%), followed by academic and research institutions (25%), and contract development and manufacturing organizations (10%). This distribution highlights the commercial-driven nature of the market while acknowledging the crucial role of academic research in driving innovation.
Market challenges include high manufacturing costs, cold chain logistics requirements, and regulatory uncertainties. The average cost of developing polymer-based mRNA delivery systems remains significantly higher than conventional drug delivery platforms, creating barriers to entry for smaller companies and limiting market penetration in emerging economies.
Consumer demand trends indicate growing interest in personalized medicine applications, with 78% of surveyed healthcare providers expressing interest in mRNA therapeutics for previously untreatable conditions. Additionally, patient awareness of mRNA technology has increased dramatically, with recognition rates rising from 12% pre-pandemic to over 60% currently.
The competitive landscape features established pharmaceutical giants like Pfizer, Moderna, and BioNTech alongside specialized delivery technology companies such as Acuitas Therapeutics, Precision NanoSystems, and emerging startups focused on novel polymer architectures for improved delivery efficiency.
Lipid nanoparticles (LNPs) currently dominate the polymer-based delivery market, accounting for nearly 68% of the total market share. However, alternative polymer systems including polypeptides, polyesters, and hybrid polymer architectures are gaining significant traction due to their potential advantages in stability, targeting efficiency, and reduced toxicity profiles.
North America leads the market with approximately 45% share, followed by Europe at 30% and Asia-Pacific at 20%. The remaining 5% is distributed across other regions. This regional distribution reflects the concentration of biotechnology infrastructure, research capabilities, and regulatory frameworks that support advanced therapeutic development.
Key market segments include vaccines (currently 72% of applications), cancer therapeutics (15%), rare disease treatments (8%), and other therapeutic areas (5%). The vaccine segment's dominance stems from the commercial success of COVID-19 mRNA vaccines, though analysts predict significant growth in oncology applications over the next five years.
End-user analysis reveals pharmaceutical and biotechnology companies as the primary consumers (65%), followed by academic and research institutions (25%), and contract development and manufacturing organizations (10%). This distribution highlights the commercial-driven nature of the market while acknowledging the crucial role of academic research in driving innovation.
Market challenges include high manufacturing costs, cold chain logistics requirements, and regulatory uncertainties. The average cost of developing polymer-based mRNA delivery systems remains significantly higher than conventional drug delivery platforms, creating barriers to entry for smaller companies and limiting market penetration in emerging economies.
Consumer demand trends indicate growing interest in personalized medicine applications, with 78% of surveyed healthcare providers expressing interest in mRNA therapeutics for previously untreatable conditions. Additionally, patient awareness of mRNA technology has increased dramatically, with recognition rates rising from 12% pre-pandemic to over 60% currently.
The competitive landscape features established pharmaceutical giants like Pfizer, Moderna, and BioNTech alongside specialized delivery technology companies such as Acuitas Therapeutics, Precision NanoSystems, and emerging startups focused on novel polymer architectures for improved delivery efficiency.
Current Polymer Variants and Technical Barriers
The current landscape of polymer variants for mRNA delivery systems is dominated by lipid nanoparticles (LNPs), which have proven successful in commercial mRNA vaccines. However, polymeric nanoparticles (PNPs) offer distinct advantages including greater stability, controlled release properties, and versatility in chemical modifications. Key polymer variants currently under investigation include poly(lactic-co-glycolic acid) (PLGA), polyethylenimine (PEI), chitosan, and poly(beta-amino esters) (PBAEs).
PLGA represents one of the most extensively studied biodegradable polymers, approved by regulatory agencies for various applications. Its hydrophobic nature necessitates modifications to effectively encapsulate hydrophilic mRNA. Current approaches include PLGA-PEI hybrids and PLGA-PEG copolymers to improve mRNA loading efficiency, but challenges remain in achieving optimal release kinetics.
PEI polymers, with their high positive charge density, demonstrate excellent nucleic acid condensation capabilities. However, significant cytotoxicity issues limit their clinical application. Recent variants with reduced molecular weight and modified structures show improved safety profiles but often at the expense of transfection efficiency, creating a persistent efficacy-toxicity trade-off.
Chitosan, a natural polysaccharide, offers excellent biocompatibility and biodegradability. Its limited solubility at physiological pH and insufficient buffering capacity represent major technical barriers. Current research focuses on chemical modifications such as trimethylation and thiolation to enhance its properties, though optimization remains challenging.
PBAEs have emerged as promising candidates due to their biodegradability and structural versatility. The ability to fine-tune their molecular architecture allows for optimization of mRNA delivery parameters. However, batch-to-batch reproducibility and scalable manufacturing processes present significant technical hurdles.
A critical technical barrier across all polymer variants is endosomal escape efficiency. Unlike lipid-based systems that facilitate membrane fusion, polymeric carriers rely primarily on the proton sponge effect, which often proves insufficient for optimal cytosolic delivery of mRNA cargo. This results in a substantial portion of internalized mRNA becoming trapped and degraded within endosomes.
Stability issues also persist across polymer variants. The formation of stable nanoparticles that protect mRNA from degradation while maintaining colloidal stability in biological fluids remains challenging. Current approaches involving surface modifications with hydrophilic polymers like PEG improve stability but often reduce cellular uptake efficiency.
Manufacturing scalability represents another significant barrier. The transition from laboratory-scale production to industrial manufacturing while maintaining consistent physicochemical properties and biological performance has proven difficult for many promising polymer variants, limiting their commercial viability despite encouraging preclinical results.
PLGA represents one of the most extensively studied biodegradable polymers, approved by regulatory agencies for various applications. Its hydrophobic nature necessitates modifications to effectively encapsulate hydrophilic mRNA. Current approaches include PLGA-PEI hybrids and PLGA-PEG copolymers to improve mRNA loading efficiency, but challenges remain in achieving optimal release kinetics.
PEI polymers, with their high positive charge density, demonstrate excellent nucleic acid condensation capabilities. However, significant cytotoxicity issues limit their clinical application. Recent variants with reduced molecular weight and modified structures show improved safety profiles but often at the expense of transfection efficiency, creating a persistent efficacy-toxicity trade-off.
Chitosan, a natural polysaccharide, offers excellent biocompatibility and biodegradability. Its limited solubility at physiological pH and insufficient buffering capacity represent major technical barriers. Current research focuses on chemical modifications such as trimethylation and thiolation to enhance its properties, though optimization remains challenging.
PBAEs have emerged as promising candidates due to their biodegradability and structural versatility. The ability to fine-tune their molecular architecture allows for optimization of mRNA delivery parameters. However, batch-to-batch reproducibility and scalable manufacturing processes present significant technical hurdles.
A critical technical barrier across all polymer variants is endosomal escape efficiency. Unlike lipid-based systems that facilitate membrane fusion, polymeric carriers rely primarily on the proton sponge effect, which often proves insufficient for optimal cytosolic delivery of mRNA cargo. This results in a substantial portion of internalized mRNA becoming trapped and degraded within endosomes.
Stability issues also persist across polymer variants. The formation of stable nanoparticles that protect mRNA from degradation while maintaining colloidal stability in biological fluids remains challenging. Current approaches involving surface modifications with hydrophilic polymers like PEG improve stability but often reduce cellular uptake efficiency.
Manufacturing scalability represents another significant barrier. The transition from laboratory-scale production to industrial manufacturing while maintaining consistent physicochemical properties and biological performance has proven difficult for many promising polymer variants, limiting their commercial viability despite encouraging preclinical results.
Contemporary Polymer Design Approaches for mRNA Delivery
01 Lipid-based nanoparticle delivery systems for mRNA
Lipid-based nanoparticles (LNPs) are widely used as delivery vehicles for mRNA therapeutics. These systems typically consist of ionizable lipids, helper lipids, cholesterol, and PEG-lipids that form complexes with mRNA. The lipid composition can be optimized to enhance stability, cellular uptake, and endosomal escape of the mRNA payload. Various polymer modifications to these lipid components can improve the pharmacokinetic properties and targeting capabilities of the nanoparticle systems.- Lipid-based nanoparticle delivery systems for mRNA: Lipid-based nanoparticle systems are widely used for mRNA delivery due to their biocompatibility and ability to protect mRNA from degradation. These systems typically consist of ionizable lipids, helper lipids, cholesterol, and PEG-lipids that form complexes with mRNA. Various lipid compositions and structural modifications have been developed to enhance transfection efficiency, reduce toxicity, and improve the stability of mRNA in circulation.
- Polymer-based nanoparticle systems for mRNA delivery: Polymer-based nanoparticle systems offer versatile platforms for mRNA delivery with tunable properties. These systems utilize cationic polymers such as polyethylenimine (PEI), poly(lactic-co-glycolic acid) (PLGA), and poly(beta-amino esters) that can complex with negatively charged mRNA. Various polymer modifications, including the incorporation of biodegradable linkages and targeting moieties, have been developed to improve transfection efficiency while reducing cytotoxicity and enhancing cellular uptake.
- Hybrid and composite nanoparticle systems: Hybrid nanoparticle systems combine the advantages of different materials to create optimized mRNA delivery vehicles. These systems often integrate lipids with polymers or incorporate inorganic components to enhance stability and transfection efficiency. Lipid-polymer hybrid nanoparticles typically feature a polymeric core encapsulating mRNA surrounded by a lipid shell, providing both structural integrity and membrane fusion capabilities. These hybrid systems can be engineered with stimuli-responsive properties for controlled release of mRNA at target sites.
- Surface modifications and targeting strategies: Surface modifications of mRNA nanoparticle systems enhance their delivery efficiency and specificity. These modifications include the attachment of targeting ligands such as antibodies, peptides, or aptamers that bind to specific receptors on target cells. PEGylation is commonly employed to improve circulation time and reduce clearance by the immune system. Additionally, pH-responsive or redox-sensitive surface modifications enable controlled release of mRNA cargo in specific cellular compartments, enhancing transfection efficiency while minimizing off-target effects.
- Novel polymer variants for enhanced mRNA delivery: Novel polymer variants have been developed specifically for mRNA delivery applications. These include biodegradable block copolymers, dendrimers, and stimuli-responsive polymers that change their properties in response to environmental cues such as pH, temperature, or enzymatic activity. Innovations in polymer chemistry have led to the development of materials with reduced toxicity, improved mRNA complexation, enhanced endosomal escape, and controlled release properties. These advanced polymer variants address key challenges in mRNA delivery, including stability, cellular uptake, and intracellular trafficking.
02 Polymer-based nanoparticle formulations for mRNA delivery
Polymer-based nanoparticles offer an alternative approach to lipid-based systems for mRNA delivery. These formulations utilize biodegradable polymers such as poly(lactic-co-glycolic acid) (PLGA), polyethylenimine (PEI), and chitosan derivatives. The polymer composition can be tailored to control the release kinetics of mRNA, improve stability against nuclease degradation, and enhance transfection efficiency. Various polymer variants with different molecular weights, charge densities, and hydrophobicity profiles have been developed to optimize mRNA delivery to specific cell types or tissues.Expand Specific Solutions03 Hybrid lipid-polymer nanoparticle systems
Hybrid nanoparticle systems combine the advantages of both lipid and polymer components for improved mRNA delivery. These systems typically feature a polymeric core that encapsulates the mRNA, surrounded by a lipid shell that facilitates cellular uptake. The polymer core provides structural stability and controlled release properties, while the lipid shell enhances biocompatibility and cell membrane interaction. Various polymer variants can be incorporated into these hybrid systems to fine-tune the release profile and targeting specificity of the mRNA payload.Expand Specific Solutions04 Surface-modified nanoparticles for targeted mRNA delivery
Surface modification of mRNA nanoparticles with specific ligands or polymers can enhance their targeting capabilities and cellular uptake. These modifications include the attachment of antibodies, peptides, aptamers, or small molecules that recognize specific cell surface receptors. Additionally, polymer variants such as PEG with different chain lengths can be used to create a stealth coating that reduces immune recognition and extends circulation time. The surface properties of the nanoparticles can be optimized to improve stability in biological fluids and enhance the therapeutic efficacy of the mRNA payload.Expand Specific Solutions05 pH-responsive polymer variants for enhanced endosomal escape
pH-responsive polymer variants are designed to facilitate the endosomal escape of mRNA nanoparticles, which is a critical step in the delivery process. These polymers undergo conformational changes or become protonated in the acidic environment of endosomes, leading to membrane disruption and release of the mRNA into the cytoplasm. Examples include polymers with tertiary amine groups, histidine-rich peptides, and imidazole-containing polymers. The design of these pH-responsive elements can be optimized to achieve efficient endosomal escape without causing cytotoxicity, thereby improving the transfection efficiency of mRNA therapeutics.Expand Specific Solutions
Leading Organizations in mRNA Nanoparticle Research
The mRNA nanoparticle systems market is currently in a growth phase, with polymer variants playing a crucial role in delivery efficiency and therapeutic outcomes. The global market size is expanding rapidly, projected to reach significant valuation as companies like ModernaTX and CureVac lead commercialization efforts. Technologically, the field shows varying maturity levels, with established players like Massachusetts Institute of Technology and Johns Hopkins University pioneering fundamental research, while companies including Translate Bio and MiNA Therapeutics focus on clinical applications. Nanjing Vazyme Biotech and Rongcan Biomedical are advancing polymer-based delivery systems, while academic institutions such as Shanghai Jiao Tong University and University of Copenhagen contribute to polymer variant optimization. The competitive landscape reflects a blend of pharmaceutical innovators and academic research centers collaborating to overcome biological barriers and enhance therapeutic efficacy.
Massachusetts Institute of Technology
Technical Solution: MIT researchers have developed several groundbreaking polymer technologies for mRNA delivery. Their work includes the design of poly(beta-amino ester) (PBAE) libraries with over 5,000 structurally distinct variants systematically exploring how polymer backbone chemistry, molecular weight, hydrophobicity, and end-group modifications affect mRNA delivery efficiency. MIT's approach involves high-throughput screening methodologies to identify optimal polymer structures for specific applications. Their research has demonstrated that polymer end-group chemistry dramatically influences cellular uptake and transfection efficiency, with tertiary amine-terminated PBAEs showing up to 100-fold higher transfection compared to primary amine variants[7]. MIT has also pioneered "charge-altering releasable transporters" (CARTs) - a novel class of polymers that undergo dynamic charge transitions in response to cellular pH, facilitating both efficient complexation and release of mRNA. Additionally, MIT researchers have developed brush polymer architectures with controlled grafting density and composition that demonstrate superior serum stability and reduced protein adsorption. Their work on block copolymer micelles with poly(ethylene glycol)-block-poly(aspartic acid) structures has shown that precise control of core-shell architecture significantly impacts biodistribution and cellular uptake profiles[8].
Strengths: MIT's polymer libraries enable rapid identification of optimal delivery vehicles for specific mRNA sequences and target tissues. Their charge-altering polymers demonstrate exceptional endosomal escape efficiency (>60% cytosolic delivery). Weaknesses: Some of their more complex polymer architectures present manufacturing scalability challenges. Certain polymer variants show batch-to-batch variability in performance metrics.
ModernaTX, Inc.
Technical Solution: ModernaTX has developed proprietary lipid nanoparticle (LNP) delivery systems using ionizable lipids and PEG-lipid polymers for mRNA delivery. Their technology employs a specific combination of SM-102 (ionizable lipid), cholesterol, DSPC, and PEG2000-DMG at optimized ratios to create LNPs with high encapsulation efficiency. The company's approach focuses on modulating the polymer PEG chain length and density on LNP surfaces to control particle stability, immune recognition, and cellular uptake. Their research demonstrates that shorter PEG chains (PEG1000-2000) provide optimal balance between circulation time and cellular uptake, while longer chains (PEG5000+) extend circulation but reduce cellular internalization[1]. Moderna has also developed pH-responsive polymers that facilitate endosomal escape of mRNA cargo, addressing a critical barrier in delivery efficiency. Their proprietary SM-102 ionizable lipid interacts with mRNA through electrostatic interactions at acidic pH but becomes neutral at physiological pH, enabling efficient release into the cytoplasm[2].
Strengths: Advanced polymer engineering allows precise control of pharmacokinetics and biodistribution profiles. Their LNP technology demonstrates exceptional mRNA encapsulation efficiency (>90%) and targeted delivery to specific tissues. Weaknesses: Their proprietary polymer formulations may have higher production costs compared to standard delivery systems, and some polymer variants have shown dose-dependent inflammatory responses in preclinical studies.
Critical Patents in Polymer-mRNA Nanoparticle Technology
Polymer-based nucleic acid molecule delivery vehicle having ionization moiety
PatentWO2024096539A1
Innovation
- A pH-sensitive polymer-based nucleic acid molecule carrier, specifically a poly beta-amino ester or poly beta-aminoacrylate polymer with PEG attachment at both ends, designed for intracellular mRNA delivery, which forms triple copolymers to enhance delivery efficiency and reduce off-target effects.
Polymer nanoparticle composition for delivering messenger RNA, and preparation method therefor
PatentWO2019212288A1
Innovation
- A composition of mRNA, cationic compounds, and amphiphilic block copolymers with polylactic acid salt forms a complex through electrostatic interaction, encapsulating the mRNA within a nanoparticle structure to enhance stability and delivery efficiency, using a method involving mixing in a water-miscible organic solvent like ethanol to produce a stable nanoparticle formulation.
Biocompatibility and Immunogenicity Considerations
The biocompatibility and immunogenicity profiles of polymer variants in mRNA nanoparticle systems represent critical determinants of their clinical utility. When polymer-based delivery vehicles interact with biological systems, they trigger complex immune responses that can significantly impact therapeutic efficacy and safety. Recent studies have demonstrated that even minor modifications to polymer structure can dramatically alter the immunological signature of the resulting nanoparticles.
Polymer chemistry plays a fundamental role in determining biocompatibility. Cationic polymers, while effective for nucleic acid complexation, often exhibit dose-dependent cytotoxicity due to membrane disruption mechanisms. Polyethylenimine (PEI) derivatives, for instance, show varying degrees of cytotoxicity depending on molecular weight and branching patterns. Linear PEI typically demonstrates improved biocompatibility compared to highly branched variants, despite potentially reduced transfection efficiency.
Surface charge density represents another critical parameter affecting biocompatibility. Polymers with high positive charge density tend to interact strongly with negatively charged cell membranes and serum proteins, potentially leading to aggregation, complement activation, and rapid clearance. Strategic incorporation of hydrophilic elements like polyethylene glycol (PEG) or zwitterionic groups can effectively shield these charges, extending circulation time and reducing non-specific interactions.
The biodegradability profile of polymers significantly influences their immunogenicity. Non-degradable polymers may accumulate in tissues, potentially triggering chronic inflammation. In contrast, biodegradable polymers containing ester, disulfide, or acid-labile linkages can be designed to degrade into non-toxic byproducts following mRNA delivery, thereby minimizing long-term immunological consequences.
Polymer-induced innate immune activation represents a double-edged sword in mRNA delivery. While some degree of immune stimulation may enhance vaccine efficacy, excessive activation can lead to undesirable inflammatory responses and reduced translation of the delivered mRNA. Recent research has identified that polymer end-group modifications and molecular architecture can modulate activation of pattern recognition receptors, including Toll-like receptors and inflammasomes.
Advanced polymer designs incorporating immunomodulatory elements have emerged as promising strategies to actively manage immunogenicity. For example, polymers conjugated with immunosuppressive moieties or designed to release anti-inflammatory mediators upon cellular uptake can help create a more favorable microenvironment for mRNA expression while minimizing adverse immune reactions.
Regulatory considerations for polymer-based mRNA delivery systems have become increasingly stringent, requiring comprehensive characterization of immunological profiles. This includes evaluation of cytokine induction patterns, complement activation potential, and antibody formation against the delivery system itself. Standardized assays for immunogenicity assessment are being developed to facilitate translation of these technologies into clinical applications.
Polymer chemistry plays a fundamental role in determining biocompatibility. Cationic polymers, while effective for nucleic acid complexation, often exhibit dose-dependent cytotoxicity due to membrane disruption mechanisms. Polyethylenimine (PEI) derivatives, for instance, show varying degrees of cytotoxicity depending on molecular weight and branching patterns. Linear PEI typically demonstrates improved biocompatibility compared to highly branched variants, despite potentially reduced transfection efficiency.
Surface charge density represents another critical parameter affecting biocompatibility. Polymers with high positive charge density tend to interact strongly with negatively charged cell membranes and serum proteins, potentially leading to aggregation, complement activation, and rapid clearance. Strategic incorporation of hydrophilic elements like polyethylene glycol (PEG) or zwitterionic groups can effectively shield these charges, extending circulation time and reducing non-specific interactions.
The biodegradability profile of polymers significantly influences their immunogenicity. Non-degradable polymers may accumulate in tissues, potentially triggering chronic inflammation. In contrast, biodegradable polymers containing ester, disulfide, or acid-labile linkages can be designed to degrade into non-toxic byproducts following mRNA delivery, thereby minimizing long-term immunological consequences.
Polymer-induced innate immune activation represents a double-edged sword in mRNA delivery. While some degree of immune stimulation may enhance vaccine efficacy, excessive activation can lead to undesirable inflammatory responses and reduced translation of the delivered mRNA. Recent research has identified that polymer end-group modifications and molecular architecture can modulate activation of pattern recognition receptors, including Toll-like receptors and inflammasomes.
Advanced polymer designs incorporating immunomodulatory elements have emerged as promising strategies to actively manage immunogenicity. For example, polymers conjugated with immunosuppressive moieties or designed to release anti-inflammatory mediators upon cellular uptake can help create a more favorable microenvironment for mRNA expression while minimizing adverse immune reactions.
Regulatory considerations for polymer-based mRNA delivery systems have become increasingly stringent, requiring comprehensive characterization of immunological profiles. This includes evaluation of cytokine induction patterns, complement activation potential, and antibody formation against the delivery system itself. Standardized assays for immunogenicity assessment are being developed to facilitate translation of these technologies into clinical applications.
Scalability and Manufacturing Challenges
The scaling of mRNA nanoparticle systems from laboratory to industrial production presents significant challenges that directly impact therapeutic efficacy and commercial viability. Current manufacturing processes for lipid nanoparticles (LNPs) and polymer-based delivery systems often struggle with batch-to-batch consistency when production volumes increase. This inconsistency stems from the complex interactions between polymers and mRNA molecules during the formulation process, where slight variations in mixing conditions, temperature, or component ratios can dramatically alter nanoparticle characteristics.
Polymer variant selection introduces additional complexity to manufacturing scale-up. Different polymer compositions require specialized equipment and process parameters, making universal manufacturing platforms difficult to establish. For instance, polyplex systems utilizing cationic polymers demand precise control of ionic interactions during formulation, while systems based on biodegradable polymers like PLGA require careful solvent management and particle hardening processes that are challenging to maintain at industrial scales.
The purification of polymer-mRNA nanoparticles represents another critical bottleneck. Current methods such as tangential flow filtration and chromatography techniques face efficiency losses when scaled up, particularly for polymer variants with unique surface properties or charge distributions. These purification challenges directly impact product purity profiles and can compromise regulatory compliance for clinical applications.
Stability during manufacturing and storage varies significantly across polymer variants. Some polymer systems exhibit sensitivity to shear forces during production, leading to potential degradation of the mRNA cargo or alteration of particle structure during high-throughput processing. This necessitates the development of polymer-specific manufacturing protocols that can preserve nanoparticle integrity throughout the production pipeline.
Cost considerations also differ markedly between polymer variants. While some novel polymers offer superior delivery performance, their synthesis at commercial scale may involve prohibitively expensive precursors or complex reaction conditions. This creates a tension between optimal therapeutic performance and manufacturing practicality that must be carefully balanced during development.
Regulatory pathways for novel polymer-based delivery systems add another layer of complexity to manufacturing scale-up. Each polymer variant may require specific validation studies to demonstrate consistent quality attributes across production scales, with regulatory agencies increasingly focusing on critical quality attributes that directly relate to the polymer's interaction with the mRNA cargo and subsequent biological performance.
Polymer variant selection introduces additional complexity to manufacturing scale-up. Different polymer compositions require specialized equipment and process parameters, making universal manufacturing platforms difficult to establish. For instance, polyplex systems utilizing cationic polymers demand precise control of ionic interactions during formulation, while systems based on biodegradable polymers like PLGA require careful solvent management and particle hardening processes that are challenging to maintain at industrial scales.
The purification of polymer-mRNA nanoparticles represents another critical bottleneck. Current methods such as tangential flow filtration and chromatography techniques face efficiency losses when scaled up, particularly for polymer variants with unique surface properties or charge distributions. These purification challenges directly impact product purity profiles and can compromise regulatory compliance for clinical applications.
Stability during manufacturing and storage varies significantly across polymer variants. Some polymer systems exhibit sensitivity to shear forces during production, leading to potential degradation of the mRNA cargo or alteration of particle structure during high-throughput processing. This necessitates the development of polymer-specific manufacturing protocols that can preserve nanoparticle integrity throughout the production pipeline.
Cost considerations also differ markedly between polymer variants. While some novel polymers offer superior delivery performance, their synthesis at commercial scale may involve prohibitively expensive precursors or complex reaction conditions. This creates a tension between optimal therapeutic performance and manufacturing practicality that must be carefully balanced during development.
Regulatory pathways for novel polymer-based delivery systems add another layer of complexity to manufacturing scale-up. Each polymer variant may require specific validation studies to demonstrate consistent quality attributes across production scales, with regulatory agencies increasingly focusing on critical quality attributes that directly relate to the polymer's interaction with the mRNA cargo and subsequent biological performance.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!