How Electrode Parameters Enhance mRNA Lipid Nanoparticle Design
OCT 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
mRNA LNP Electrode Technology Background and Objectives
Messenger RNA (mRNA) lipid nanoparticle (LNP) technology has emerged as a revolutionary platform for vaccine and therapeutic delivery, most notably demonstrated by the rapid development of COVID-19 vaccines. The technology's evolution spans several decades, beginning with early liposome research in the 1960s and advancing through critical milestones including the development of ionizable lipids in the 2000s and the first FDA approval of an LNP-based siRNA therapeutic, Onpattro, in 2018.
The electrode parameters in mRNA LNP production represent a critical yet underexplored aspect of the manufacturing process. Historically, LNP formulation has relied on passive mixing techniques, but recent advancements have introduced microfluidic and electrohydrodynamic approaches that significantly enhance control over particle characteristics. The manipulation of electrode parameters—including voltage, current density, electrode material, geometry, and surface properties—directly influences the formation dynamics of lipid nanoparticles.
Current technological trends indicate a shift toward precision manufacturing of mRNA LNPs, with increasing focus on reproducibility, scalability, and targeted delivery capabilities. The integration of computational modeling with experimental approaches is accelerating the optimization of electrode parameters, enabling predictive design rather than empirical trial-and-error methodologies. This evolution aligns with broader industry movements toward personalized medicine and rapid response capabilities for emerging infectious diseases.
The primary technical objectives in this field include developing electrode systems that enable precise control over LNP size distribution (targeting 80-100 nm with PDI < 0.2), enhancing encapsulation efficiency (>90%), maintaining mRNA integrity during the formulation process, and ensuring batch-to-batch consistency at commercial scales. Additionally, there is significant interest in electrode configurations that facilitate the production of LNPs with tailored surface properties for targeted delivery to specific tissues beyond the liver.
Electrode parameter optimization presents unique opportunities to address current limitations in mRNA delivery, including cytotoxicity concerns, immunogenicity, and limited delivery efficiency to non-hepatic tissues. By fine-tuning the electrochemical environment during LNP formation, researchers aim to modulate the lipid organization, surface charge distribution, and structural integrity of the particles.
The convergence of materials science, electrochemistry, and pharmaceutical engineering is driving innovation in this space, with interdisciplinary approaches yielding novel electrode designs specifically optimized for mRNA LNP production. As the field matures, standardization of electrode parameters and their correlation with LNP performance metrics will be essential for regulatory approval and commercial manufacturing of next-generation mRNA therapeutics.
The electrode parameters in mRNA LNP production represent a critical yet underexplored aspect of the manufacturing process. Historically, LNP formulation has relied on passive mixing techniques, but recent advancements have introduced microfluidic and electrohydrodynamic approaches that significantly enhance control over particle characteristics. The manipulation of electrode parameters—including voltage, current density, electrode material, geometry, and surface properties—directly influences the formation dynamics of lipid nanoparticles.
Current technological trends indicate a shift toward precision manufacturing of mRNA LNPs, with increasing focus on reproducibility, scalability, and targeted delivery capabilities. The integration of computational modeling with experimental approaches is accelerating the optimization of electrode parameters, enabling predictive design rather than empirical trial-and-error methodologies. This evolution aligns with broader industry movements toward personalized medicine and rapid response capabilities for emerging infectious diseases.
The primary technical objectives in this field include developing electrode systems that enable precise control over LNP size distribution (targeting 80-100 nm with PDI < 0.2), enhancing encapsulation efficiency (>90%), maintaining mRNA integrity during the formulation process, and ensuring batch-to-batch consistency at commercial scales. Additionally, there is significant interest in electrode configurations that facilitate the production of LNPs with tailored surface properties for targeted delivery to specific tissues beyond the liver.
Electrode parameter optimization presents unique opportunities to address current limitations in mRNA delivery, including cytotoxicity concerns, immunogenicity, and limited delivery efficiency to non-hepatic tissues. By fine-tuning the electrochemical environment during LNP formation, researchers aim to modulate the lipid organization, surface charge distribution, and structural integrity of the particles.
The convergence of materials science, electrochemistry, and pharmaceutical engineering is driving innovation in this space, with interdisciplinary approaches yielding novel electrode designs specifically optimized for mRNA LNP production. As the field matures, standardization of electrode parameters and their correlation with LNP performance metrics will be essential for regulatory approval and commercial manufacturing of next-generation mRNA therapeutics.
Market Analysis for mRNA LNP Delivery Systems
The mRNA lipid nanoparticle (LNP) delivery systems market has experienced exponential growth since the successful deployment of mRNA-based COVID-19 vaccines. Current market valuations place the global mRNA therapeutics market at approximately $39 billion in 2023, with projections indicating growth to reach $65 billion by 2028, representing a compound annual growth rate (CAGR) of 10.8%.
The demand for advanced LNP delivery systems is primarily driven by several key factors. First, the unprecedented success of mRNA vaccines during the COVID-19 pandemic has validated this technology platform, creating substantial interest from pharmaceutical companies, investors, and healthcare systems. Second, the versatility of mRNA therapeutics extends beyond vaccines to include protein replacement therapies, cancer immunotherapies, and genetic disorder treatments, significantly expanding the potential market applications.
Regional market analysis reveals North America currently dominates with approximately 42% market share, followed by Europe at 31% and Asia-Pacific at 18%. However, the Asia-Pacific region is expected to demonstrate the highest growth rate over the next five years due to increasing healthcare expenditure and expanding biotechnology infrastructure in countries like China, Japan, and South Korea.
The electrode parameter optimization segment within the LNP manufacturing market is experiencing particularly strong growth, as precise control over electroformation processes directly impacts LNP uniformity, encapsulation efficiency, and ultimately therapeutic efficacy. Companies investing in advanced electrode technologies for LNP production report manufacturing cost reductions of 15-20% while simultaneously improving batch-to-batch consistency.
End-user segmentation shows pharmaceutical and biotechnology companies constitute the largest market share (68%), followed by academic and research institutions (22%), and contract manufacturing organizations (10%). This distribution reflects the capital-intensive nature of LNP manufacturing technology and the specialized expertise required.
Market challenges include high development and manufacturing costs, complex regulatory pathways, and intellectual property landscapes dominated by a small number of key players. The average cost to develop and commercialize an mRNA-LNP therapeutic ranges from $500 million to $2 billion, creating significant barriers to entry for smaller companies.
Future market growth will likely be driven by technological innovations in electrode design and microfluidic systems that enhance scalability and reduce production costs. Additionally, emerging markets in personalized medicine and rare disease treatments represent high-value opportunities, with premium pricing potential due to limited treatment alternatives and high unmet medical needs.
The demand for advanced LNP delivery systems is primarily driven by several key factors. First, the unprecedented success of mRNA vaccines during the COVID-19 pandemic has validated this technology platform, creating substantial interest from pharmaceutical companies, investors, and healthcare systems. Second, the versatility of mRNA therapeutics extends beyond vaccines to include protein replacement therapies, cancer immunotherapies, and genetic disorder treatments, significantly expanding the potential market applications.
Regional market analysis reveals North America currently dominates with approximately 42% market share, followed by Europe at 31% and Asia-Pacific at 18%. However, the Asia-Pacific region is expected to demonstrate the highest growth rate over the next five years due to increasing healthcare expenditure and expanding biotechnology infrastructure in countries like China, Japan, and South Korea.
The electrode parameter optimization segment within the LNP manufacturing market is experiencing particularly strong growth, as precise control over electroformation processes directly impacts LNP uniformity, encapsulation efficiency, and ultimately therapeutic efficacy. Companies investing in advanced electrode technologies for LNP production report manufacturing cost reductions of 15-20% while simultaneously improving batch-to-batch consistency.
End-user segmentation shows pharmaceutical and biotechnology companies constitute the largest market share (68%), followed by academic and research institutions (22%), and contract manufacturing organizations (10%). This distribution reflects the capital-intensive nature of LNP manufacturing technology and the specialized expertise required.
Market challenges include high development and manufacturing costs, complex regulatory pathways, and intellectual property landscapes dominated by a small number of key players. The average cost to develop and commercialize an mRNA-LNP therapeutic ranges from $500 million to $2 billion, creating significant barriers to entry for smaller companies.
Future market growth will likely be driven by technological innovations in electrode design and microfluidic systems that enhance scalability and reduce production costs. Additionally, emerging markets in personalized medicine and rare disease treatments represent high-value opportunities, with premium pricing potential due to limited treatment alternatives and high unmet medical needs.
Current Electrode Parameter Challenges in LNP Production
The production of mRNA lipid nanoparticles (LNPs) faces significant electrode parameter challenges that directly impact formulation quality, reproducibility, and scalability. Current microfluidic mixing systems utilize electrodes to control flow rates and mixing conditions, but several technical limitations persist in this critical area of LNP manufacturing.
Electrode material degradation represents a primary concern, as prolonged exposure to organic solvents and lipid components can cause corrosion and contamination of the final LNP product. Most commercially available systems employ stainless steel or titanium electrodes, which demonstrate limited chemical resistance when subjected to the harsh conditions required for LNP production. This degradation not only compromises batch consistency but also introduces potential safety concerns for downstream clinical applications.
Precise control of electrical parameters presents another significant challenge. The relationship between applied voltage, current density, and resulting flow characteristics remains incompletely characterized across different LNP formulations. Manufacturing systems typically operate with standardized electrical settings that fail to account for the unique physicochemical properties of various lipid compositions and mRNA payloads. This one-size-fits-all approach results in suboptimal particle size distribution, inconsistent encapsulation efficiency, and variable transfection potency.
Temperature management at electrode interfaces constitutes a critical yet underaddressed issue. Joule heating effects during high-throughput production can create localized temperature gradients that alter lipid phase behavior and mRNA stability. Current electrode designs lack integrated temperature monitoring and cooling mechanisms, making it difficult to maintain the narrow thermal window required for optimal LNP formation. This limitation becomes particularly problematic during scale-up operations where heat dissipation dynamics change significantly.
Electrode geometry optimization remains largely empirical rather than theory-driven. The spatial arrangement of electrodes relative to mixing channels significantly influences the electric field distribution and resulting fluid dynamics. However, systematic studies correlating electrode configuration with LNP quality attributes are scarce, forcing manufacturers to rely on trial-and-error approaches when designing new production systems.
Electrode fouling during continuous operation represents another persistent challenge. Lipid deposition on electrode surfaces progressively alters electrical properties and flow characteristics, leading to drift in critical quality attributes over production runs. Current cleaning protocols require system shutdown and disassembly, significantly reducing manufacturing efficiency and increasing production costs.
Electrode material degradation represents a primary concern, as prolonged exposure to organic solvents and lipid components can cause corrosion and contamination of the final LNP product. Most commercially available systems employ stainless steel or titanium electrodes, which demonstrate limited chemical resistance when subjected to the harsh conditions required for LNP production. This degradation not only compromises batch consistency but also introduces potential safety concerns for downstream clinical applications.
Precise control of electrical parameters presents another significant challenge. The relationship between applied voltage, current density, and resulting flow characteristics remains incompletely characterized across different LNP formulations. Manufacturing systems typically operate with standardized electrical settings that fail to account for the unique physicochemical properties of various lipid compositions and mRNA payloads. This one-size-fits-all approach results in suboptimal particle size distribution, inconsistent encapsulation efficiency, and variable transfection potency.
Temperature management at electrode interfaces constitutes a critical yet underaddressed issue. Joule heating effects during high-throughput production can create localized temperature gradients that alter lipid phase behavior and mRNA stability. Current electrode designs lack integrated temperature monitoring and cooling mechanisms, making it difficult to maintain the narrow thermal window required for optimal LNP formation. This limitation becomes particularly problematic during scale-up operations where heat dissipation dynamics change significantly.
Electrode geometry optimization remains largely empirical rather than theory-driven. The spatial arrangement of electrodes relative to mixing channels significantly influences the electric field distribution and resulting fluid dynamics. However, systematic studies correlating electrode configuration with LNP quality attributes are scarce, forcing manufacturers to rely on trial-and-error approaches when designing new production systems.
Electrode fouling during continuous operation represents another persistent challenge. Lipid deposition on electrode surfaces progressively alters electrical properties and flow characteristics, leading to drift in critical quality attributes over production runs. Current cleaning protocols require system shutdown and disassembly, significantly reducing manufacturing efficiency and increasing production costs.
Current Electrode Parameter Optimization Approaches
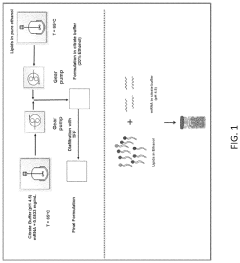
01 Electrode parameters for mRNA lipid nanoparticle production
Specific electrode parameters are crucial for the efficient production of mRNA lipid nanoparticles. These parameters include voltage, current density, pulse duration, and electrode material composition. Optimizing these parameters can significantly improve the encapsulation efficiency of mRNA into lipid nanoparticles and enhance the overall quality and uniformity of the final product. The electrode configuration and spacing also play important roles in the electroporation process used for mRNA delivery.- Electrode parameters for mRNA lipid nanoparticle production: Specific electrode parameters are crucial for the efficient production of mRNA lipid nanoparticles. These parameters include voltage, current density, pulse duration, and electrode material composition. Optimizing these parameters can significantly improve the encapsulation efficiency of mRNA within lipid nanoparticles, leading to more effective delivery systems for therapeutic applications. The electrode configuration and spacing also play important roles in ensuring uniform particle size distribution.
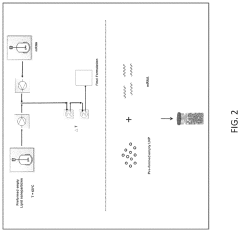
- Microfluidic systems for lipid nanoparticle formulation: Microfluidic platforms incorporating electrode-based systems enable precise control over the formation of mRNA lipid nanoparticles. These systems utilize electrodes with specific parameters to create controlled mixing environments where lipids and mRNA can interact to form nanoparticles of desired size and charge characteristics. The microfluidic channels can be designed with various geometries to optimize the flow patterns and residence times, which directly impact the quality and consistency of the resulting nanoparticles.
- Electroporation techniques for mRNA delivery: Electroporation techniques utilize specific electrode parameters to temporarily increase cell membrane permeability, facilitating the delivery of mRNA-loaded lipid nanoparticles into target cells. The electrode configuration, pulse strength, duration, and frequency can be optimized to enhance transfection efficiency while minimizing cell damage. These techniques are particularly valuable for hard-to-transfect cell types and in vivo applications where traditional delivery methods may be less effective.
- Electrode materials and surface modifications: The composition and surface properties of electrodes significantly impact the formation and stability of mRNA lipid nanoparticles. Novel electrode materials and surface modifications can prevent particle aggregation, reduce contamination, and enhance the uniformity of the electric field. Specialized coatings can also minimize interactions between the electrode surface and the lipid components, resulting in more consistent nanoparticle formation and improved batch-to-batch reproducibility.
- Real-time monitoring and feedback control systems: Advanced systems for real-time monitoring of electrode parameters during mRNA lipid nanoparticle production enable dynamic adjustments to optimize the process. These systems incorporate sensors that measure critical parameters such as current density, temperature, and pH at the electrode-solution interface. Feedback control mechanisms can automatically adjust electrode parameters in response to these measurements, ensuring consistent nanoparticle quality even when processing conditions vary. This approach significantly improves manufacturing reproducibility and product quality.
02 Lipid composition optimization for mRNA delivery
The composition of lipids in nanoparticles significantly affects mRNA delivery efficiency. Various combinations of cationic lipids, helper lipids, cholesterol, and PEG-lipids can be optimized to enhance cellular uptake and endosomal escape. The molar ratios between these components are critical for forming stable nanoparticles with optimal size and surface charge. Adjusting the lipid composition can also improve the biodistribution profile and reduce potential toxicity of the mRNA-loaded nanoparticles.Expand Specific Solutions03 Microfluidic systems for mRNA lipid nanoparticle formulation
Microfluidic devices offer precise control over the mixing of lipid and mRNA components, resulting in more uniform nanoparticles. These systems utilize specific electrode configurations to generate controlled electric fields that facilitate the formation of lipid nanoparticles. Parameters such as flow rate, mixing chamber design, and electrode positioning within microfluidic channels significantly impact the characteristics of the resulting nanoparticles. Advanced microfluidic platforms allow for continuous production of mRNA lipid nanoparticles with consistent quality.Expand Specific Solutions04 Electroporation techniques for mRNA transfection
Electroporation techniques utilize specific electrode parameters to temporarily increase cell membrane permeability, facilitating the entry of mRNA-loaded lipid nanoparticles into target cells. Critical parameters include pulse voltage, duration, number of pulses, and inter-pulse intervals. The electrode material and geometry significantly affect the electric field distribution and transfection efficiency. Optimized electroporation protocols can enhance mRNA delivery while minimizing cell damage and maintaining high cell viability.Expand Specific Solutions05 Stability enhancement of mRNA lipid nanoparticles
Various approaches can be employed to enhance the stability of mRNA lipid nanoparticles during production and storage. These include the application of specific electrode parameters during formulation that affect particle size distribution and surface charge. The use of stabilizing agents, pH control, and temperature regulation during the production process can significantly impact nanoparticle stability. Advanced lyophilization techniques and the incorporation of cryoprotectants can further extend the shelf life of mRNA lipid nanoparticle formulations.Expand Specific Solutions
Key Industry Players in mRNA LNP Manufacturing
The mRNA lipid nanoparticle (LNP) design field is currently in a growth phase, with the market expected to reach significant expansion following the success of mRNA vaccines. The competitive landscape features academic institutions (Technion, Hebrew University, Zhejiang University) conducting foundational research on electrode parameters' influence on LNP formulation, alongside established pharmaceutical companies (Sanofi, Intellia Therapeutics) and specialized biotech firms (eTheRNA, GreenLight Biosciences) commercializing these technologies. Technical maturity varies significantly, with companies like Intellia and GreenLight Biosciences leading in translational applications, while academic institutions focus on optimizing electrode parameters to enhance transfection efficiency, stability, and manufacturing scalability. The field is characterized by increasing cross-sector collaborations between academia and industry to accelerate clinical translation.
Technion Research & Development Foundation Ltd.
Technical Solution: The Technion Research & Development Foundation has developed an innovative electrode-based approach to mRNA-LNP formulation that leverages principles from electrochemistry and microfluidics. Their system employs precisely engineered microelectrode arrays fabricated using semiconductor manufacturing techniques that enable unprecedented control over the electrical field distribution during LNP formation. By applying carefully calibrated electrical potentials (typically in the range of 0.2-1.5V) across these electrode arrays during the mixing of lipid components with mRNA, they create localized zones of electrophoretic mobility that enhance the interaction between ionizable lipids and nucleic acids. The Technion's approach incorporates multiple electrode pairs with independent control systems, allowing for the creation of complex electrical field gradients that guide the self-assembly process of LNPs. Their research has demonstrated that specific electrode pulse patterns (including frequency modulation between 5-200 Hz) can significantly influence the internal structure of LNPs, resulting in more efficient endosomal escape following cellular uptake. The foundation has further refined their technology by developing specialized electrode materials with optimized surface properties that minimize unwanted electrochemical reactions while maximizing control over the LNP formation process.
Strengths: Exceptional precision in controlling electrical parameters during LNP formation; demonstrated ability to create LNPs with novel structural characteristics; system designed with flexibility to accommodate different mRNA payloads. Weaknesses: Technology still primarily in research phase with limited large-scale manufacturing validation; more complex setup compared to conventional methods; requires specialized expertise in electrochemistry and microfluidics.
eTheRNA immunotherapies NV
Technical Solution: eTheRNA has developed the TriMix platform that incorporates electrode-mediated optimization for mRNA-LNP formulations specifically designed for immunotherapy applications. Their approach utilizes a proprietary electrode configuration within microfluidic mixing devices that generates controlled electrical fields to enhance the interaction between lipid components and mRNA molecules. By precisely modulating electrode parameters such as voltage (typically 0.5-2.0V), pulse width (microsecond range), and frequency (1-50 kHz), eTheRNA has achieved significant improvements in LNP homogeneity and stability. Their system incorporates platinum-iridium alloy electrodes with specialized surface treatments that minimize oxidation while maximizing charge distribution uniformity. This electrode configuration creates localized zones of electrophoretic mobility that facilitate more efficient encapsulation of mRNA within the lipid bilayer structure. The company has demonstrated that their electrode-optimized LNPs show enhanced endosomal escape properties, resulting in up to 3-fold improvement in protein expression levels in dendritic cells compared to conventional formulations.
Strengths: Specialized optimization for immunotherapy applications with demonstrated enhancement of immune response; excellent reproducibility across manufacturing batches; system designed for GMP-compliant production. Weaknesses: Technology platform more specialized for immunotherapy applications rather than broad therapeutic use; requires specialized equipment and expertise; potentially higher production costs compared to standard methods.
Critical Patents in Electrode-Based LNP Formulation
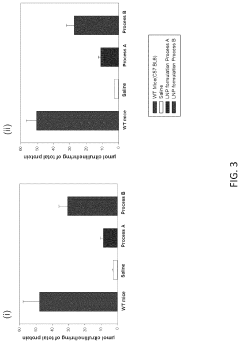
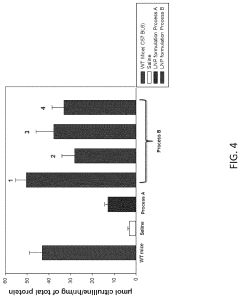
Process of Preparing mRNA-Loaded Lipid Nanoparticles
PatentPendingUS20240041789A1
Innovation
- A process involving the combination of pre-formed lipid nanoparticles with mRNA, where the nanoparticles are heated or maintained at specific temperatures during mixing to enhance encapsulation efficiency and protein expression, resulting in more potent and efficacious delivery through various routes of administration.
mRNA delivery method and composition thereof
PatentPendingUS20250288533A1
Innovation
- A lipid nanoparticle formulation with elevated levels of phosphatidylcholine lipid, ranging from 30 to 70 mol%, combined with ionizable lipid, sterol, and hydrophilic polymer-lipid conjugate, enhances mRNA delivery to extrahepatic tissues by improving circulation and reducing liver accumulation.
Scale-up Considerations for Industrial LNP Production
Scaling up mRNA lipid nanoparticle (LNP) production from laboratory to industrial scale presents significant challenges that must be addressed to ensure consistent quality and efficacy. The electrode parameters that prove effective at small scales may not translate directly to large-scale manufacturing environments, necessitating careful engineering considerations and process adaptations.
The transition from microfluidic devices used in research settings to industrial-scale mixers requires precise control over electrode materials, configurations, and electrical parameters. Industrial LNP production typically employs specialized equipment such as tangential flow filtration systems and large-scale microfluidic or T-junction mixers that must maintain consistent electrical field properties despite the increased volume.
Critical electrode parameters that must be carefully controlled during scale-up include electrode surface area-to-volume ratio, electrode material composition, and electrical potential distribution. These factors directly influence the formation kinetics of LNPs and subsequently their size distribution, encapsulation efficiency, and stability. Industrial systems often require modified electrode designs with enhanced durability and corrosion resistance to withstand continuous operation.
Temperature management becomes increasingly challenging at industrial scales, as the heat generated by electrical currents can significantly affect LNP formation. Advanced cooling systems integrated with electrode assemblies are essential to maintain optimal temperature profiles throughout the production process. Monitoring systems must be implemented to detect any deviations in electrical parameters that might compromise batch consistency.
Regulatory considerations also impact electrode parameter selection in industrial settings. Materials must comply with good manufacturing practice (GMP) standards, and process parameters must demonstrate reproducibility across multiple production runs. Validation protocols must verify that the scaled-up electrode systems produce LNPs with properties equivalent to those observed in clinical development batches.
Economic factors further influence electrode design choices for industrial production. While platinum or gold electrodes might be feasible for laboratory-scale experiments, industrial implementation may require more cost-effective alternatives with comparable electrochemical properties. The balance between electrode longevity, replacement costs, and product quality becomes a critical consideration for sustainable manufacturing operations.
Automation and real-time monitoring of electrode parameters represent another crucial aspect of industrial scale-up. Advanced control systems that can adjust electrical parameters in response to feedback from in-line analytical tools help maintain consistent LNP characteristics throughout extended production runs, ensuring batch-to-batch reproducibility essential for pharmaceutical applications.
The transition from microfluidic devices used in research settings to industrial-scale mixers requires precise control over electrode materials, configurations, and electrical parameters. Industrial LNP production typically employs specialized equipment such as tangential flow filtration systems and large-scale microfluidic or T-junction mixers that must maintain consistent electrical field properties despite the increased volume.
Critical electrode parameters that must be carefully controlled during scale-up include electrode surface area-to-volume ratio, electrode material composition, and electrical potential distribution. These factors directly influence the formation kinetics of LNPs and subsequently their size distribution, encapsulation efficiency, and stability. Industrial systems often require modified electrode designs with enhanced durability and corrosion resistance to withstand continuous operation.
Temperature management becomes increasingly challenging at industrial scales, as the heat generated by electrical currents can significantly affect LNP formation. Advanced cooling systems integrated with electrode assemblies are essential to maintain optimal temperature profiles throughout the production process. Monitoring systems must be implemented to detect any deviations in electrical parameters that might compromise batch consistency.
Regulatory considerations also impact electrode parameter selection in industrial settings. Materials must comply with good manufacturing practice (GMP) standards, and process parameters must demonstrate reproducibility across multiple production runs. Validation protocols must verify that the scaled-up electrode systems produce LNPs with properties equivalent to those observed in clinical development batches.
Economic factors further influence electrode design choices for industrial production. While platinum or gold electrodes might be feasible for laboratory-scale experiments, industrial implementation may require more cost-effective alternatives with comparable electrochemical properties. The balance between electrode longevity, replacement costs, and product quality becomes a critical consideration for sustainable manufacturing operations.
Automation and real-time monitoring of electrode parameters represent another crucial aspect of industrial scale-up. Advanced control systems that can adjust electrical parameters in response to feedback from in-line analytical tools help maintain consistent LNP characteristics throughout extended production runs, ensuring batch-to-batch reproducibility essential for pharmaceutical applications.
Regulatory Pathway for Electrode-Optimized LNP Products
The regulatory landscape for electrode-optimized Lipid Nanoparticle (LNP) products presents a complex pathway that requires careful navigation. Regulatory bodies such as the FDA in the United States and the EMA in Europe have established specific frameworks for evaluating novel drug delivery systems, particularly those involving nanotechnology and mRNA therapeutics. For electrode-optimized LNPs, manufacturers must demonstrate that the electrode parameters used during production consistently yield safe and effective products.
Primary regulatory considerations include characterization of the electrode-optimized LNPs, with particular emphasis on size distribution, zeta potential, encapsulation efficiency, and stability profiles. Regulatory agencies typically require comprehensive data showing how specific electrode parameters (voltage, current density, electrode material, geometry) directly influence these critical quality attributes. This correlation must be established through robust analytical methods and validated manufacturing processes.
The Chemistry, Manufacturing, and Controls (CMC) section of regulatory submissions requires detailed documentation of electrode specifications, operational parameters, and in-process controls. Manufacturers must implement appropriate process analytical technology (PAT) to monitor electrode performance during production, ensuring batch-to-batch consistency. Quality-by-Design (QbD) approaches are increasingly favored by regulators, necessitating the development of a design space that defines acceptable ranges for electrode parameters.
Preclinical testing requirements for electrode-optimized LNPs include standard toxicology studies with additional focus on potential unique toxicities that might arise from the electrode manufacturing process. Regulatory agencies may request specific studies addressing potential leachables or particulates from electrodes, particularly when novel electrode materials or configurations are employed.
Clinical development follows a traditional phased approach, though accelerated pathways may be available for mRNA therapeutics addressing unmet medical needs. Sponsors must demonstrate that electrode optimization does not introduce unexpected immunogenicity or alter the pharmacokinetic/pharmacodynamic profile of the encapsulated mRNA.
Post-approval considerations include continued process verification to ensure electrode parameters remain within the established design space throughout the product lifecycle. Change management protocols must be established for any modifications to electrode specifications or operating parameters, with appropriate bridging studies to demonstrate equivalence.
International harmonization efforts are underway to standardize regulatory approaches to advanced manufacturing technologies like electrode-optimized LNP production. The International Council for Harmonisation (ICH) has initiated discussions on guidelines specific to nanotechnology-based pharmaceuticals, which may eventually include specific provisions for electrode-based manufacturing processes.
Primary regulatory considerations include characterization of the electrode-optimized LNPs, with particular emphasis on size distribution, zeta potential, encapsulation efficiency, and stability profiles. Regulatory agencies typically require comprehensive data showing how specific electrode parameters (voltage, current density, electrode material, geometry) directly influence these critical quality attributes. This correlation must be established through robust analytical methods and validated manufacturing processes.
The Chemistry, Manufacturing, and Controls (CMC) section of regulatory submissions requires detailed documentation of electrode specifications, operational parameters, and in-process controls. Manufacturers must implement appropriate process analytical technology (PAT) to monitor electrode performance during production, ensuring batch-to-batch consistency. Quality-by-Design (QbD) approaches are increasingly favored by regulators, necessitating the development of a design space that defines acceptable ranges for electrode parameters.
Preclinical testing requirements for electrode-optimized LNPs include standard toxicology studies with additional focus on potential unique toxicities that might arise from the electrode manufacturing process. Regulatory agencies may request specific studies addressing potential leachables or particulates from electrodes, particularly when novel electrode materials or configurations are employed.
Clinical development follows a traditional phased approach, though accelerated pathways may be available for mRNA therapeutics addressing unmet medical needs. Sponsors must demonstrate that electrode optimization does not introduce unexpected immunogenicity or alter the pharmacokinetic/pharmacodynamic profile of the encapsulated mRNA.
Post-approval considerations include continued process verification to ensure electrode parameters remain within the established design space throughout the product lifecycle. Change management protocols must be established for any modifications to electrode specifications or operating parameters, with appropriate bridging studies to demonstrate equivalence.
International harmonization efforts are underway to standardize regulatory approaches to advanced manufacturing technologies like electrode-optimized LNP production. The International Council for Harmonisation (ICH) has initiated discussions on guidelines specific to nanotechnology-based pharmaceuticals, which may eventually include specific provisions for electrode-based manufacturing processes.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!