Comparison of Thermal Properties in Lipid Nanoparticle Systems
OCT 10, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Lipid Nanoparticle Thermal Properties Background and Objectives
Lipid nanoparticles (LNPs) have emerged as revolutionary delivery systems for various therapeutic agents, particularly nucleic acids such as mRNA and siRNA. The thermal properties of these nanoparticle systems play a crucial role in determining their stability, drug encapsulation efficiency, release kinetics, and ultimately their therapeutic efficacy. The evolution of LNP technology can be traced back to the early liposomal formulations in the 1960s, with significant advancements occurring in the past two decades that have culminated in the successful deployment of mRNA-LNP vaccines during the COVID-19 pandemic.
The thermal behavior of LNPs is governed by the physicochemical properties of their constituent lipids, including phase transition temperatures, enthalpy changes, and thermal stability. These properties are influenced by lipid composition, structural organization, and interactions with encapsulated therapeutic agents. Understanding these thermal characteristics is essential for optimizing LNP formulations for specific applications and ensuring their stability during manufacturing, storage, and administration.
Recent technological advancements have enabled more precise characterization of LNP thermal properties through techniques such as differential scanning calorimetry (DSC), thermogravimetric analysis (TGA), and temperature-dependent spectroscopic methods. These analytical approaches have revealed complex thermal behaviors in multi-component LNP systems that significantly impact their functional performance.
The field is witnessing a trend toward rational design of LNPs with tailored thermal properties to address specific delivery challenges. This includes the development of thermosensitive LNPs that respond to external thermal stimuli for controlled release applications, as well as formulations with enhanced thermal stability for improved shelf-life and resistance to temperature fluctuations during distribution.
The primary objective of this technical research is to comprehensively compare the thermal properties across different LNP systems, identifying key determinants of thermal behavior and establishing correlations between thermal characteristics and functional performance. This analysis aims to provide insights that can guide the rational design of next-generation LNP formulations with optimized thermal profiles for specific therapeutic applications.
Additional goals include mapping the relationship between lipid composition and thermal properties, evaluating the impact of manufacturing processes on thermal characteristics, and assessing how thermal properties influence the biological performance of LNPs in various physiological environments. By establishing these structure-property-function relationships, we seek to develop predictive models that can accelerate the development of LNP formulations with desired thermal behaviors.
This research is particularly timely given the expanding applications of LNPs beyond vaccine delivery to include gene therapy, protein replacement, and targeted drug delivery, each presenting unique requirements for thermal stability and responsiveness.
The thermal behavior of LNPs is governed by the physicochemical properties of their constituent lipids, including phase transition temperatures, enthalpy changes, and thermal stability. These properties are influenced by lipid composition, structural organization, and interactions with encapsulated therapeutic agents. Understanding these thermal characteristics is essential for optimizing LNP formulations for specific applications and ensuring their stability during manufacturing, storage, and administration.
Recent technological advancements have enabled more precise characterization of LNP thermal properties through techniques such as differential scanning calorimetry (DSC), thermogravimetric analysis (TGA), and temperature-dependent spectroscopic methods. These analytical approaches have revealed complex thermal behaviors in multi-component LNP systems that significantly impact their functional performance.
The field is witnessing a trend toward rational design of LNPs with tailored thermal properties to address specific delivery challenges. This includes the development of thermosensitive LNPs that respond to external thermal stimuli for controlled release applications, as well as formulations with enhanced thermal stability for improved shelf-life and resistance to temperature fluctuations during distribution.
The primary objective of this technical research is to comprehensively compare the thermal properties across different LNP systems, identifying key determinants of thermal behavior and establishing correlations between thermal characteristics and functional performance. This analysis aims to provide insights that can guide the rational design of next-generation LNP formulations with optimized thermal profiles for specific therapeutic applications.
Additional goals include mapping the relationship between lipid composition and thermal properties, evaluating the impact of manufacturing processes on thermal characteristics, and assessing how thermal properties influence the biological performance of LNPs in various physiological environments. By establishing these structure-property-function relationships, we seek to develop predictive models that can accelerate the development of LNP formulations with desired thermal behaviors.
This research is particularly timely given the expanding applications of LNPs beyond vaccine delivery to include gene therapy, protein replacement, and targeted drug delivery, each presenting unique requirements for thermal stability and responsiveness.
Market Applications and Demand Analysis for Thermally Optimized LNPs
The global market for lipid nanoparticle (LNP) systems has experienced significant growth, primarily driven by their application in mRNA vaccine delivery during the COVID-19 pandemic. This breakthrough has catalyzed interest in thermally optimized LNPs across multiple sectors, with the pharmaceutical industry leading adoption for drug delivery applications.
Current market analysis indicates that the global LNP market reached approximately $5.5 billion in 2022 and is projected to grow at a compound annual growth rate of 15.7% through 2030. Thermally optimized LNPs represent a specialized segment with particularly strong demand in advanced therapeutic applications where precise thermal stability is critical for maintaining drug efficacy.
The pharmaceutical sector demonstrates the most robust demand, with applications extending beyond vaccines to include cancer therapeutics, gene therapy, and treatment of rare genetic disorders. Companies developing mRNA-based therapeutics particularly value thermally stable LNPs that can protect fragile genetic material across varying temperature conditions, extending shelf life and reducing cold chain requirements.
Diagnostic applications represent another significant market segment, where thermally optimized LNPs enable more stable reagents for point-of-care testing and advanced molecular diagnostics. The market for LNP-based diagnostics is growing at 18.3% annually, with thermal optimization being a key differentiator for products targeting resource-limited settings.
Cosmetic and dermatological applications are emerging as a rapidly expanding market segment, with demand for thermally stable LNPs that can deliver active ingredients through the skin barrier. Premium skincare brands are increasingly incorporating these advanced delivery systems, with the market segment growing at 22.1% annually.
Regional analysis reveals North America currently dominates the market with 42% share, followed by Europe at 31% and Asia-Pacific at 21%. However, the Asia-Pacific region is experiencing the fastest growth rate at 19.8% annually, driven by expanding pharmaceutical manufacturing capabilities and increasing healthcare expenditure.
Customer requirements analysis indicates three primary demand drivers: extended temperature stability range, reduced cold chain dependency, and enhanced payload protection. End-users are willing to pay premium prices for LNP systems that demonstrate superior thermal stability profiles, particularly those that maintain integrity at temperatures between 2-8°C for extended periods.
Market forecasts suggest that thermally optimized LNPs will increasingly penetrate emerging therapeutic areas including immunotherapy, regenerative medicine, and personalized medicine applications. The ability to engineer LNPs with customized thermal properties for specific therapeutic payloads represents a significant competitive advantage in these high-value markets.
Current market analysis indicates that the global LNP market reached approximately $5.5 billion in 2022 and is projected to grow at a compound annual growth rate of 15.7% through 2030. Thermally optimized LNPs represent a specialized segment with particularly strong demand in advanced therapeutic applications where precise thermal stability is critical for maintaining drug efficacy.
The pharmaceutical sector demonstrates the most robust demand, with applications extending beyond vaccines to include cancer therapeutics, gene therapy, and treatment of rare genetic disorders. Companies developing mRNA-based therapeutics particularly value thermally stable LNPs that can protect fragile genetic material across varying temperature conditions, extending shelf life and reducing cold chain requirements.
Diagnostic applications represent another significant market segment, where thermally optimized LNPs enable more stable reagents for point-of-care testing and advanced molecular diagnostics. The market for LNP-based diagnostics is growing at 18.3% annually, with thermal optimization being a key differentiator for products targeting resource-limited settings.
Cosmetic and dermatological applications are emerging as a rapidly expanding market segment, with demand for thermally stable LNPs that can deliver active ingredients through the skin barrier. Premium skincare brands are increasingly incorporating these advanced delivery systems, with the market segment growing at 22.1% annually.
Regional analysis reveals North America currently dominates the market with 42% share, followed by Europe at 31% and Asia-Pacific at 21%. However, the Asia-Pacific region is experiencing the fastest growth rate at 19.8% annually, driven by expanding pharmaceutical manufacturing capabilities and increasing healthcare expenditure.
Customer requirements analysis indicates three primary demand drivers: extended temperature stability range, reduced cold chain dependency, and enhanced payload protection. End-users are willing to pay premium prices for LNP systems that demonstrate superior thermal stability profiles, particularly those that maintain integrity at temperatures between 2-8°C for extended periods.
Market forecasts suggest that thermally optimized LNPs will increasingly penetrate emerging therapeutic areas including immunotherapy, regenerative medicine, and personalized medicine applications. The ability to engineer LNPs with customized thermal properties for specific therapeutic payloads represents a significant competitive advantage in these high-value markets.
Current Thermal Characterization Challenges in LNP Systems
Despite significant advancements in lipid nanoparticle (LNP) technology, thermal characterization remains one of the most challenging aspects in LNP system development and optimization. Current differential scanning calorimetry (DSC) methods often struggle with the nanoscale dimensions of LNPs, resulting in signal-to-noise ratio issues that complicate accurate phase transition detection. This is particularly problematic when analyzing heterogeneous LNP formulations where multiple lipid components interact in complex ways.
Traditional thermal analysis techniques frequently fail to distinguish between bulk lipid behavior and the unique thermal properties that emerge at the nanoscale. The interfacial effects between different lipid components and between lipids and encapsulated materials (such as mRNA or small molecules) create thermal signatures that conventional equipment may not adequately resolve. Furthermore, the presence of stabilizers, cryoprotectants, and buffer components introduces additional variables that can mask or alter the thermal behavior of the core LNP structure.
Sample preparation inconsistencies represent another significant challenge in thermal characterization. Minor variations in concentration, hydration state, or thermal history can dramatically affect measurement outcomes, making reproducibility difficult to achieve across different laboratories or manufacturing batches. This becomes especially problematic during scale-up processes where maintaining consistent thermal properties is critical for product stability and efficacy.
The dynamic nature of LNPs presents additional complications for thermal analysis. These systems can undergo time-dependent structural reorganization, particularly in response to temperature changes during measurement. Current methodologies often fail to capture these kinetic aspects, providing only static snapshots rather than comprehensive thermal behavior profiles. This limitation is particularly relevant for understanding LNP stability during storage and administration.
Correlation between thermal properties and functional performance remains poorly established. While researchers can measure thermal transitions, translating these data into predictive models for drug release kinetics, cellular uptake efficiency, or in vivo stability continues to challenge the field. The lack of standardized protocols for thermal characterization further complicates cross-study comparisons and technology transfer processes.
Emerging techniques such as nanocalorimetry and modulated temperature DSC offer potential solutions but face implementation barriers including high equipment costs, specialized expertise requirements, and limited accessibility. Additionally, computational modeling approaches that could complement experimental thermal characterization remain underdeveloped for complex LNP systems, with current models struggling to account for the multifactorial interactions that determine thermal behavior.
Traditional thermal analysis techniques frequently fail to distinguish between bulk lipid behavior and the unique thermal properties that emerge at the nanoscale. The interfacial effects between different lipid components and between lipids and encapsulated materials (such as mRNA or small molecules) create thermal signatures that conventional equipment may not adequately resolve. Furthermore, the presence of stabilizers, cryoprotectants, and buffer components introduces additional variables that can mask or alter the thermal behavior of the core LNP structure.
Sample preparation inconsistencies represent another significant challenge in thermal characterization. Minor variations in concentration, hydration state, or thermal history can dramatically affect measurement outcomes, making reproducibility difficult to achieve across different laboratories or manufacturing batches. This becomes especially problematic during scale-up processes where maintaining consistent thermal properties is critical for product stability and efficacy.
The dynamic nature of LNPs presents additional complications for thermal analysis. These systems can undergo time-dependent structural reorganization, particularly in response to temperature changes during measurement. Current methodologies often fail to capture these kinetic aspects, providing only static snapshots rather than comprehensive thermal behavior profiles. This limitation is particularly relevant for understanding LNP stability during storage and administration.
Correlation between thermal properties and functional performance remains poorly established. While researchers can measure thermal transitions, translating these data into predictive models for drug release kinetics, cellular uptake efficiency, or in vivo stability continues to challenge the field. The lack of standardized protocols for thermal characterization further complicates cross-study comparisons and technology transfer processes.
Emerging techniques such as nanocalorimetry and modulated temperature DSC offer potential solutions but face implementation barriers including high equipment costs, specialized expertise requirements, and limited accessibility. Additionally, computational modeling approaches that could complement experimental thermal characterization remain underdeveloped for complex LNP systems, with current models struggling to account for the multifactorial interactions that determine thermal behavior.
Methodologies for Thermal Property Comparison in LNP Systems
01 Thermal stability of lipid nanoparticles
Lipid nanoparticle systems can be formulated to maintain stability across various temperature ranges. The thermal properties of these systems are critical for preserving the integrity of encapsulated active ingredients during storage and transportation. Specific lipid compositions and stabilizers can be incorporated to prevent phase separation, aggregation, or degradation when exposed to temperature fluctuations. These formulations often undergo thermal cycling tests to ensure their robustness under different environmental conditions.- Thermal stability of lipid nanoparticle formulations: Lipid nanoparticle systems can be engineered to maintain structural integrity and functional properties across various temperature ranges. These formulations often incorporate specific lipid compositions and stabilizers that prevent degradation during temperature fluctuations. Thermal stability is critical for preserving the encapsulated active ingredients and ensuring consistent performance in different environmental conditions. Advanced formulation techniques can enhance the temperature resistance of lipid nanoparticles, making them suitable for applications requiring thermal processing or storage at various temperatures.
- Phase transition behavior in lipid nanoparticles: Lipid nanoparticles exhibit distinct phase transition behaviors that significantly impact their functionality. These transitions, including solid-to-liquid crystalline transformations, occur at specific temperatures and influence drug release kinetics, stability, and cellular interactions. Understanding and controlling these phase transitions is essential for designing lipid nanoparticle systems with predictable performance characteristics. The melting and crystallization properties of the lipid components determine the physical state of the nanoparticles at physiological temperatures, affecting their ability to encapsulate and deliver therapeutic payloads.
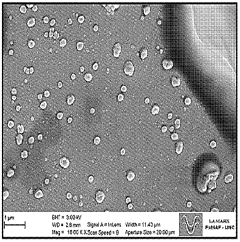
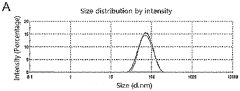
- Thermal analysis techniques for lipid nanoparticle characterization: Various thermal analysis methods are employed to characterize the thermal properties of lipid nanoparticle systems. Differential scanning calorimetry (DSC), thermogravimetric analysis (TGA), and isothermal titration calorimetry provide valuable insights into the thermal behavior of these nanostructures. These techniques help determine critical parameters such as phase transition temperatures, enthalpy changes, and thermal stability profiles. The thermal characterization data guides formulation optimization and quality control processes, ensuring consistent performance of lipid nanoparticle-based products.
- Temperature-responsive lipid nanoparticle systems: Temperature-responsive lipid nanoparticles are designed to undergo structural or functional changes in response to thermal stimuli. These smart delivery systems can release their payload at specific temperatures, enabling targeted delivery to tissues with altered thermal profiles, such as tumor microenvironments. The temperature-triggered release mechanism typically relies on phase transitions or conformational changes in the lipid bilayer structure. By incorporating thermosensitive lipids or polymers, these nanoparticles can achieve precise control over the timing and location of therapeutic agent release, enhancing treatment efficacy while reducing side effects.
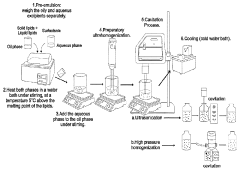
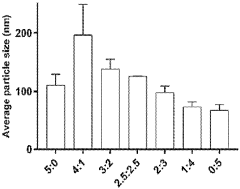
- Thermal processing methods for lipid nanoparticle production: Thermal processing plays a crucial role in the manufacturing of lipid nanoparticle systems. Techniques such as high-pressure homogenization, microfluidics, and hot melt extrusion utilize controlled temperature conditions to form nanoparticles with desired characteristics. The thermal history during production significantly influences particle size distribution, morphology, and encapsulation efficiency. Precise temperature control during processing is essential for ensuring batch-to-batch consistency and product quality. Advanced thermal processing methods enable the scalable production of lipid nanoparticles with tailored thermal properties for various pharmaceutical and biomedical applications.
02 Phase transition behavior of lipid nanoparticles
The phase transition behavior of lipid nanoparticles is a key thermal property that affects their functionality. Different lipid compositions exhibit unique melting points, crystallization patterns, and polymorphic transitions that can be characterized using differential scanning calorimetry and other thermal analysis techniques. Understanding these phase transitions is essential for designing lipid nanoparticle systems with predictable release profiles and stability characteristics. The transition temperature can be modified by adjusting the ratio of solid to liquid lipids or incorporating specific additives.Expand Specific Solutions03 Thermosensitive lipid nanoparticles for controlled release
Thermosensitive lipid nanoparticle systems can be designed to respond to temperature changes for controlled release applications. These systems utilize lipids with specific melting points that undergo structural reorganization when exposed to thermal stimuli, triggering the release of encapsulated compounds. This property is particularly valuable for targeted drug delivery applications where release can be activated by external heat sources or the naturally elevated temperatures in diseased tissues. The temperature-responsive behavior can be fine-tuned by incorporating specific lipid blends and surface modifiers.Expand Specific Solutions04 Thermal processing methods for lipid nanoparticle production
Various thermal processing methods are employed in the production of lipid nanoparticle systems, including hot homogenization, microemulsion, and solvent evaporation techniques. The thermal conditions during manufacturing significantly impact the particle size distribution, encapsulation efficiency, and long-term stability of the resulting nanoparticles. Precise temperature control during production is essential to ensure consistent quality attributes. Post-production thermal treatments can also be applied to modify crystallinity and improve stability characteristics of the lipid nanoparticle systems.Expand Specific Solutions05 Thermal characterization techniques for lipid nanoparticles
Advanced thermal characterization techniques are essential for analyzing the properties of lipid nanoparticle systems. These include differential scanning calorimetry (DSC), thermogravimetric analysis (TGA), and isothermal titration calorimetry (ITC), which provide insights into melting behavior, thermal stability, and interaction energetics. These analytical methods help in understanding the relationship between lipid composition, structural organization, and thermal properties. The thermal characterization data is crucial for quality control, formulation optimization, and predicting the in vivo performance of lipid nanoparticle systems.Expand Specific Solutions
Leading Research Groups and Companies in LNP Thermal Engineering
The thermal properties of lipid nanoparticle (LNP) systems are currently at a critical development stage, with the market expanding rapidly due to mRNA vaccine applications. The competitive landscape is characterized by a mix of specialized biotech firms (Translate Bio, Axelyf, Abogen Biosciences), pharmaceutical companies (Metis Pharmaceuticals), and academic institutions (King's College London, Rice University, Vanderbilt). Technical maturity varies significantly, with companies like Translate Bio and Abogen leading in commercial applications, while research institutions focus on fundamental thermal characterization. The field is transitioning from early-stage research to commercial implementation, with thermal optimization becoming crucial for stability, efficacy, and manufacturing of LNP-based therapeutics, creating opportunities for cross-sector collaborations between industry leaders and academic pioneers.
Suzhou Abogen Biosciences Co., Ltd.
Technical Solution: Abogen has developed proprietary lipid nanoparticle (LNP) formulations with optimized thermal properties for mRNA delivery. Their technology focuses on the rational design of lipid components with specific phase transition temperatures to enhance stability during storage and controlled release upon administration. The company's approach involves systematic characterization of thermal behaviors including differential scanning calorimetry (DSC) to measure phase transitions, thermogravimetric analysis (TGA) to assess thermal stability, and dynamic light scattering (DLS) to monitor temperature-dependent size changes. Abogen's LNPs incorporate ionizable lipids with tailored pKa values that respond to temperature changes, allowing for temperature-triggered release mechanisms. Their formulations demonstrate superior thermal stability at 2-8°C for extended periods and can withstand brief exposure to temperatures up to 25°C without significant degradation of encapsulated mRNA.
Strengths: Advanced thermal stability profiles allowing for less stringent cold chain requirements compared to competitors; proprietary ionizable lipid compositions with optimized phase transition temperatures. Weaknesses: Limited published data on long-term stability under various thermal stress conditions; potential challenges in scaling up production while maintaining consistent thermal properties.
William Marsh Rice University
Technical Solution: Rice University has established a significant research program focused on the thermal characterization and optimization of lipid nanoparticle systems. Their approach combines advanced biophysical techniques with innovative nanomaterials science to develop LNPs with precisely controlled thermal properties. The university's research team has pioneered the use of temperature-dependent fluorescence spectroscopy to monitor lipid phase transitions in real-time, providing insights into how thermal fluctuations affect nanoparticle structure and cargo release kinetics. Their work has revealed critical correlations between lipid composition, phase transition temperatures, and delivery efficiency across different physiological environments. Rice researchers have developed novel temperature-responsive lipid formulations incorporating thermosensitive polymers that undergo conformational changes at specific temperatures, enabling triggered release of encapsulated compounds. Their thermal analysis platform includes high-precision differential scanning calorimetry coupled with small-angle neutron scattering to characterize the nanoscale organization of lipid bilayers as a function of temperature. This multidisciplinary approach has yielded fundamental insights into how thermal properties can be manipulated to enhance stability, cellular uptake, and controlled release in LNP-based delivery systems.
Strengths: Strong fundamental science approach providing mechanistic understanding of thermal behavior in LNPs; innovative integration of responsive polymers with lipid systems; access to specialized characterization techniques through academic collaborations. Weaknesses: Research primarily at laboratory scale with limited translation to industrial production; focus on fundamental mechanisms rather than specific therapeutic applications; potential intellectual property constraints in commercial development.
Critical Thermal Parameters and Their Impact on LNP Performance
Lipid nanoparticles as active molecule carriers in ophthalmic, dermatological, and/or cosmetic applications, and process for production thereof
PatentWO2024102798A1
Innovation
- Lipid nanoparticles comprising solid and liquid lipids with non-ionic surfactants, produced through a cavitation process, achieving an average particle size of around 80 nm, enhancing drug and cosmetic permeability and therapeutic efficacy.
Polyethylene glycol lipid and use thereof
PatentPendingUS20240350640A1
Innovation
- A new type of polyethylene glycol lipid with non-cleavable linkers, capable of being positively charged in specific pH environments, forms stable particles with bioactive substances for enhanced delivery efficacy.
Regulatory Considerations for Thermally Modified LNP Products
The regulatory landscape for thermally modified Lipid Nanoparticle (LNP) products presents unique challenges that require careful navigation. Regulatory bodies, including the FDA, EMA, and other international agencies, have established specific frameworks for evaluating the safety and efficacy of LNP-based therapeutics. When thermal properties are intentionally modified, these regulatory considerations become more complex.
Temperature-modified LNPs must meet stringent quality control standards that specifically address thermal stability profiles. Regulatory agencies typically require comprehensive data demonstrating that thermal modifications do not adversely affect critical quality attributes such as particle size distribution, encapsulation efficiency, and drug release kinetics across the intended storage and administration temperature range.
Thermal characterization documentation has become increasingly important in regulatory submissions. Manufacturers must provide detailed thermal analysis data, including differential scanning calorimetry (DSC) profiles, thermogravimetric analysis, and stability studies under various temperature conditions. These data must demonstrate consistent thermal behavior batch-to-batch and support shelf-life claims.
Accelerated stability testing protocols for thermally modified LNPs often require customization beyond standard ICH guidelines. Regulatory agencies may request additional temperature excursion studies to evaluate product robustness under real-world conditions, particularly for products with novel thermal transition properties or those requiring specialized storage conditions.
Manufacturing process validation for thermally sensitive LNPs demands particular attention to temperature-controlled critical process parameters. Regulatory expectations include thorough risk assessments identifying potential thermal variability points in the manufacturing process and appropriate control strategies. Process analytical technology (PAT) implementation for real-time thermal monitoring is increasingly viewed favorably by regulators.
Clinical trial design for thermally modified LNP products must address potential temperature-related safety concerns. Regulatory agencies typically require preclinical studies that specifically evaluate the impact of thermal transitions on biodistribution, cellular uptake, and potential toxicity. Clinical protocols must include appropriate temperature management procedures and monitoring throughout the supply chain.
Post-approval commitments often include ongoing thermal stability monitoring programs and temperature excursion studies. Manufacturers may be required to implement temperature-monitoring systems throughout the distribution network and establish clear protocols for handling temperature deviations, with specific reporting requirements to regulatory authorities when predefined thermal limits are exceeded.
Temperature-modified LNPs must meet stringent quality control standards that specifically address thermal stability profiles. Regulatory agencies typically require comprehensive data demonstrating that thermal modifications do not adversely affect critical quality attributes such as particle size distribution, encapsulation efficiency, and drug release kinetics across the intended storage and administration temperature range.
Thermal characterization documentation has become increasingly important in regulatory submissions. Manufacturers must provide detailed thermal analysis data, including differential scanning calorimetry (DSC) profiles, thermogravimetric analysis, and stability studies under various temperature conditions. These data must demonstrate consistent thermal behavior batch-to-batch and support shelf-life claims.
Accelerated stability testing protocols for thermally modified LNPs often require customization beyond standard ICH guidelines. Regulatory agencies may request additional temperature excursion studies to evaluate product robustness under real-world conditions, particularly for products with novel thermal transition properties or those requiring specialized storage conditions.
Manufacturing process validation for thermally sensitive LNPs demands particular attention to temperature-controlled critical process parameters. Regulatory expectations include thorough risk assessments identifying potential thermal variability points in the manufacturing process and appropriate control strategies. Process analytical technology (PAT) implementation for real-time thermal monitoring is increasingly viewed favorably by regulators.
Clinical trial design for thermally modified LNP products must address potential temperature-related safety concerns. Regulatory agencies typically require preclinical studies that specifically evaluate the impact of thermal transitions on biodistribution, cellular uptake, and potential toxicity. Clinical protocols must include appropriate temperature management procedures and monitoring throughout the supply chain.
Post-approval commitments often include ongoing thermal stability monitoring programs and temperature excursion studies. Manufacturers may be required to implement temperature-monitoring systems throughout the distribution network and establish clear protocols for handling temperature deviations, with specific reporting requirements to regulatory authorities when predefined thermal limits are exceeded.
Scale-up Challenges in Maintaining Thermal Properties of LNPs
The scale-up of lipid nanoparticle (LNP) production from laboratory to industrial scale presents significant challenges in maintaining consistent thermal properties. As production volumes increase, heat transfer dynamics change dramatically, affecting critical parameters such as phase transition temperatures, crystallization behavior, and thermal stability of the nanoparticle systems.
One of the primary challenges is the non-linear scaling of heat distribution in larger reaction vessels. Laboratory-scale production typically involves volumes of 10-100 mL, while industrial production may require batches of hundreds of liters. This volume increase creates temperature gradients within the mixture that can lead to heterogeneous nucleation and inconsistent particle formation, ultimately affecting the thermal characteristics of the final LNP product.
Equipment design presents another significant hurdle. Industrial-scale mixing systems cannot replicate the precise thermal control achieved in laboratory settings. The increased distance between heating/cooling elements and the reaction mixture in larger vessels results in delayed thermal response times. This lag can critically impact the lipid phase transitions that determine nanoparticle structure and stability, particularly for temperature-sensitive lipid components like ionizable cationic lipids used in mRNA delivery systems.
Process parameters require substantial recalibration during scale-up. The heating and cooling rates that yield optimal thermal properties at small scale cannot be directly translated to larger systems. For instance, rapid cooling techniques that produce desirable crystalline structures in laboratory settings become impractical at industrial scale, necessitating alternative approaches to achieve comparable thermal profiles.
Energy input considerations also change dramatically with scale. The power required for maintaining precise temperature control increases non-proportionally with batch size. This creates challenges in designing energy-efficient processes that can still deliver the narrow temperature ranges required for consistent LNP formation without compromising the thermal properties that influence drug encapsulation efficiency and release kinetics.
Monitoring and control systems face increased complexity during scale-up. Real-time thermal mapping becomes more challenging in larger vessels, making it difficult to ensure uniform temperature distribution. Advanced thermal imaging and multiple-point sensing technologies are often required to maintain the thermal homogeneity necessary for consistent LNP quality, adding significant complexity and cost to the manufacturing process.
Regulatory considerations further complicate scale-up efforts, as authorities require demonstration that the thermal properties critical to product performance remain consistent between development and commercial manufacturing scales. This necessitates comprehensive thermal characterization studies and robust process analytical technologies to ensure that scaled-up processes deliver LNPs with thermal properties equivalent to those proven effective in clinical studies.
One of the primary challenges is the non-linear scaling of heat distribution in larger reaction vessels. Laboratory-scale production typically involves volumes of 10-100 mL, while industrial production may require batches of hundreds of liters. This volume increase creates temperature gradients within the mixture that can lead to heterogeneous nucleation and inconsistent particle formation, ultimately affecting the thermal characteristics of the final LNP product.
Equipment design presents another significant hurdle. Industrial-scale mixing systems cannot replicate the precise thermal control achieved in laboratory settings. The increased distance between heating/cooling elements and the reaction mixture in larger vessels results in delayed thermal response times. This lag can critically impact the lipid phase transitions that determine nanoparticle structure and stability, particularly for temperature-sensitive lipid components like ionizable cationic lipids used in mRNA delivery systems.
Process parameters require substantial recalibration during scale-up. The heating and cooling rates that yield optimal thermal properties at small scale cannot be directly translated to larger systems. For instance, rapid cooling techniques that produce desirable crystalline structures in laboratory settings become impractical at industrial scale, necessitating alternative approaches to achieve comparable thermal profiles.
Energy input considerations also change dramatically with scale. The power required for maintaining precise temperature control increases non-proportionally with batch size. This creates challenges in designing energy-efficient processes that can still deliver the narrow temperature ranges required for consistent LNP formation without compromising the thermal properties that influence drug encapsulation efficiency and release kinetics.
Monitoring and control systems face increased complexity during scale-up. Real-time thermal mapping becomes more challenging in larger vessels, making it difficult to ensure uniform temperature distribution. Advanced thermal imaging and multiple-point sensing technologies are often required to maintain the thermal homogeneity necessary for consistent LNP quality, adding significant complexity and cost to the manufacturing process.
Regulatory considerations further complicate scale-up efforts, as authorities require demonstration that the thermal properties critical to product performance remain consistent between development and commercial manufacturing scales. This necessitates comprehensive thermal characterization studies and robust process analytical technologies to ensure that scaled-up processes deliver LNPs with thermal properties equivalent to those proven effective in clinical studies.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!