What Regulations Exist for mRNA Nanoparticle Distribution
OCT 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
mRNA Nanoparticle Regulatory Background and Objectives
Messenger RNA (mRNA) nanoparticle technology has emerged as a revolutionary platform in modern medicine, most notably demonstrated by the rapid development and deployment of COVID-19 vaccines. This technology represents the convergence of molecular biology, nanotechnology, and pharmaceutical science, enabling targeted delivery of genetic instructions to cells for therapeutic protein production. The evolution of mRNA therapeutics spans several decades, beginning with fundamental research in the 1990s on lipid nanoparticles (LNPs) as delivery vehicles, progressing through preclinical studies in the 2000s, and culminating in breakthrough clinical applications in the 2020s.
The regulatory landscape for mRNA nanoparticle distribution has developed reactively rather than proactively, with frameworks being established or modified in response to technological advancements. Initially, these products were evaluated under existing biologics and advanced therapy medicinal products (ATMPs) regulations, but their unique characteristics have prompted regulatory authorities worldwide to develop more specific guidance.
Key regulatory milestones include the FDA's 2017 guidance on liposomal drug products, the EMA's reflection paper on nanotechnology-based medicinal products, and the accelerated regulatory pathways established during the COVID-19 pandemic. These developments highlight the dynamic nature of regulation in this field, balancing innovation facilitation with safety assurance.
The primary technical objectives in the regulatory domain for mRNA nanoparticle distribution include establishing standardized characterization methods for nanoparticle formulations, developing validated stability testing protocols specific to mRNA-LNP complexes, and creating harmonized guidelines for manufacturing process validation. Additionally, there is a pressing need for consensus on biodistribution assessment methodologies and long-term safety monitoring frameworks.
Regulatory convergence represents another critical objective, as current disparities between major regulatory bodies (FDA, EMA, PMDA, NMPA) create challenges for global development programs. Industry stakeholders and regulatory authorities are increasingly collaborating through initiatives like the International Council for Harmonisation (ICH) to develop globally applicable standards.
The evolution of regulations is expected to follow a risk-based approach, with increasing granularity as the technology matures. Future regulatory frameworks will likely incorporate advanced analytics for real-time release testing, novel biocompatibility assessment methods, and specialized pharmacovigilance systems tailored to genetic medicines.
Understanding this regulatory background is essential for anticipating compliance requirements, identifying potential regulatory hurdles, and developing strategic approaches to navigate the evolving landscape of mRNA nanoparticle distribution regulations.
The regulatory landscape for mRNA nanoparticle distribution has developed reactively rather than proactively, with frameworks being established or modified in response to technological advancements. Initially, these products were evaluated under existing biologics and advanced therapy medicinal products (ATMPs) regulations, but their unique characteristics have prompted regulatory authorities worldwide to develop more specific guidance.
Key regulatory milestones include the FDA's 2017 guidance on liposomal drug products, the EMA's reflection paper on nanotechnology-based medicinal products, and the accelerated regulatory pathways established during the COVID-19 pandemic. These developments highlight the dynamic nature of regulation in this field, balancing innovation facilitation with safety assurance.
The primary technical objectives in the regulatory domain for mRNA nanoparticle distribution include establishing standardized characterization methods for nanoparticle formulations, developing validated stability testing protocols specific to mRNA-LNP complexes, and creating harmonized guidelines for manufacturing process validation. Additionally, there is a pressing need for consensus on biodistribution assessment methodologies and long-term safety monitoring frameworks.
Regulatory convergence represents another critical objective, as current disparities between major regulatory bodies (FDA, EMA, PMDA, NMPA) create challenges for global development programs. Industry stakeholders and regulatory authorities are increasingly collaborating through initiatives like the International Council for Harmonisation (ICH) to develop globally applicable standards.
The evolution of regulations is expected to follow a risk-based approach, with increasing granularity as the technology matures. Future regulatory frameworks will likely incorporate advanced analytics for real-time release testing, novel biocompatibility assessment methods, and specialized pharmacovigilance systems tailored to genetic medicines.
Understanding this regulatory background is essential for anticipating compliance requirements, identifying potential regulatory hurdles, and developing strategic approaches to navigate the evolving landscape of mRNA nanoparticle distribution regulations.
Market Analysis for mRNA Nanoparticle Therapeutics
The mRNA nanoparticle therapeutics market has experienced unprecedented growth following the successful deployment of COVID-19 vaccines. Current market valuations indicate that the global mRNA therapeutics market reached approximately $40 billion in 2021, with projections suggesting a compound annual growth rate (CAGR) of 13.2% through 2030. This remarkable expansion is driven by both established pharmaceutical giants and emerging biotech companies investing heavily in this transformative technology.
Market segmentation reveals diverse applications beyond vaccines, including cancer immunotherapy, protein replacement therapies, and treatments for rare genetic disorders. Oncology represents the fastest-growing segment, with over 30% of mRNA clinical trials focusing on cancer treatments. Geographically, North America dominates with approximately 45% market share, followed by Europe at 30% and Asia-Pacific showing the highest growth rate at 15.8% annually.
Consumer demand analysis indicates strong acceptance of mRNA technology following COVID-19 vaccine successes, with 78% of healthcare providers expressing confidence in expanding mRNA applications. However, challenges remain in public perception regarding novel therapeutic modalities, particularly in developing markets where regulatory frameworks are still evolving.
Pricing structures for mRNA therapeutics reflect their complex manufacturing requirements and specialized delivery systems. Current treatments command premium pricing, with cancer therapeutics averaging $100,000-$350,000 per treatment course. This presents accessibility challenges that regulatory bodies worldwide are actively addressing through various pricing control mechanisms and reimbursement pathways.
Supply chain considerations significantly impact market dynamics, with cold chain requirements for mRNA products creating distribution barriers, particularly in regions with limited infrastructure. Regulatory requirements for temperature-controlled transportation add approximately 30% to distribution costs compared to conventional pharmaceuticals.
Competitive analysis reveals a market dominated by key players including Moderna, BioNTech, CureVac, and Arcturus Therapeutics, alongside pharmaceutical giants like Pfizer, Merck, and Sanofi who have established strategic partnerships. Market concentration remains high, with the top five companies controlling 70% of intellectual property in the space.
Investment trends show continued capital influx, with venture capital funding for mRNA startups exceeding $6 billion in 2022 alone. This reflects strong investor confidence in the technology's potential to revolutionize multiple therapeutic areas beyond infectious diseases.
Market segmentation reveals diverse applications beyond vaccines, including cancer immunotherapy, protein replacement therapies, and treatments for rare genetic disorders. Oncology represents the fastest-growing segment, with over 30% of mRNA clinical trials focusing on cancer treatments. Geographically, North America dominates with approximately 45% market share, followed by Europe at 30% and Asia-Pacific showing the highest growth rate at 15.8% annually.
Consumer demand analysis indicates strong acceptance of mRNA technology following COVID-19 vaccine successes, with 78% of healthcare providers expressing confidence in expanding mRNA applications. However, challenges remain in public perception regarding novel therapeutic modalities, particularly in developing markets where regulatory frameworks are still evolving.
Pricing structures for mRNA therapeutics reflect their complex manufacturing requirements and specialized delivery systems. Current treatments command premium pricing, with cancer therapeutics averaging $100,000-$350,000 per treatment course. This presents accessibility challenges that regulatory bodies worldwide are actively addressing through various pricing control mechanisms and reimbursement pathways.
Supply chain considerations significantly impact market dynamics, with cold chain requirements for mRNA products creating distribution barriers, particularly in regions with limited infrastructure. Regulatory requirements for temperature-controlled transportation add approximately 30% to distribution costs compared to conventional pharmaceuticals.
Competitive analysis reveals a market dominated by key players including Moderna, BioNTech, CureVac, and Arcturus Therapeutics, alongside pharmaceutical giants like Pfizer, Merck, and Sanofi who have established strategic partnerships. Market concentration remains high, with the top five companies controlling 70% of intellectual property in the space.
Investment trends show continued capital influx, with venture capital funding for mRNA startups exceeding $6 billion in 2022 alone. This reflects strong investor confidence in the technology's potential to revolutionize multiple therapeutic areas beyond infectious diseases.
Global Regulatory Landscape and Technical Barriers
The global regulatory landscape for mRNA nanoparticle distribution remains in a developmental phase, with significant variations across different regions. In the United States, the FDA has established a framework through its Center for Biologics Evaluation and Research (CBER) and Center for Drug Evaluation and Research (CDER), which jointly oversee mRNA-based therapeutics. These regulatory bodies apply existing frameworks for biological products and nanomedicines, requiring comprehensive safety data, manufacturing consistency, and clinical efficacy evidence.
The European Medicines Agency (EMA) has implemented the Advanced Therapy Medicinal Products (ATMP) regulation, which encompasses mRNA therapeutics. Additionally, the EMA's Innovation Task Force provides scientific advice specifically for novel technologies like mRNA nanoparticles. European regulations place particular emphasis on environmental risk assessments and long-term safety monitoring protocols.
In Asia, regulatory approaches vary significantly. Japan's Pharmaceuticals and Medical Devices Agency (PMDA) has established the Sakigake designation system to expedite innovative therapies, including mRNA technologies. China's National Medical Products Administration (NMPA) has recently updated its guidelines to accommodate novel therapeutic modalities, though specific provisions for mRNA nanoparticles remain limited.
Technical barriers to regulatory compliance include standardization challenges in characterization methods for lipid nanoparticles (LNPs). Current analytical techniques struggle to provide consistent measurements of particle size distribution, encapsulation efficiency, and stability across different manufacturing batches. This inconsistency complicates regulatory submissions and approval processes.
Manufacturing scale-up represents another significant barrier, as processes that work effectively at laboratory scale often encounter unforeseen challenges during industrial production. Regulatory bodies increasingly require demonstration of manufacturing consistency across multiple batches and production scales.
Stability and cold-chain requirements pose both regulatory and logistical challenges. Current mRNA formulations typically require ultra-cold storage conditions (-70°C to -20°C), which complicates distribution networks and raises regulatory concerns about product integrity throughout the supply chain.
Safety monitoring frameworks remain underdeveloped for mRNA nanoparticles, with regulatory agencies still determining appropriate post-market surveillance requirements. The novelty of the technology means that long-term effects are not fully understood, leading to cautious regulatory approaches that may delay market access.
Cross-border harmonization efforts are emerging through initiatives like the International Council for Harmonisation (ICH), but significant gaps remain in aligning regulatory requirements across different jurisdictions, creating additional complexity for global distribution strategies.
The European Medicines Agency (EMA) has implemented the Advanced Therapy Medicinal Products (ATMP) regulation, which encompasses mRNA therapeutics. Additionally, the EMA's Innovation Task Force provides scientific advice specifically for novel technologies like mRNA nanoparticles. European regulations place particular emphasis on environmental risk assessments and long-term safety monitoring protocols.
In Asia, regulatory approaches vary significantly. Japan's Pharmaceuticals and Medical Devices Agency (PMDA) has established the Sakigake designation system to expedite innovative therapies, including mRNA technologies. China's National Medical Products Administration (NMPA) has recently updated its guidelines to accommodate novel therapeutic modalities, though specific provisions for mRNA nanoparticles remain limited.
Technical barriers to regulatory compliance include standardization challenges in characterization methods for lipid nanoparticles (LNPs). Current analytical techniques struggle to provide consistent measurements of particle size distribution, encapsulation efficiency, and stability across different manufacturing batches. This inconsistency complicates regulatory submissions and approval processes.
Manufacturing scale-up represents another significant barrier, as processes that work effectively at laboratory scale often encounter unforeseen challenges during industrial production. Regulatory bodies increasingly require demonstration of manufacturing consistency across multiple batches and production scales.
Stability and cold-chain requirements pose both regulatory and logistical challenges. Current mRNA formulations typically require ultra-cold storage conditions (-70°C to -20°C), which complicates distribution networks and raises regulatory concerns about product integrity throughout the supply chain.
Safety monitoring frameworks remain underdeveloped for mRNA nanoparticles, with regulatory agencies still determining appropriate post-market surveillance requirements. The novelty of the technology means that long-term effects are not fully understood, leading to cautious regulatory approaches that may delay market access.
Cross-border harmonization efforts are emerging through initiatives like the International Council for Harmonisation (ICH), but significant gaps remain in aligning regulatory requirements across different jurisdictions, creating additional complexity for global distribution strategies.
Current Regulatory Frameworks for mRNA Nanoparticles
01 Lipid nanoparticle formulations for mRNA delivery
Lipid nanoparticles (LNPs) serve as effective delivery vehicles for mRNA therapeutics. These formulations typically include ionizable lipids, helper lipids, cholesterol, and PEG-lipids that encapsulate and protect mRNA molecules. The lipid composition can be optimized to enhance cellular uptake, endosomal escape, and targeted delivery to specific tissues, improving the efficacy of mRNA-based treatments while reducing potential side effects.- Lipid nanoparticle formulations for mRNA delivery: Lipid nanoparticles (LNPs) serve as effective delivery vehicles for mRNA therapeutics. These formulations typically consist of ionizable lipids, helper lipids, cholesterol, and PEG-lipids that encapsulate and protect mRNA molecules. The lipid composition can be optimized to enhance cellular uptake, endosomal escape, and overall transfection efficiency. These specialized formulations enable targeted delivery of mRNA to specific tissues while protecting the genetic material from degradation in the bloodstream.
- Polymer-based nanoparticles for mRNA delivery: Polymer-based nanoparticles represent an alternative approach to lipid systems for mRNA delivery. These formulations utilize biodegradable polymers such as PLGA, PEI, or chitosan derivatives that can complex with mRNA through electrostatic interactions. The polymer composition and structure can be engineered to control release kinetics, improve stability, and reduce toxicity. These systems offer advantages including tunable degradation profiles and versatile surface modification options for targeted delivery applications.
- mRNA nanoparticle stabilization techniques: Various stabilization techniques are employed to enhance the stability and efficacy of mRNA nanoparticles. These include chemical modifications of the mRNA structure (such as pseudouridine substitutions), lyophilization processes, and the incorporation of stabilizing excipients. Advanced formulation approaches may involve pH-responsive elements, temperature-sensitive components, or cross-linking strategies to protect the mRNA cargo from degradation and improve shelf-life while maintaining biological activity upon delivery.
- Targeted delivery systems for mRNA nanoparticles: Targeted delivery systems for mRNA nanoparticles incorporate specific ligands or surface modifications to enhance delivery to intended tissues or cells. These targeting strategies include antibody conjugation, peptide functionalization, or receptor-specific ligands that facilitate cellular recognition and uptake. The surface engineering of nanoparticles can improve biodistribution profiles, reduce off-target effects, and enhance therapeutic efficacy by ensuring the mRNA payload reaches the desired site of action.
- Manufacturing and quality control of mRNA nanoparticles: Manufacturing processes and quality control methods for mRNA nanoparticles are critical for clinical translation. These include microfluidic mixing techniques, controlled precipitation methods, and extrusion processes that ensure consistent particle size distribution and encapsulation efficiency. Advanced analytical methods such as dynamic light scattering, electron microscopy, and chromatographic techniques are employed to characterize physical properties and ensure batch-to-batch consistency. Sterility assurance and endotoxin control measures are also implemented to meet regulatory requirements for pharmaceutical applications.
02 Polymer-based nanoparticles for mRNA delivery
Polymer-based nanoparticles offer an alternative approach for mRNA delivery. These systems utilize biodegradable polymers such as PLGA, PEI, or chitosan to form complexes with mRNA. The polymer composition can be engineered to control release kinetics, improve stability, and enhance transfection efficiency. These nanoparticles can be surface-modified to reduce immunogenicity and increase circulation time in the bloodstream.Expand Specific Solutions03 Targeted mRNA nanoparticle delivery systems
Targeted delivery systems incorporate ligands or antibodies on the nanoparticle surface to direct mRNA to specific cell types or tissues. These targeting moieties can include peptides, aptamers, or small molecules that bind to receptors overexpressed on target cells. This approach enhances therapeutic efficacy while minimizing off-target effects and reducing the required dose. The targeting strategy can be customized for various applications including cancer therapy, genetic disorders, and vaccination.Expand Specific Solutions04 mRNA nanoparticle stability and storage innovations
Innovations in mRNA nanoparticle formulations focus on improving stability during storage and administration. These approaches include lyophilization techniques, cryoprotectants, and pH-responsive elements that protect the mRNA cargo from degradation. Modified nucleosides and optimized buffer systems can enhance the shelf-life of mRNA nanoparticles, allowing for room temperature storage and simplified distribution logistics, which is particularly important for vaccine applications in resource-limited settings.Expand Specific Solutions05 Manufacturing processes for mRNA nanoparticles
Advanced manufacturing processes for mRNA nanoparticles include microfluidic mixing, extrusion techniques, and controlled precipitation methods. These processes enable precise control over particle size, polydispersity, and encapsulation efficiency. Scalable production methods incorporate continuous flow systems and automated quality control measures to ensure batch-to-batch consistency. Innovations in manufacturing technology focus on increasing production capacity while maintaining product quality for clinical and commercial applications.Expand Specific Solutions
Key Regulatory Bodies and Industry Stakeholders
The mRNA nanoparticle distribution regulatory landscape is currently in a dynamic growth phase, with the market expanding rapidly following COVID-19 vaccine successes. Regulatory frameworks remain fragmented globally, with different approaches in the US, EU, and Asia. Key players like Moderna, BioNTech, and Translate Bio are driving innovation while navigating evolving regulatory requirements. Established pharmaceutical companies such as Sanofi are partnering with technology developers, while academic institutions (Peking University, Yale, Zhejiang University) contribute significant research. The regulatory environment is maturing but still developing, with challenges in standardization of lipid nanoparticle characterization, biodistribution assessment, and long-term safety monitoring across different therapeutic applications beyond vaccines.
ModernaTX, Inc.
Technical Solution: Moderna has developed a proprietary lipid nanoparticle (LNP) delivery system for mRNA therapeutics and vaccines that complies with FDA and EMA regulations. Their regulatory approach includes comprehensive characterization of LNP components, manufacturing consistency protocols, and stability testing to meet GMP standards. Moderna's regulatory strategy addresses biodistribution concerns through extensive preclinical studies demonstrating tissue distribution patterns and clearance kinetics. They've established specific quality control parameters for particle size distribution (maintaining 80-100nm diameter range), encapsulation efficiency (>90%), and lipid composition consistency. Moderna has worked closely with regulatory agencies to develop novel assessment frameworks for mRNA-LNP products, contributing to the establishment of industry standards for this emerging therapeutic modality.
Strengths: Extensive experience navigating regulatory pathways for mRNA-LNP products with successful EUA and full approvals; established relationships with global regulatory bodies; comprehensive internal quality systems. Weaknesses: Regulatory frameworks remain evolving and inconsistent across different regions; long-term safety monitoring requirements create ongoing compliance challenges.
BioNTech SE
Technical Solution: BioNTech has implemented a regulatory compliance framework for their LNP-mRNA products that addresses the unique challenges of nanoparticle distribution. Their approach includes standardized analytical methods for characterizing nanoparticle size distribution, zeta potential, and polydispersity index to ensure batch-to-batch consistency. BioNTech has developed specialized toxicology protocols to assess biodistribution patterns of their LNPs, with particular attention to accumulation in liver, spleen, and other organs of concern to regulators. Their regulatory strategy incorporates ICH guidelines for pharmaceutical development while adapting them to the specific requirements of mRNA therapeutics. BioNTech works with regulatory agencies to establish appropriate stability testing protocols that account for the unique degradation pathways of mRNA-LNPs, including monitoring lipid oxidation and RNA integrity over time under various storage conditions.
Strengths: Strong partnership with Pfizer enhancing regulatory expertise and global compliance capabilities; established precedent for mRNA-LNP approval through COVID-19 vaccine; robust manufacturing controls. Weaknesses: Regulatory requirements for non-vaccine mRNA therapeutics remain less defined; temperature stability challenges create additional regulatory hurdles for distribution compliance.
Critical Regulatory Guidelines and Scientific Literature
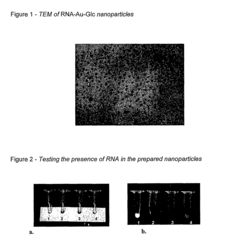
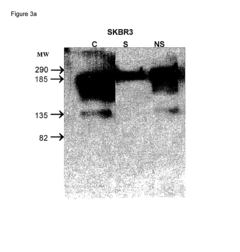
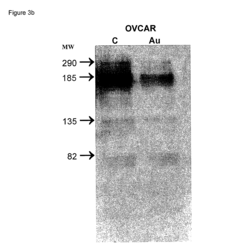
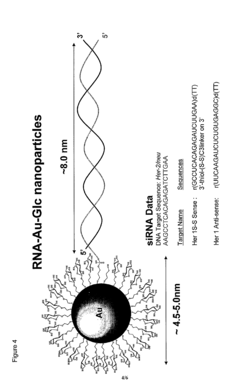
Nanoparticles comprising RNA ligands
PatentInactiveEP2330208A1
Innovation
- Nanoparticles with cores made of metal and/or semiconductor atoms covalently linked to RNA ligands, such as siRNA and miRNA, which are designed to mimic short interfering RNA sequences, enabling targeted gene silencing, mRNA degradation, and imaging, with optional additional ligands for enhanced targeting and stability.
Microrna modulators and method for identifying and using the same
PatentWO2010051048A1
Innovation
- A method involving a screening assay that contacts cells with a microRNA binding sequence linked to a reporter protein to identify small molecule modulators, specifically diazobenzene, indenoisoquinoline, and cyclopentaphenanthrene compounds that inhibit or enhance microRNA activity, allowing for targeted treatment of diseases related to microRNA expression.
Safety and Toxicity Assessment Requirements
The safety and toxicity assessment requirements for mRNA nanoparticle distribution are governed by comprehensive regulatory frameworks established by various international and national regulatory bodies. These requirements are designed to ensure that mRNA-based therapeutics and vaccines delivered via nanoparticle systems meet stringent safety standards before human administration.
Regulatory agencies, including the FDA, EMA, and PMDA, mandate extensive in vitro and in vivo toxicological evaluations for mRNA nanoparticle formulations. These assessments typically include acute, sub-acute, and chronic toxicity studies to evaluate potential adverse effects across different exposure durations and dosage levels. The unique nature of lipid nanoparticles (LNPs) used in mRNA delivery necessitates specialized toxicity evaluations focusing on their biodistribution patterns and potential accumulation in tissues.
Genotoxicity and carcinogenicity assessments are required for novel lipid components in the nanoparticle formulations, particularly when these components have not been previously approved for human use. Regulatory guidelines specify that these assessments should evaluate potential DNA damage, mutagenicity, and long-term carcinogenic potential through standardized testing protocols such as Ames test, chromosomal aberration assays, and rodent carcinogenicity studies.
Immunotoxicity evaluations represent a critical component of safety assessment for mRNA nanoparticles due to their potential to trigger immune responses. Manufacturers must demonstrate that their formulations do not induce unintended immunostimulatory effects, hypersensitivity reactions, or autoimmune responses. This includes evaluating cytokine release profiles, complement activation, and potential impacts on immune cell populations.
Developmental and reproductive toxicity (DART) studies are mandatory for mRNA therapeutics intended for use in populations of reproductive age or pregnant individuals. These studies assess potential impacts on fertility, embryo-fetal development, and postnatal development, with particular attention to the potential transfer of nanoparticles across the placental barrier.
The regulatory landscape also emphasizes the importance of characterizing the pharmacokinetic and biodistribution profiles of mRNA nanoparticles. Manufacturers must provide data on tissue distribution, metabolism, and elimination pathways, with special focus on potential accumulation in critical organs such as liver, spleen, and reproductive tissues. Advanced imaging techniques and quantitative PCR methods are commonly employed to track the distribution and persistence of both the lipid components and the mRNA cargo.
Regulatory requirements continue to evolve as scientific understanding of mRNA nanoparticle safety advances. Recent regulatory guidance has placed increased emphasis on evaluating the potential for delayed adverse effects and understanding the mechanisms underlying observed toxicities, reflecting the relatively novel nature of this therapeutic modality in widespread clinical use.
Regulatory agencies, including the FDA, EMA, and PMDA, mandate extensive in vitro and in vivo toxicological evaluations for mRNA nanoparticle formulations. These assessments typically include acute, sub-acute, and chronic toxicity studies to evaluate potential adverse effects across different exposure durations and dosage levels. The unique nature of lipid nanoparticles (LNPs) used in mRNA delivery necessitates specialized toxicity evaluations focusing on their biodistribution patterns and potential accumulation in tissues.
Genotoxicity and carcinogenicity assessments are required for novel lipid components in the nanoparticle formulations, particularly when these components have not been previously approved for human use. Regulatory guidelines specify that these assessments should evaluate potential DNA damage, mutagenicity, and long-term carcinogenic potential through standardized testing protocols such as Ames test, chromosomal aberration assays, and rodent carcinogenicity studies.
Immunotoxicity evaluations represent a critical component of safety assessment for mRNA nanoparticles due to their potential to trigger immune responses. Manufacturers must demonstrate that their formulations do not induce unintended immunostimulatory effects, hypersensitivity reactions, or autoimmune responses. This includes evaluating cytokine release profiles, complement activation, and potential impacts on immune cell populations.
Developmental and reproductive toxicity (DART) studies are mandatory for mRNA therapeutics intended for use in populations of reproductive age or pregnant individuals. These studies assess potential impacts on fertility, embryo-fetal development, and postnatal development, with particular attention to the potential transfer of nanoparticles across the placental barrier.
The regulatory landscape also emphasizes the importance of characterizing the pharmacokinetic and biodistribution profiles of mRNA nanoparticles. Manufacturers must provide data on tissue distribution, metabolism, and elimination pathways, with special focus on potential accumulation in critical organs such as liver, spleen, and reproductive tissues. Advanced imaging techniques and quantitative PCR methods are commonly employed to track the distribution and persistence of both the lipid components and the mRNA cargo.
Regulatory requirements continue to evolve as scientific understanding of mRNA nanoparticle safety advances. Recent regulatory guidance has placed increased emphasis on evaluating the potential for delayed adverse effects and understanding the mechanisms underlying observed toxicities, reflecting the relatively novel nature of this therapeutic modality in widespread clinical use.
Cross-Border Distribution Compliance Challenges
The cross-border distribution of mRNA nanoparticle technologies faces a complex regulatory landscape that varies significantly between jurisdictions. Companies operating in this space must navigate a patchwork of regulations that govern pharmaceutical products, biological materials, and novel delivery technologies across different countries and regions.
In the United States, the FDA has established specific guidance for lipid nanoparticle (LNP) delivery systems used in mRNA therapeutics, focusing on characterization, manufacturing consistency, and stability. Meanwhile, the European Medicines Agency (EMA) has implemented its Advanced Therapy Medicinal Products (ATMP) framework, which includes specific provisions for novel delivery technologies like those used in mRNA therapeutics.
Regulatory divergence presents significant challenges for global distribution networks. Temperature control requirements, for instance, vary between jurisdictions, with some requiring continuous temperature monitoring while others accept periodic verification. These differences necessitate customized logistics solutions for each market, increasing operational complexity and costs.
Import and export controls represent another major hurdle. Many countries classify mRNA technologies as dual-use items with potential biosecurity implications, subjecting them to additional scrutiny and licensing requirements. The classification of these products—whether as biologics, advanced therapies, or novel pharmaceutical entities—differs across borders, creating compliance challenges for manufacturers and distributors.
Data protection regulations further complicate cross-border distribution. Patient information associated with personalized mRNA therapies must comply with regulations like GDPR in Europe and HIPAA in the US, requiring robust data management systems that can adapt to different jurisdictional requirements.
Harmonization efforts are underway through organizations like the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) and the World Health Organization (WHO). These initiatives aim to develop standardized approaches to quality control, stability testing, and safety monitoring for advanced therapeutic products including mRNA nanoparticles.
Companies must develop comprehensive regulatory intelligence capabilities to monitor evolving requirements across markets. This includes establishing dedicated cross-functional teams that combine expertise in regulatory affairs, supply chain management, and quality assurance to ensure compliance throughout the distribution process.
Strategic partnerships with local regulatory experts and distribution networks have emerged as a key approach to navigating these challenges. These collaborations provide valuable insights into local regulatory nuances and help companies develop market-specific compliance strategies that balance global standardization with local requirements.
In the United States, the FDA has established specific guidance for lipid nanoparticle (LNP) delivery systems used in mRNA therapeutics, focusing on characterization, manufacturing consistency, and stability. Meanwhile, the European Medicines Agency (EMA) has implemented its Advanced Therapy Medicinal Products (ATMP) framework, which includes specific provisions for novel delivery technologies like those used in mRNA therapeutics.
Regulatory divergence presents significant challenges for global distribution networks. Temperature control requirements, for instance, vary between jurisdictions, with some requiring continuous temperature monitoring while others accept periodic verification. These differences necessitate customized logistics solutions for each market, increasing operational complexity and costs.
Import and export controls represent another major hurdle. Many countries classify mRNA technologies as dual-use items with potential biosecurity implications, subjecting them to additional scrutiny and licensing requirements. The classification of these products—whether as biologics, advanced therapies, or novel pharmaceutical entities—differs across borders, creating compliance challenges for manufacturers and distributors.
Data protection regulations further complicate cross-border distribution. Patient information associated with personalized mRNA therapies must comply with regulations like GDPR in Europe and HIPAA in the US, requiring robust data management systems that can adapt to different jurisdictional requirements.
Harmonization efforts are underway through organizations like the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) and the World Health Organization (WHO). These initiatives aim to develop standardized approaches to quality control, stability testing, and safety monitoring for advanced therapeutic products including mRNA nanoparticles.
Companies must develop comprehensive regulatory intelligence capabilities to monitor evolving requirements across markets. This includes establishing dedicated cross-functional teams that combine expertise in regulatory affairs, supply chain management, and quality assurance to ensure compliance throughout the distribution process.
Strategic partnerships with local regulatory experts and distribution networks have emerged as a key approach to navigating these challenges. These collaborations provide valuable insights into local regulatory nuances and help companies develop market-specific compliance strategies that balance global standardization with local requirements.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!