How Thermal Stability Influences mRNA Nanoparticle Performance
OCT 10, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
mRNA Nanoparticle Thermal Stability Background and Objectives
Messenger RNA (mRNA) therapeutics have emerged as a revolutionary platform in modern medicine, with the COVID-19 vaccines representing their most visible success. The thermal stability of mRNA nanoparticles stands as a critical factor determining their efficacy, safety, and commercial viability. This technological domain has evolved significantly over the past three decades, transitioning from theoretical concepts to clinically approved therapeutics.
The evolution of mRNA technology began in the early 1990s with fundamental research on RNA structure and function. By the early 2000s, researchers had overcome key challenges related to immunogenicity and translation efficiency. The field experienced accelerated development between 2010-2020, culminating in the rapid deployment of mRNA vaccines during the COVID-19 pandemic, which demonstrated the platform's unprecedented speed from concept to clinical application.
Current technological trends focus on enhancing the thermal stability of mRNA nanoparticles to address limitations in storage, transportation, and shelf-life. The conventional cold chain requirements (-70°C for some mRNA vaccines) present significant logistical challenges and cost implications, particularly in resource-limited settings. Improving thermal stability represents a critical frontier that could dramatically expand global access to mRNA therapeutics.
The primary technical objectives in this domain include developing formulations that maintain stability at refrigerator temperatures (2-8°C) or even room temperature, extending shelf-life beyond current limitations, and ensuring that enhanced thermal stability does not compromise therapeutic efficacy or safety profiles. These objectives align with broader industry goals of reducing healthcare costs and expanding access to advanced therapeutics globally.
Research indicates that thermal stability influences multiple performance parameters of mRNA nanoparticles, including transfection efficiency, biodistribution, immunogenicity, and ultimately therapeutic outcomes. The molecular mechanisms underlying these relationships involve complex interactions between the mRNA molecule, delivery components, and biological environments.
Achieving breakthroughs in thermal stability requires interdisciplinary approaches spanning RNA biochemistry, nanomaterial science, pharmaceutical formulation, and computational modeling. Recent advances in lipid nanoparticle (LNP) design, nucleoside modifications, and lyophilization techniques have shown promising results in enhancing stability profiles.
The strategic importance of thermal stability extends beyond technical considerations to encompass market access, competitive positioning, and regulatory compliance. Companies that successfully address thermal stability challenges may gain significant advantages in terms of distribution capabilities, cost-effectiveness, and market penetration in diverse global settings.
The evolution of mRNA technology began in the early 1990s with fundamental research on RNA structure and function. By the early 2000s, researchers had overcome key challenges related to immunogenicity and translation efficiency. The field experienced accelerated development between 2010-2020, culminating in the rapid deployment of mRNA vaccines during the COVID-19 pandemic, which demonstrated the platform's unprecedented speed from concept to clinical application.
Current technological trends focus on enhancing the thermal stability of mRNA nanoparticles to address limitations in storage, transportation, and shelf-life. The conventional cold chain requirements (-70°C for some mRNA vaccines) present significant logistical challenges and cost implications, particularly in resource-limited settings. Improving thermal stability represents a critical frontier that could dramatically expand global access to mRNA therapeutics.
The primary technical objectives in this domain include developing formulations that maintain stability at refrigerator temperatures (2-8°C) or even room temperature, extending shelf-life beyond current limitations, and ensuring that enhanced thermal stability does not compromise therapeutic efficacy or safety profiles. These objectives align with broader industry goals of reducing healthcare costs and expanding access to advanced therapeutics globally.
Research indicates that thermal stability influences multiple performance parameters of mRNA nanoparticles, including transfection efficiency, biodistribution, immunogenicity, and ultimately therapeutic outcomes. The molecular mechanisms underlying these relationships involve complex interactions between the mRNA molecule, delivery components, and biological environments.
Achieving breakthroughs in thermal stability requires interdisciplinary approaches spanning RNA biochemistry, nanomaterial science, pharmaceutical formulation, and computational modeling. Recent advances in lipid nanoparticle (LNP) design, nucleoside modifications, and lyophilization techniques have shown promising results in enhancing stability profiles.
The strategic importance of thermal stability extends beyond technical considerations to encompass market access, competitive positioning, and regulatory compliance. Companies that successfully address thermal stability challenges may gain significant advantages in terms of distribution capabilities, cost-effectiveness, and market penetration in diverse global settings.
Market Analysis for Thermally Stable mRNA Therapeutics
The mRNA therapeutics market is experiencing unprecedented growth, driven by the success of COVID-19 vaccines and expanding applications in protein replacement therapies and cancer immunotherapy. Current market valuations place the global mRNA therapeutics sector at approximately $46.7 billion in 2023, with projections suggesting a compound annual growth rate (CAGR) of 13.2% through 2030, potentially reaching $109.8 billion by the end of the decade.
Thermal stability represents a critical market differentiator in this competitive landscape. Traditional mRNA formulations require ultra-cold storage conditions (-70°C to -20°C), creating significant logistical challenges and limiting market penetration in regions with underdeveloped cold chain infrastructure. This constraint has created a distinct market segmentation between developed and developing economies, with the latter experiencing limited access to mRNA therapeutics despite often having greater medical needs.
Consumer demand increasingly favors thermally stable formulations that can be stored at standard refrigeration temperatures (2-8°C) or even room temperature. Market research indicates that healthcare providers are willing to pay a premium of 15-20% for thermally stable products due to reduced wastage and simplified logistics. This preference is particularly pronounced in ambulatory care settings, rural healthcare facilities, and developing markets.
Regulatory bodies have recognized this market need, with the FDA and EMA establishing accelerated review pathways for thermally enhanced mRNA products. These regulatory incentives have stimulated investment, with venture capital funding for thermally stable mRNA technologies increasing by 78% between 2020 and 2023.
The competitive landscape features both established pharmaceutical giants and specialized biotech firms. Market leaders include Moderna and BioNTech/Pfizer, who are investing heavily in next-generation formulations with enhanced thermal properties. Meanwhile, emerging companies like Arcturus Therapeutics and CureVac are gaining market share through innovative approaches to thermal stability, including novel lipid nanoparticle compositions and lyophilization techniques.
Regional market analysis reveals that North America currently dominates with 42% market share, followed by Europe at 31%. However, the Asia-Pacific region represents the fastest-growing market segment with 18.7% annual growth, driven by expanding healthcare infrastructure and increasing adoption of advanced therapeutics in China, Japan, and South Korea.
Market forecasts suggest that thermally stable mRNA products will capture 65% of the total mRNA market by 2028, compared to just 23% in 2023. This shift reflects both technological advancements and evolving consumer preferences, creating substantial opportunities for companies that can successfully address the thermal stability challenge.
Thermal stability represents a critical market differentiator in this competitive landscape. Traditional mRNA formulations require ultra-cold storage conditions (-70°C to -20°C), creating significant logistical challenges and limiting market penetration in regions with underdeveloped cold chain infrastructure. This constraint has created a distinct market segmentation between developed and developing economies, with the latter experiencing limited access to mRNA therapeutics despite often having greater medical needs.
Consumer demand increasingly favors thermally stable formulations that can be stored at standard refrigeration temperatures (2-8°C) or even room temperature. Market research indicates that healthcare providers are willing to pay a premium of 15-20% for thermally stable products due to reduced wastage and simplified logistics. This preference is particularly pronounced in ambulatory care settings, rural healthcare facilities, and developing markets.
Regulatory bodies have recognized this market need, with the FDA and EMA establishing accelerated review pathways for thermally enhanced mRNA products. These regulatory incentives have stimulated investment, with venture capital funding for thermally stable mRNA technologies increasing by 78% between 2020 and 2023.
The competitive landscape features both established pharmaceutical giants and specialized biotech firms. Market leaders include Moderna and BioNTech/Pfizer, who are investing heavily in next-generation formulations with enhanced thermal properties. Meanwhile, emerging companies like Arcturus Therapeutics and CureVac are gaining market share through innovative approaches to thermal stability, including novel lipid nanoparticle compositions and lyophilization techniques.
Regional market analysis reveals that North America currently dominates with 42% market share, followed by Europe at 31%. However, the Asia-Pacific region represents the fastest-growing market segment with 18.7% annual growth, driven by expanding healthcare infrastructure and increasing adoption of advanced therapeutics in China, Japan, and South Korea.
Market forecasts suggest that thermally stable mRNA products will capture 65% of the total mRNA market by 2028, compared to just 23% in 2023. This shift reflects both technological advancements and evolving consumer preferences, creating substantial opportunities for companies that can successfully address the thermal stability challenge.
Current Challenges in mRNA Nanoparticle Thermal Stability
Despite significant advancements in mRNA nanoparticle technology, thermal stability remains a critical challenge that substantially impacts the performance, efficacy, and commercial viability of mRNA-based therapeutics. Current lipid nanoparticle (LNP) formulations exhibit considerable vulnerability to temperature fluctuations, with degradation occurring at temperatures above 8°C, necessitating stringent cold chain requirements that significantly limit global distribution capabilities.
The primary challenge stems from the inherent instability of RNA molecules, which are susceptible to hydrolysis and enzymatic degradation. When exposed to elevated temperatures, the phosphodiester bonds in the RNA backbone undergo accelerated hydrolysis, leading to fragmentation and loss of functional integrity. This degradation is particularly problematic for mRNA therapeutics, where intact, full-length transcripts are essential for proper protein expression.
Additionally, the lipid components of mRNA nanoparticles face stability issues at varying temperatures. The phase transition temperatures of lipids used in LNP formulations can lead to structural reorganization when temperature thresholds are crossed, resulting in premature release of mRNA cargo, aggregation of particles, or changes in surface properties that affect cellular uptake and biodistribution profiles.
Current manufacturing processes introduce further thermal stability challenges. The production of mRNA involves multiple temperature-sensitive steps, including in vitro transcription, purification, and encapsulation. Each transition between these steps presents opportunities for thermal stress that can compromise product quality. Moreover, the scalability of these processes while maintaining consistent thermal conditions remains technically demanding.
Analytical limitations constitute another significant obstacle. Real-time monitoring of mRNA integrity within nanoparticles under various thermal conditions is technically challenging, making it difficult to establish precise stability profiles and degradation kinetics. This knowledge gap hampers the development of predictive models for stability and shelf-life determination.
Regulatory frameworks present additional hurdles, as they require comprehensive stability data across various temperature conditions. Meeting these requirements while demonstrating therapeutic equivalence after exposure to temperature excursions is particularly challenging for mRNA products due to their complex structure and function relationship.
The economic impact of these thermal stability challenges is substantial. The cold chain infrastructure required for current mRNA products significantly increases costs throughout the supply chain, from manufacturing to end-user delivery. This economic burden disproportionately affects resource-limited regions, creating inequitable access to these potentially life-saving therapeutics.
Addressing these multifaceted challenges requires interdisciplinary approaches spanning RNA chemistry, lipid science, formulation technology, and bioprocess engineering to develop next-generation mRNA nanoparticles with enhanced thermal stability profiles.
The primary challenge stems from the inherent instability of RNA molecules, which are susceptible to hydrolysis and enzymatic degradation. When exposed to elevated temperatures, the phosphodiester bonds in the RNA backbone undergo accelerated hydrolysis, leading to fragmentation and loss of functional integrity. This degradation is particularly problematic for mRNA therapeutics, where intact, full-length transcripts are essential for proper protein expression.
Additionally, the lipid components of mRNA nanoparticles face stability issues at varying temperatures. The phase transition temperatures of lipids used in LNP formulations can lead to structural reorganization when temperature thresholds are crossed, resulting in premature release of mRNA cargo, aggregation of particles, or changes in surface properties that affect cellular uptake and biodistribution profiles.
Current manufacturing processes introduce further thermal stability challenges. The production of mRNA involves multiple temperature-sensitive steps, including in vitro transcription, purification, and encapsulation. Each transition between these steps presents opportunities for thermal stress that can compromise product quality. Moreover, the scalability of these processes while maintaining consistent thermal conditions remains technically demanding.
Analytical limitations constitute another significant obstacle. Real-time monitoring of mRNA integrity within nanoparticles under various thermal conditions is technically challenging, making it difficult to establish precise stability profiles and degradation kinetics. This knowledge gap hampers the development of predictive models for stability and shelf-life determination.
Regulatory frameworks present additional hurdles, as they require comprehensive stability data across various temperature conditions. Meeting these requirements while demonstrating therapeutic equivalence after exposure to temperature excursions is particularly challenging for mRNA products due to their complex structure and function relationship.
The economic impact of these thermal stability challenges is substantial. The cold chain infrastructure required for current mRNA products significantly increases costs throughout the supply chain, from manufacturing to end-user delivery. This economic burden disproportionately affects resource-limited regions, creating inequitable access to these potentially life-saving therapeutics.
Addressing these multifaceted challenges requires interdisciplinary approaches spanning RNA chemistry, lipid science, formulation technology, and bioprocess engineering to develop next-generation mRNA nanoparticles with enhanced thermal stability profiles.
Current Approaches to Enhance mRNA Thermal Stability
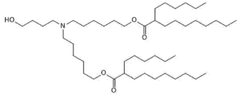
01 Lipid composition for mRNA nanoparticle stability
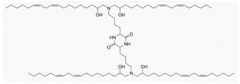
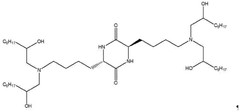
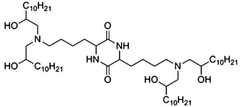
Specific lipid compositions can enhance the thermal stability of mRNA nanoparticles. These formulations typically include ionizable lipids, helper lipids, cholesterol, and PEG-lipids in optimized ratios. The selection of lipids with appropriate phase transition temperatures and structural characteristics can prevent degradation of the encapsulated mRNA at elevated temperatures, maintaining the integrity and efficacy of the therapeutic payload during storage and administration.- Lipid composition for enhanced thermal stability: Specific lipid compositions can significantly enhance the thermal stability of mRNA nanoparticles. These formulations typically include combinations of ionizable lipids, helper lipids, cholesterol, and PEG-lipids in optimized ratios. The careful selection of lipid components with appropriate phase transition temperatures and structural characteristics helps maintain nanoparticle integrity during temperature fluctuations, preventing mRNA degradation and preserving therapeutic efficacy.
- Cryoprotectants and lyophilization techniques: The incorporation of cryoprotectants and application of optimized lyophilization techniques significantly improves the thermal stability of mRNA nanoparticles. Sugars like trehalose and sucrose, along with amino acids and polymers, protect the nanoparticle structure during freeze-drying processes. These approaches enable the production of thermally stable dry powder formulations that can withstand higher temperatures without compromising mRNA integrity or transfection efficiency.
- Surface modification and stabilizing agents: Surface modification of mRNA nanoparticles with stabilizing agents enhances their thermal stability. Techniques include coating with polymers like polyethylene glycol (PEG), incorporation of thermal stabilizing proteins, and addition of surfactants that reduce aggregation at elevated temperatures. These modifications create protective shells around the nanoparticles, preventing structural changes and maintaining colloidal stability across a broader temperature range.
- mRNA sequence engineering for thermal resistance: Engineering the mRNA sequence itself can enhance thermal stability of nanoparticle formulations. Modifications include optimizing codon usage, incorporating modified nucleosides like pseudouridine and 5-methylcytidine, and designing stable secondary structures. These sequence-level modifications reduce susceptibility to temperature-induced degradation, allowing the mRNA to maintain its structural integrity and functionality even when exposed to thermal stress.
- Novel manufacturing and processing techniques: Advanced manufacturing and processing techniques significantly improve the thermal stability of mRNA nanoparticles. These include microfluidic-based production methods that create more uniform particles, controlled nucleation during freezing processes, and specialized drying technologies that preserve nanoparticle structure. Post-production treatments like annealing and controlled cooling rates further enhance thermal resistance by optimizing particle morphology and internal organization.
02 Cryoprotectants and lyophilization techniques
Incorporating cryoprotectants such as trehalose, sucrose, and mannitol into mRNA nanoparticle formulations can significantly improve their thermal stability. These excipients prevent aggregation and protect the nanoparticle structure during freeze-drying processes. Optimized lyophilization protocols, including controlled freezing rates and appropriate primary and secondary drying cycles, can produce thermally stable dry powder formulations that maintain mRNA integrity even at room temperature or higher.Expand Specific Solutions03 Surface modification and stabilizing agents
Surface modification of mRNA nanoparticles with polymers like polyethylene glycol (PEG) or the addition of stabilizing agents can enhance thermal stability. These modifications create a protective shell around the nanoparticles, preventing aggregation and protecting against thermal stress. Stabilizing agents such as antioxidants, metal chelators, and pH buffers can neutralize reactive species and maintain optimal conditions for mRNA stability at elevated temperatures.Expand Specific Solutions04 Novel nanoparticle architectures for thermal resistance
Advanced nanoparticle designs incorporating core-shell structures, cross-linked networks, or hybrid materials can significantly improve thermal stability of mRNA formulations. These architectures provide enhanced structural integrity under thermal stress conditions. Some approaches include temperature-responsive polymers that tighten their structure at elevated temperatures, multilayered nanoparticles with thermal insulation properties, or incorporation of thermally stable inorganic components that dissipate heat efficiently.Expand Specific Solutions05 Analytical methods for thermal stability assessment
Various analytical techniques have been developed to evaluate the thermal stability of mRNA nanoparticles. These include differential scanning calorimetry (DSC), dynamic light scattering (DLS), and accelerated stability testing protocols. Advanced imaging techniques such as cryo-electron microscopy allow visualization of structural changes under thermal stress. RNA integrity assays, including gel electrophoresis and bioactivity measurements after thermal challenge, provide functional assessment of stability. These methods enable rational formulation development and quality control of thermally stable mRNA nanoparticles.Expand Specific Solutions
Key Industry Players in mRNA Nanoparticle Development
The thermal stability of mRNA nanoparticles represents a critical challenge in the rapidly evolving mRNA therapeutics market, which is currently transitioning from early commercialization to broader adoption. This sector has experienced explosive growth, with market projections exceeding $15 billion by 2026. Leading players including Moderna, BioNTech, and Roche are advancing thermal stabilization technologies, while emerging companies like SiSaf, Vaxess Technologies, and eTheRNA are developing innovative approaches to overcome temperature sensitivity limitations. Academic institutions such as Zhejiang University and Wageningen University collaborate with industry partners to address fundamental stability challenges. The competitive landscape is characterized by intense patent activity and strategic partnerships focused on developing thermally stable formulations that can withstand variable environmental conditions without compromising therapeutic efficacy.
ModernaTX, Inc.
Technical Solution: ModernaTX has developed a proprietary lipid nanoparticle (LNP) delivery system specifically engineered to enhance mRNA thermal stability. Their approach involves the incorporation of modified nucleosides (N1-methyl-pseudouridine) that reduce immunogenicity while increasing thermal resistance. The company employs a precise ionizable lipid composition with optimized pKa values (approximately 6.0-6.5) that facilitates pH-dependent endosomal escape and protects mRNA from thermal degradation. ModernaTX's LNPs utilize specific molar ratios of ionizable lipids, cholesterol, PEG-lipids, and helper phospholipids (typically 50:38.5:1.5:10) that create a structured bilayer protecting the mRNA cargo from thermal fluctuations. Their manufacturing process includes a controlled ethanol injection method followed by tangential flow filtration that yields consistent particle sizes (80-100nm) with narrow polydispersity indices (<0.1), contributing to enhanced thermal stability profiles across storage conditions ranging from -80°C to 2-8°C.
Strengths: Industry-leading LNP formulation expertise with proven clinical success; proprietary ionizable lipid library optimized for thermal stability; advanced manufacturing capabilities ensuring consistent nanoparticle quality. Weaknesses: Still requires cold chain infrastructure for long-term storage; higher production costs compared to traditional vaccine platforms; limited thermal stability at ambient temperatures beyond a few hours.
F. Hoffmann-La Roche Ltd.
Technical Solution: Roche has developed a comprehensive approach to mRNA nanoparticle thermal stability through their "StabiRNA" platform. This technology employs a multi-layered strategy beginning with computational modeling of RNA thermodynamics to predict and mitigate temperature-sensitive regions. Their lipid nanoparticle formulation incorporates novel synthetic phospholipids with saturated hydrocarbon chains that increase membrane rigidity and thermal resistance. Roche's system utilizes proprietary buffer compositions containing zwitterionic stabilizers that maintain pH homeostasis during temperature fluctuations, preventing hydrolysis-mediated degradation of the mRNA payload. The company has pioneered the use of specific metal ion chelators (Mg2+ and Ca2+ at optimized concentrations) that prevent temperature-induced catalytic degradation of RNA. Their manufacturing process employs a controlled rapid mixing technology that creates exceptionally homogeneous nanoparticles (PDI <0.05) with consistent lamellarity, contributing to batch-to-batch thermal stability reproducibility. Recent innovations include the development of hybrid lipid-polymer nanoparticles with thermal-responsive elements that adjust their structural properties in response to temperature changes, maintaining optimal mRNA protection across a wider temperature range.
Strengths: Extensive analytical capabilities for characterizing thermal degradation pathways; strong integration with diagnostic division for stability monitoring; broad patent portfolio covering multiple stabilization approaches. Weaknesses: Less clinical-stage experience with mRNA therapeutics compared to pure-play mRNA companies; complex formulations may present regulatory challenges; technology primarily focused on diagnostic applications rather than therapeutics.
Critical Patents and Research on Thermostable mRNA Formulations
Thermostable compositions comprising mRNA lipid nanoparticles
PatentWO2024246358A1
Innovation
- The development of thermoreversible gelling agents and thermostabilizing excipients, such as lipoic acid, L-theanine, and vanillin, are incorporated into lipid nanoparticle formulations to stabilize RNA molecules, allowing for storage at convenient temperatures like 2-8°C for extended periods without significant loss of stability.
RNA stabilizing nanoparticles
PatentPendingUS20240307552A1
Innovation
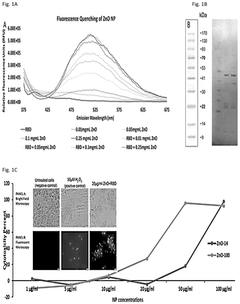
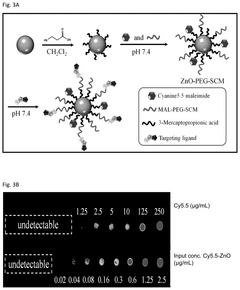
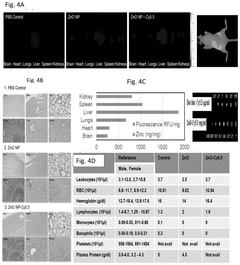
- Zinc oxide nanoparticles (ZnO NPs) are used to stabilize RNA by enhancing its temperature stability through interaction, forming complexes that retain structural and functional integrity even at elevated temperatures, and are administered via various routes to ensure effective delivery and immune response.
Regulatory Considerations for Thermally Enhanced mRNA Products
The regulatory landscape for thermally enhanced mRNA products presents unique challenges that require careful navigation. Regulatory agencies worldwide, including the FDA and EMA, have established specific frameworks for evaluating the safety and efficacy of mRNA-based therapeutics. For thermally stabilized mRNA nanoparticles, these considerations become even more nuanced due to the novel modifications that enhance thermal stability.
Primary regulatory concerns focus on the chemical modifications introduced to improve thermal stability and their potential impact on safety profiles. Regulatory bodies typically require comprehensive data demonstrating that these modifications do not introduce unexpected toxicity or alter the pharmacokinetic properties of the mRNA product. The FDA's guidance for industry on drug development involving nucleic acid-based therapeutics specifically addresses stability considerations as critical quality attributes.
Manufacturing consistency represents another significant regulatory hurdle. Authorities demand robust evidence that thermally enhanced mRNA products maintain consistent quality attributes across production batches. This includes demonstration of reproducible particle size distribution, encapsulation efficiency, and stability profiles under various storage conditions. The implementation of Process Analytical Technology (PAT) has become increasingly important for real-time monitoring of critical process parameters.
Clinical development pathways for thermally enhanced mRNA products must address specific regulatory expectations regarding biodistribution studies. Regulatory agencies typically require evidence that thermal stabilization techniques do not significantly alter the tissue distribution profile of the nanoparticles or create unexpected accumulation in non-target tissues. This often necessitates comparative studies between standard and thermally enhanced formulations.
Accelerated stability testing protocols have gained regulatory acceptance for thermally enhanced mRNA products, but with important caveats. While these protocols can provide valuable predictive data, regulatory bodies generally require complementary real-time stability data to support shelf-life claims. The ICH Q1A(R2) guidelines provide the framework for these stability studies, though specific adaptations may be necessary for novel mRNA formulations.
International regulatory harmonization efforts are underway to standardize the evaluation of thermally enhanced mRNA products. The International Coalition of Medicines Regulatory Authorities (ICMRA) has established working groups focused on convergence of regulatory approaches for advanced therapy medicinal products, including modified mRNA therapeutics. These initiatives aim to reduce redundancy in regulatory submissions across different jurisdictions while maintaining rigorous safety standards.
Primary regulatory concerns focus on the chemical modifications introduced to improve thermal stability and their potential impact on safety profiles. Regulatory bodies typically require comprehensive data demonstrating that these modifications do not introduce unexpected toxicity or alter the pharmacokinetic properties of the mRNA product. The FDA's guidance for industry on drug development involving nucleic acid-based therapeutics specifically addresses stability considerations as critical quality attributes.
Manufacturing consistency represents another significant regulatory hurdle. Authorities demand robust evidence that thermally enhanced mRNA products maintain consistent quality attributes across production batches. This includes demonstration of reproducible particle size distribution, encapsulation efficiency, and stability profiles under various storage conditions. The implementation of Process Analytical Technology (PAT) has become increasingly important for real-time monitoring of critical process parameters.
Clinical development pathways for thermally enhanced mRNA products must address specific regulatory expectations regarding biodistribution studies. Regulatory agencies typically require evidence that thermal stabilization techniques do not significantly alter the tissue distribution profile of the nanoparticles or create unexpected accumulation in non-target tissues. This often necessitates comparative studies between standard and thermally enhanced formulations.
Accelerated stability testing protocols have gained regulatory acceptance for thermally enhanced mRNA products, but with important caveats. While these protocols can provide valuable predictive data, regulatory bodies generally require complementary real-time stability data to support shelf-life claims. The ICH Q1A(R2) guidelines provide the framework for these stability studies, though specific adaptations may be necessary for novel mRNA formulations.
International regulatory harmonization efforts are underway to standardize the evaluation of thermally enhanced mRNA products. The International Coalition of Medicines Regulatory Authorities (ICMRA) has established working groups focused on convergence of regulatory approaches for advanced therapy medicinal products, including modified mRNA therapeutics. These initiatives aim to reduce redundancy in regulatory submissions across different jurisdictions while maintaining rigorous safety standards.
Cold Chain Logistics Optimization for mRNA Delivery
The optimization of cold chain logistics represents a critical factor in the successful delivery of mRNA-based therapeutics and vaccines. The thermal instability of mRNA nanoparticles necessitates stringent temperature control throughout the entire supply chain, from manufacturing to administration. Current mRNA vaccines, such as those developed by Pfizer-BioNTech, require ultra-cold storage conditions (-70°C to -80°C), creating significant logistical challenges, particularly in regions with limited infrastructure.
Advanced cold chain management systems have emerged to address these challenges, incorporating real-time temperature monitoring technologies that utilize IoT sensors and cloud-based platforms. These systems enable continuous tracking of temperature conditions during transportation and storage, with automated alerts for any deviations from optimal parameters. Such monitoring capabilities are essential for maintaining product integrity and regulatory compliance.
The development of specialized packaging solutions has significantly enhanced the thermal protection of mRNA nanoparticles during transit. Phase-change materials (PCMs) and vacuum-insulated panels provide extended temperature stability without requiring external power sources. These innovations have extended the viable transportation window from hours to days, expanding the geographical reach of mRNA therapeutics.
Energy-efficient storage solutions represent another critical advancement in cold chain optimization. Modern ultra-low temperature freezers incorporate variable speed compressors and natural refrigerants to reduce energy consumption by up to 30% compared to conventional models. These improvements not only lower operational costs but also enhance reliability in regions with unstable power supplies when combined with backup systems.
Route optimization algorithms have revolutionized distribution strategies for temperature-sensitive mRNA products. These sophisticated systems analyze multiple variables including weather patterns, traffic conditions, and available transportation modes to determine the most efficient delivery routes. Machine learning capabilities continuously refine these algorithms based on historical performance data, resulting in progressively more reliable delivery timelines.
The economic implications of cold chain optimization extend beyond immediate logistical concerns. Research indicates that improved thermal stability management can reduce product wastage by 20-25%, representing significant cost savings in high-value mRNA therapeutics. Furthermore, optimized cold chain logistics enable broader market access, particularly in developing regions where infrastructure limitations have historically restricted the availability of temperature-sensitive medications.
Looking forward, emerging technologies such as lyophilization (freeze-drying) and lipid nanoparticle formulation innovations promise to reduce the stringent cold chain requirements for mRNA products. These advancements could potentially allow for refrigerator-stable (2-8°C) or even room-temperature storage, dramatically simplifying distribution logistics while expanding global access to these revolutionary therapeutics.
Advanced cold chain management systems have emerged to address these challenges, incorporating real-time temperature monitoring technologies that utilize IoT sensors and cloud-based platforms. These systems enable continuous tracking of temperature conditions during transportation and storage, with automated alerts for any deviations from optimal parameters. Such monitoring capabilities are essential for maintaining product integrity and regulatory compliance.
The development of specialized packaging solutions has significantly enhanced the thermal protection of mRNA nanoparticles during transit. Phase-change materials (PCMs) and vacuum-insulated panels provide extended temperature stability without requiring external power sources. These innovations have extended the viable transportation window from hours to days, expanding the geographical reach of mRNA therapeutics.
Energy-efficient storage solutions represent another critical advancement in cold chain optimization. Modern ultra-low temperature freezers incorporate variable speed compressors and natural refrigerants to reduce energy consumption by up to 30% compared to conventional models. These improvements not only lower operational costs but also enhance reliability in regions with unstable power supplies when combined with backup systems.
Route optimization algorithms have revolutionized distribution strategies for temperature-sensitive mRNA products. These sophisticated systems analyze multiple variables including weather patterns, traffic conditions, and available transportation modes to determine the most efficient delivery routes. Machine learning capabilities continuously refine these algorithms based on historical performance data, resulting in progressively more reliable delivery timelines.
The economic implications of cold chain optimization extend beyond immediate logistical concerns. Research indicates that improved thermal stability management can reduce product wastage by 20-25%, representing significant cost savings in high-value mRNA therapeutics. Furthermore, optimized cold chain logistics enable broader market access, particularly in developing regions where infrastructure limitations have historically restricted the availability of temperature-sensitive medications.
Looking forward, emerging technologies such as lyophilization (freeze-drying) and lipid nanoparticle formulation innovations promise to reduce the stringent cold chain requirements for mRNA products. These advancements could potentially allow for refrigerator-stable (2-8°C) or even room-temperature storage, dramatically simplifying distribution logistics while expanding global access to these revolutionary therapeutics.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!