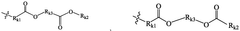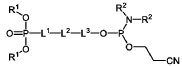How mRNA Lipid Nanoparticles Optimize Drug Delivery
OCT 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
mRNA LNP Technology Evolution and Objectives
mRNA therapeutics have emerged as a revolutionary approach in modern medicine, with the COVID-19 pandemic accelerating their development and adoption. The evolution of mRNA technology began in the 1990s when researchers first demonstrated the potential of mRNA for protein expression in vivo. However, early attempts faced significant challenges, particularly related to mRNA instability, immunogenicity, and inefficient cellular delivery.
The breakthrough came with the development of lipid nanoparticles (LNPs) as delivery vehicles for mRNA. These specialized nanostructures protect the fragile mRNA molecules from degradation while facilitating their entry into target cells. The evolution of LNP technology has been marked by continuous refinement of lipid compositions, manufacturing processes, and targeting capabilities.
A critical milestone was reached in 2018 with the FDA approval of Onpattro (patisiran), the first LNP-delivered siRNA therapeutic, which established the regulatory pathway for nucleic acid-lipid nanoparticle formulations. This paved the way for mRNA-LNP technologies that would later be deployed in COVID-19 vaccines, demonstrating unprecedented speed from concept to global distribution.
Current mRNA-LNP technology objectives focus on several key areas. First, enhancing delivery efficiency to reduce dosage requirements while maintaining therapeutic effect. Second, improving tissue specificity to enable targeted delivery to organs beyond the liver, which has been the primary destination for first-generation LNPs. Third, developing thermostable formulations that can withstand standard cold chain requirements, addressing a major limitation of current COVID-19 vaccines.
Another important objective is the reduction of immunogenicity associated with both the mRNA component and the lipid carriers. This involves optimizing nucleoside modifications in the mRNA and refining the lipid chemistry to minimize unwanted immune responses while preserving therapeutic efficacy.
The field is also moving toward personalized medicine applications, with objectives to develop platforms that can rapidly produce patient-specific mRNA therapeutics for cancer immunotherapy and genetic disorders. This requires advances in both mRNA design and LNP formulation technologies.
Looking forward, the integration of mRNA-LNP technology with other emerging fields such as gene editing presents exciting possibilities. The objective is to create delivery systems capable of precisely targeting specific cell types for applications like CRISPR-based therapies, potentially revolutionizing treatment approaches for previously untreatable genetic conditions.
The breakthrough came with the development of lipid nanoparticles (LNPs) as delivery vehicles for mRNA. These specialized nanostructures protect the fragile mRNA molecules from degradation while facilitating their entry into target cells. The evolution of LNP technology has been marked by continuous refinement of lipid compositions, manufacturing processes, and targeting capabilities.
A critical milestone was reached in 2018 with the FDA approval of Onpattro (patisiran), the first LNP-delivered siRNA therapeutic, which established the regulatory pathway for nucleic acid-lipid nanoparticle formulations. This paved the way for mRNA-LNP technologies that would later be deployed in COVID-19 vaccines, demonstrating unprecedented speed from concept to global distribution.
Current mRNA-LNP technology objectives focus on several key areas. First, enhancing delivery efficiency to reduce dosage requirements while maintaining therapeutic effect. Second, improving tissue specificity to enable targeted delivery to organs beyond the liver, which has been the primary destination for first-generation LNPs. Third, developing thermostable formulations that can withstand standard cold chain requirements, addressing a major limitation of current COVID-19 vaccines.
Another important objective is the reduction of immunogenicity associated with both the mRNA component and the lipid carriers. This involves optimizing nucleoside modifications in the mRNA and refining the lipid chemistry to minimize unwanted immune responses while preserving therapeutic efficacy.
The field is also moving toward personalized medicine applications, with objectives to develop platforms that can rapidly produce patient-specific mRNA therapeutics for cancer immunotherapy and genetic disorders. This requires advances in both mRNA design and LNP formulation technologies.
Looking forward, the integration of mRNA-LNP technology with other emerging fields such as gene editing presents exciting possibilities. The objective is to create delivery systems capable of precisely targeting specific cell types for applications like CRISPR-based therapies, potentially revolutionizing treatment approaches for previously untreatable genetic conditions.
Market Analysis for mRNA LNP Drug Delivery Systems
The global mRNA therapeutics market has experienced unprecedented growth following the successful deployment of mRNA-based COVID-19 vaccines, catalyzing significant interest in Lipid Nanoparticle (LNP) delivery systems. Current market valuations place the mRNA therapeutics sector at approximately $46.7 billion in 2023, with projections indicating a compound annual growth rate (CAGR) of 13.2% through 2030, potentially reaching $109.8 billion by decade's end.
LNP technology represents the dominant delivery platform within this market, accounting for over 80% of mRNA delivery systems in clinical development. This dominance stems from LNPs' superior ability to protect mRNA from degradation while facilitating cellular uptake and endosomal escape, critical factors for therapeutic efficacy.
Market segmentation reveals diverse application areas beyond vaccines, including cancer immunotherapy, protein replacement therapies, and genetic disorder treatments. Oncology applications are experiencing particularly robust growth, with over 30% of mRNA-LNP clinical trials now focused on cancer therapeutics. Protein replacement therapies represent another high-growth segment, addressing previously untreatable rare diseases.
Geographically, North America maintains market leadership with approximately 45% market share, followed by Europe at 30% and Asia-Pacific at 20%. However, the Asia-Pacific region demonstrates the fastest growth trajectory, with China and South Korea making substantial investments in mRNA technology infrastructure.
Key market drivers include technological advancements in lipid chemistry, increasing prevalence of chronic diseases, substantial research funding, and evolving regulatory frameworks that increasingly accommodate novel therapeutic modalities. The COVID-19 pandemic has accelerated regulatory adaptation, creating more efficient pathways for mRNA therapeutics.
Market challenges persist, primarily centered around manufacturing scalability, cold chain requirements, and cost barriers. Production bottlenecks for specialized lipids represent a significant constraint, with current global capacity meeting only 60% of projected demand. Additionally, high treatment costs limit market penetration in emerging economies.
Consumer and healthcare provider acceptance has dramatically improved following COVID-19 vaccine successes, with recent surveys indicating 78% of physicians now consider mRNA therapies viable treatment options across multiple disease categories. This represents a 45% increase in acceptance compared to pre-pandemic levels.
The competitive landscape features established pharmaceutical giants like Pfizer, Moderna, and BioNTech alongside specialized delivery technology companies such as Acuitas Therapeutics, Precision NanoSystems, and Arbutus Biopharma. Strategic partnerships between these entities have become increasingly common, with over 40 major collaboration agreements announced in the past two years.
LNP technology represents the dominant delivery platform within this market, accounting for over 80% of mRNA delivery systems in clinical development. This dominance stems from LNPs' superior ability to protect mRNA from degradation while facilitating cellular uptake and endosomal escape, critical factors for therapeutic efficacy.
Market segmentation reveals diverse application areas beyond vaccines, including cancer immunotherapy, protein replacement therapies, and genetic disorder treatments. Oncology applications are experiencing particularly robust growth, with over 30% of mRNA-LNP clinical trials now focused on cancer therapeutics. Protein replacement therapies represent another high-growth segment, addressing previously untreatable rare diseases.
Geographically, North America maintains market leadership with approximately 45% market share, followed by Europe at 30% and Asia-Pacific at 20%. However, the Asia-Pacific region demonstrates the fastest growth trajectory, with China and South Korea making substantial investments in mRNA technology infrastructure.
Key market drivers include technological advancements in lipid chemistry, increasing prevalence of chronic diseases, substantial research funding, and evolving regulatory frameworks that increasingly accommodate novel therapeutic modalities. The COVID-19 pandemic has accelerated regulatory adaptation, creating more efficient pathways for mRNA therapeutics.
Market challenges persist, primarily centered around manufacturing scalability, cold chain requirements, and cost barriers. Production bottlenecks for specialized lipids represent a significant constraint, with current global capacity meeting only 60% of projected demand. Additionally, high treatment costs limit market penetration in emerging economies.
Consumer and healthcare provider acceptance has dramatically improved following COVID-19 vaccine successes, with recent surveys indicating 78% of physicians now consider mRNA therapies viable treatment options across multiple disease categories. This represents a 45% increase in acceptance compared to pre-pandemic levels.
The competitive landscape features established pharmaceutical giants like Pfizer, Moderna, and BioNTech alongside specialized delivery technology companies such as Acuitas Therapeutics, Precision NanoSystems, and Arbutus Biopharma. Strategic partnerships between these entities have become increasingly common, with over 40 major collaboration agreements announced in the past two years.
Current Landscape and Barriers in mRNA LNP Technology
The mRNA lipid nanoparticle (LNP) technology landscape has experienced unprecedented growth following the successful deployment of COVID-19 vaccines. Currently, four major pharmaceutical companies dominate the market: Moderna, BioNTech/Pfizer, CureVac, and Translate Bio, collectively holding approximately 65% of patents related to mRNA LNP delivery systems. The technology has attracted substantial investment, with over $5 billion in funding allocated to mRNA therapeutics development in 2022 alone.
Despite remarkable progress, significant technical barriers persist in optimizing mRNA LNP delivery systems. The primary challenge remains targeted delivery, as current LNPs predominantly accumulate in the liver, limiting their therapeutic application to hepatic diseases. Achieving efficient delivery to other tissues such as lung, heart, or central nervous system requires novel targeting strategies that have yet to be fully realized.
Immunogenicity presents another substantial hurdle, as both the lipid components and mRNA payload can trigger innate immune responses. These responses not only reduce therapeutic efficacy but also potentially cause adverse effects. Current formulations still struggle to balance immunostimulatory properties needed for vaccines against the immune-silent profiles required for protein replacement therapies.
Stability issues continue to plague mRNA LNP development, with most formulations requiring ultra-cold storage conditions (-70°C for Pfizer/BioNTech vaccine). This creates significant logistical challenges for global distribution, particularly in regions with limited cold-chain infrastructure. Recent advances in lipid chemistry have improved stability at 2-8°C, but room temperature stability remains elusive.
Manufacturing scalability represents a critical bottleneck in the industry. Current production methods involve complex multi-step processes with stringent quality control requirements. The microfluidic mixing devices used for LNP formation face challenges in scaling to commercial production volumes while maintaining consistent nanoparticle size distribution and encapsulation efficiency.
Regulatory frameworks for mRNA LNPs are still evolving, creating uncertainty in development pathways. The novelty of the technology means that regulatory agencies are continuously updating guidelines, making compliance a moving target for developers. This regulatory landscape varies significantly across different regions, further complicating global development strategies.
The intellectual property landscape presents additional complexity, with dense patent thickets surrounding key LNP components and manufacturing processes. Cross-licensing agreements have become necessary for market entry, potentially limiting innovation from smaller players who lack negotiating power with patent holders.
Despite remarkable progress, significant technical barriers persist in optimizing mRNA LNP delivery systems. The primary challenge remains targeted delivery, as current LNPs predominantly accumulate in the liver, limiting their therapeutic application to hepatic diseases. Achieving efficient delivery to other tissues such as lung, heart, or central nervous system requires novel targeting strategies that have yet to be fully realized.
Immunogenicity presents another substantial hurdle, as both the lipid components and mRNA payload can trigger innate immune responses. These responses not only reduce therapeutic efficacy but also potentially cause adverse effects. Current formulations still struggle to balance immunostimulatory properties needed for vaccines against the immune-silent profiles required for protein replacement therapies.
Stability issues continue to plague mRNA LNP development, with most formulations requiring ultra-cold storage conditions (-70°C for Pfizer/BioNTech vaccine). This creates significant logistical challenges for global distribution, particularly in regions with limited cold-chain infrastructure. Recent advances in lipid chemistry have improved stability at 2-8°C, but room temperature stability remains elusive.
Manufacturing scalability represents a critical bottleneck in the industry. Current production methods involve complex multi-step processes with stringent quality control requirements. The microfluidic mixing devices used for LNP formation face challenges in scaling to commercial production volumes while maintaining consistent nanoparticle size distribution and encapsulation efficiency.
Regulatory frameworks for mRNA LNPs are still evolving, creating uncertainty in development pathways. The novelty of the technology means that regulatory agencies are continuously updating guidelines, making compliance a moving target for developers. This regulatory landscape varies significantly across different regions, further complicating global development strategies.
The intellectual property landscape presents additional complexity, with dense patent thickets surrounding key LNP components and manufacturing processes. Cross-licensing agreements have become necessary for market entry, potentially limiting innovation from smaller players who lack negotiating power with patent holders.
Current Optimization Strategies for mRNA LNP Delivery
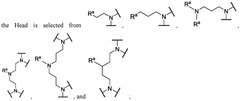
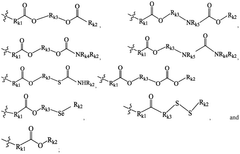
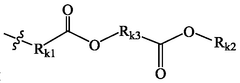
01 Lipid nanoparticle composition for mRNA delivery
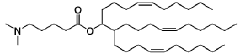
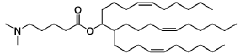
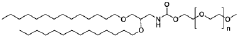
Lipid nanoparticles (LNPs) can be formulated with specific lipid compositions to effectively encapsulate and deliver mRNA. These formulations typically include ionizable lipids, helper lipids, cholesterol, and PEG-lipids in optimized ratios to enhance stability, cellular uptake, and endosomal escape. The careful selection and proportion of these components significantly impact the efficacy of mRNA delivery to target cells and subsequent protein expression.- Lipid nanoparticle composition for mRNA delivery: Specific lipid compositions can enhance the delivery efficiency of mRNA therapeutics. These formulations typically include ionizable lipids, helper lipids, cholesterol, and PEG-lipids in optimized ratios to improve stability, cellular uptake, and endosomal escape. The careful selection and proportion of these components significantly impacts transfection efficiency and the therapeutic index of the delivered mRNA.
- mRNA encapsulation techniques in lipid nanoparticles: Various methods for encapsulating mRNA within lipid nanoparticles have been developed to protect the genetic material from degradation and improve delivery efficiency. These techniques include microfluidic mixing, ethanol injection, and rapid mixing processes that control particle size and ensure uniform mRNA distribution. Optimized encapsulation methods can achieve high loading efficiency while maintaining the structural integrity and biological activity of the mRNA.
- Targeted delivery systems for mRNA lipid nanoparticles: Targeted delivery systems incorporate specific ligands or modifications to direct mRNA-loaded lipid nanoparticles to particular tissues or cell types. These targeting strategies can include antibodies, peptides, aptamers, or other molecules that recognize receptors overexpressed on target cells. Such approaches enhance therapeutic efficacy while reducing off-target effects and the required dose, improving the safety profile of mRNA therapeutics.
- Stability enhancement of mRNA lipid nanoparticles: Various approaches have been developed to improve the stability of mRNA lipid nanoparticles during storage and in biological environments. These include lyophilization techniques, cryoprotectants, pH optimization, and surface modifications that prevent aggregation and degradation. Enhanced stability extends shelf life, maintains therapeutic potency, and enables more practical distribution and administration of mRNA-based medicines.
- Manufacturing processes for mRNA lipid nanoparticles: Scalable manufacturing processes for producing consistent and high-quality mRNA lipid nanoparticles are critical for clinical translation. These processes include continuous flow microfluidic systems, controlled precipitation methods, and automated production lines that ensure batch-to-batch reproducibility. Advanced manufacturing techniques focus on controlling critical quality attributes such as particle size distribution, polydispersity, and encapsulation efficiency while maintaining sterility and regulatory compliance.
02 Modified mRNA formulations for enhanced stability and expression
Modified mRNA molecules can be incorporated into lipid nanoparticles to improve stability, reduce immunogenicity, and enhance protein expression. These modifications may include the use of pseudouridine, 5-methylcytidine, or other nucleoside analogs, as well as optimized 5' caps and poly(A) tails. Such modifications protect the mRNA from degradation and improve translation efficiency when delivered via lipid nanoparticles.Expand Specific Solutions03 Targeted delivery systems for mRNA lipid nanoparticles
Lipid nanoparticles can be functionalized with targeting ligands to enhance the delivery of mRNA to specific tissues or cell types. These targeting moieties may include antibodies, peptides, aptamers, or small molecules that bind to receptors overexpressed on target cells. By incorporating these targeting elements into the LNP surface, the specificity of mRNA delivery can be significantly improved, reducing off-target effects and enhancing therapeutic efficacy.Expand Specific Solutions04 Manufacturing processes for mRNA lipid nanoparticles
Various manufacturing techniques have been developed for the production of mRNA-loaded lipid nanoparticles with consistent quality and scalability. These methods include microfluidic mixing, ethanol injection, and other controlled mixing approaches that enable the formation of uniform nanoparticles with high encapsulation efficiency. Process parameters such as flow rates, mixing conditions, and purification steps are critical for maintaining the integrity and functionality of the final LNP product.Expand Specific Solutions05 Novel lipid structures for improved mRNA delivery
Research has focused on developing novel lipid structures specifically designed to enhance mRNA delivery efficiency. These include biodegradable ionizable lipids, lipidoids with branched structures, and pH-responsive lipids that facilitate endosomal escape. The molecular design of these lipids influences critical parameters such as pKa values, biodegradability, and membrane fusion properties, which collectively determine the transfection efficiency and safety profile of mRNA lipid nanoparticles.Expand Specific Solutions
Leading Companies in mRNA LNP Development
The mRNA lipid nanoparticle (LNP) drug delivery market is currently in a growth phase, with an estimated global market size exceeding $5 billion and projected to reach $15 billion by 2030. The technology has reached commercial maturity for certain applications, particularly vaccines, while therapeutic applications remain in early to mid-stage clinical development. Key players in this competitive landscape include established companies like Arbutus Biopharma and Translate Bio (acquired by Sanofi), alongside emerging innovators such as Orna Therapeutics, GreenLight Biosciences, and Renagade Therapeutics. Chinese companies including Stemirna Therapeutics and Suzhou Abogen are rapidly advancing their capabilities. Academic institutions like University of Pennsylvania and UNC Chapel Hill continue to drive fundamental research, while pharmaceutical giants like Merck are strategically entering the space through partnerships and acquisitions, indicating the technology's growing commercial significance.
Genevant Sciences GmbH
Technical Solution: Genevant Sciences has developed proprietary lipid nanoparticle (LNP) delivery platforms that optimize mRNA drug delivery through their Lipid Nanoparticle (LNP) technology. Their approach focuses on ionizable lipids with optimized pKa values that facilitate endosomal escape, a critical step for effective mRNA delivery. The company's LNP systems incorporate four key components: ionizable lipids that promote cellular uptake and endosomal release, helper phospholipids that stabilize the lipid bilayer structure, cholesterol that provides structural integrity, and PEG-lipids that extend circulation time and prevent aggregation[1]. Genevant has refined their formulation process to achieve precise particle size control (typically 80-100nm) and narrow size distribution, which significantly enhances biodistribution profiles and cellular uptake efficiency. Their technology allows for targeted delivery to specific tissues beyond the liver, addressing one of the major challenges in mRNA therapeutics[2].
Strengths: Proprietary ionizable lipid chemistry with optimized pKa values for enhanced endosomal escape; demonstrated clinical validation through partnerships with multiple pharmaceutical companies. Weaknesses: Potential intellectual property challenges due to competitive landscape in LNP technology; limited public data on delivery efficiency to tissues beyond liver.
Arbutus Biopharma Corp.
Technical Solution: Arbutus Biopharma has pioneered LNP technology for mRNA delivery with their proprietary lipid nanoparticle (LNP) platform that focuses on optimizing the ionizable lipid component. Their technology employs lipids with tertiary amines that remain neutral at physiological pH but become positively charged in the acidic environment of endosomes, facilitating membrane disruption and mRNA release into the cytoplasm. Arbutus has developed a library of over 300 proprietary lipids with varying structures to optimize delivery to specific tissues[3]. Their LNP formulations typically contain an ionizable amino lipid, phospholipid, cholesterol, and a PEG-lipid at optimized molar ratios. The company has refined their manufacturing process to ensure consistent particle size (70-100nm) and high encapsulation efficiency (>90%). Arbutus's technology has been instrumental in the development of approved mRNA vaccines and is being applied to various therapeutic applications beyond vaccines[4].
Strengths: Extensive patent portfolio covering fundamental LNP technology; proven technology that has been licensed for commercial products; demonstrated high encapsulation efficiency. Weaknesses: Facing increasing competition from newer LNP technologies; historical focus on liver delivery may limit applications in other tissues without further optimization.
Key Patents and Breakthroughs in LNP Formulation
Lipid nanoparticle for targeted delivery of therapeutic payloads
PatentWO2024192117A9
Innovation
- The development of lipid nanoparticles (LNPs) formulated with ionizable lipids, which are designed to protect and deliver mRNA payloads efficiently by forming stable complexes that can be taken up by cells and release the mRNA effectively.
Lipid nanoparticle formulations
PatentWO2020097540A1
Innovation
- The development of specific lipid nanoparticle formulations comprising nucleic acids, cholesterol, DSPC, PEG-C-DMA, and a cationic lipid, optimized in molar percentages to enhance stability and delivery efficacy, allowing for lower doses and improved therapeutic outcomes.
Regulatory Pathway for mRNA LNP Therapeutics
The regulatory landscape for mRNA Lipid Nanoparticle (LNP) therapeutics presents a complex framework that developers must navigate to bring these innovative treatments to market. Currently, the FDA categorizes mRNA LNP products as biologics, placing them under the regulatory oversight of the Center for Biologics Evaluation and Research (CBER). This classification necessitates adherence to specific regulatory pathways designed for biological products rather than conventional pharmaceuticals.
The approval process typically begins with preclinical studies focusing on toxicology, biodistribution, and pharmacokinetics specific to the LNP formulation and the encapsulated mRNA. These studies must address both the active mRNA component and the lipid delivery system, as each presents unique safety considerations. Regulatory agencies require comprehensive characterization of the LNP structure, size distribution, encapsulation efficiency, and stability profiles.
For clinical development, sponsors must submit an Investigational New Drug (IND) application containing detailed information about manufacturing processes, quality control measures, and preliminary safety data. The accelerated development and emergency use authorization of COVID-19 mRNA vaccines has established precedents that may influence future regulatory approaches for mRNA LNP therapeutics, potentially streamlining certain aspects of the review process.
Manufacturing considerations present significant regulatory challenges due to the complex nature of LNP production. Regulatory bodies demand robust chemistry, manufacturing, and controls (CMC) documentation, including validation of critical process parameters and analytical methods specific to mRNA LNP formulations. The FDA's approach emphasizes a quality-by-design framework that requires manufacturers to demonstrate thorough understanding of how process variables affect product quality attributes.
International regulatory harmonization remains an evolving area, with differences in requirements across major markets including the US, EU, and Japan. The European Medicines Agency (EMA) has established specific guidelines for advanced therapy medicinal products that encompass certain mRNA therapeutics, while Japan's PMDA has developed expedited pathways for regenerative medicines that may apply to some mRNA applications.
Post-approval monitoring requirements for mRNA LNP products are typically more extensive than for conventional drugs, with regulatory agencies often mandating long-term safety surveillance studies. As the field evolves, regulatory frameworks are expected to adapt, with increasing focus on establishing product-specific guidance documents and potentially specialized review pathways for different categories of mRNA therapeutics based on their intended use, target population, and risk profile.
The approval process typically begins with preclinical studies focusing on toxicology, biodistribution, and pharmacokinetics specific to the LNP formulation and the encapsulated mRNA. These studies must address both the active mRNA component and the lipid delivery system, as each presents unique safety considerations. Regulatory agencies require comprehensive characterization of the LNP structure, size distribution, encapsulation efficiency, and stability profiles.
For clinical development, sponsors must submit an Investigational New Drug (IND) application containing detailed information about manufacturing processes, quality control measures, and preliminary safety data. The accelerated development and emergency use authorization of COVID-19 mRNA vaccines has established precedents that may influence future regulatory approaches for mRNA LNP therapeutics, potentially streamlining certain aspects of the review process.
Manufacturing considerations present significant regulatory challenges due to the complex nature of LNP production. Regulatory bodies demand robust chemistry, manufacturing, and controls (CMC) documentation, including validation of critical process parameters and analytical methods specific to mRNA LNP formulations. The FDA's approach emphasizes a quality-by-design framework that requires manufacturers to demonstrate thorough understanding of how process variables affect product quality attributes.
International regulatory harmonization remains an evolving area, with differences in requirements across major markets including the US, EU, and Japan. The European Medicines Agency (EMA) has established specific guidelines for advanced therapy medicinal products that encompass certain mRNA therapeutics, while Japan's PMDA has developed expedited pathways for regenerative medicines that may apply to some mRNA applications.
Post-approval monitoring requirements for mRNA LNP products are typically more extensive than for conventional drugs, with regulatory agencies often mandating long-term safety surveillance studies. As the field evolves, regulatory frameworks are expected to adapt, with increasing focus on establishing product-specific guidance documents and potentially specialized review pathways for different categories of mRNA therapeutics based on their intended use, target population, and risk profile.
Manufacturing Challenges and Scale-up Solutions
The manufacturing of mRNA lipid nanoparticles (LNPs) presents significant challenges that must be addressed for successful commercial-scale production. Traditional laboratory-scale production methods often involve batch processes with manual interventions, which become impractical when scaling to meet global demand. One primary challenge is maintaining consistent particle size distribution and encapsulation efficiency across larger production volumes, as these parameters directly impact the therapeutic efficacy and safety profile of the final product.
Microfluidic mixing technology has emerged as a promising solution for scalable LNP production, offering precise control over mixing parameters and resulting in more uniform nanoparticles. However, implementing microfluidic systems at industrial scale requires specialized equipment and expertise. Companies must invest in custom-designed parallel microfluidic systems that can maintain the critical quality attributes while increasing throughput by orders of magnitude.
Raw material supply chain constraints represent another significant hurdle. The specialized lipids required for LNP formulation—particularly ionizable cationic lipids and PEGylated lipids—have historically been produced in limited quantities for research purposes. The sudden demand surge following COVID-19 vaccine development exposed vulnerabilities in the supply chain, with manufacturers struggling to secure consistent, high-quality lipid components in sufficient quantities.
Quality control and analytical testing become increasingly complex at larger scales. Batch-to-batch consistency must be rigorously monitored through comprehensive physicochemical characterization, including particle size analysis, zeta potential measurements, and encapsulation efficiency determination. Developing robust in-process controls and real-time monitoring capabilities is essential for maintaining product quality during continuous manufacturing operations.
Regulatory considerations add another layer of complexity to scale-up efforts. Manufacturers must demonstrate that their scaled processes produce material equivalent to that used in clinical trials, requiring extensive comparability studies. Process validation becomes more challenging as production volumes increase, necessitating careful design of validation protocols that account for the unique characteristics of LNP manufacturing.
Cold chain requirements present logistical challenges that impact manufacturing decisions. Current mRNA-LNP formulations typically require ultra-cold storage conditions (-70°C to -20°C), necessitating specialized equipment throughout the production, storage, and distribution chain. Innovations in lyophilization techniques and alternative stabilization approaches are being explored to address these limitations and simplify the manufacturing process.
Microfluidic mixing technology has emerged as a promising solution for scalable LNP production, offering precise control over mixing parameters and resulting in more uniform nanoparticles. However, implementing microfluidic systems at industrial scale requires specialized equipment and expertise. Companies must invest in custom-designed parallel microfluidic systems that can maintain the critical quality attributes while increasing throughput by orders of magnitude.
Raw material supply chain constraints represent another significant hurdle. The specialized lipids required for LNP formulation—particularly ionizable cationic lipids and PEGylated lipids—have historically been produced in limited quantities for research purposes. The sudden demand surge following COVID-19 vaccine development exposed vulnerabilities in the supply chain, with manufacturers struggling to secure consistent, high-quality lipid components in sufficient quantities.
Quality control and analytical testing become increasingly complex at larger scales. Batch-to-batch consistency must be rigorously monitored through comprehensive physicochemical characterization, including particle size analysis, zeta potential measurements, and encapsulation efficiency determination. Developing robust in-process controls and real-time monitoring capabilities is essential for maintaining product quality during continuous manufacturing operations.
Regulatory considerations add another layer of complexity to scale-up efforts. Manufacturers must demonstrate that their scaled processes produce material equivalent to that used in clinical trials, requiring extensive comparability studies. Process validation becomes more challenging as production volumes increase, necessitating careful design of validation protocols that account for the unique characteristics of LNP manufacturing.
Cold chain requirements present logistical challenges that impact manufacturing decisions. Current mRNA-LNP formulations typically require ultra-cold storage conditions (-70°C to -20°C), necessitating specialized equipment throughout the production, storage, and distribution chain. Innovations in lyophilization techniques and alternative stabilization approaches are being explored to address these limitations and simplify the manufacturing process.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!