mRNA Lipid Nanoparticle Delivery in Pharmaceuticals
OCT 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
mRNA LNP Technology Evolution and Objectives
Messenger RNA (mRNA) technology has undergone a remarkable evolution over the past four decades, transforming from a theoretical concept to a revolutionary pharmaceutical platform. The journey began in the 1980s with the first successful in vitro transcription of mRNA, followed by pioneering work in the 1990s that demonstrated the potential of mRNA for protein expression in vivo. However, early applications faced significant challenges related to mRNA instability, immunogenicity, and inefficient cellular delivery.
The breakthrough came in the early 2000s with the development of modified nucleosides that could reduce immunogenicity while enhancing translation efficiency. Researchers discovered that incorporating pseudouridine and 5-methylcytidine into mRNA structures significantly improved stability and reduced activation of innate immune responses, addressing key barriers to therapeutic applications.
Parallel to these advancements, lipid nanoparticle (LNP) delivery systems emerged as a critical enabling technology. The evolution of LNP formulations progressed from simple liposomes to sophisticated multi-component systems incorporating ionizable lipids, helper phospholipids, cholesterol, and PEGylated lipids. This technological convergence created a synergistic platform that protected mRNA from degradation while facilitating cellular uptake and endosomal escape.
The 2010s witnessed accelerated development in LNP design, with innovations in lipid chemistry yielding formulations with enhanced biodistribution profiles and reduced toxicity. Breakthrough discoveries of novel ionizable lipids with optimized pKa values dramatically improved the efficiency of mRNA delivery to target tissues, particularly the liver, which became the initial focus for many therapeutic applications.
The COVID-19 pandemic served as a catalyst for mRNA-LNP technology, demonstrating unprecedented speed from concept to global deployment of vaccines. This real-world validation has significantly accelerated investment and research interest across the pharmaceutical industry, expanding potential applications beyond vaccines to protein replacement therapies, gene editing, and immunomodulation.
The primary objectives for mRNA-LNP technology advancement now focus on several key areas: enhancing tissue-specific targeting beyond hepatic delivery; improving the therapeutic index through reduced immunogenicity and toxicity; extending mRNA half-life and protein expression duration; scaling manufacturing processes for consistent quality and reduced costs; and developing formulations with improved stability profiles that eliminate ultra-cold storage requirements.
Future evolution aims to create programmable delivery systems capable of targeting specific cell types across diverse tissues, potentially revolutionizing treatment approaches for genetic disorders, cancer, and autoimmune diseases. The convergence of mRNA design optimization and LNP engineering represents a frontier with transformative potential for pharmaceutical development and precision medicine.
The breakthrough came in the early 2000s with the development of modified nucleosides that could reduce immunogenicity while enhancing translation efficiency. Researchers discovered that incorporating pseudouridine and 5-methylcytidine into mRNA structures significantly improved stability and reduced activation of innate immune responses, addressing key barriers to therapeutic applications.
Parallel to these advancements, lipid nanoparticle (LNP) delivery systems emerged as a critical enabling technology. The evolution of LNP formulations progressed from simple liposomes to sophisticated multi-component systems incorporating ionizable lipids, helper phospholipids, cholesterol, and PEGylated lipids. This technological convergence created a synergistic platform that protected mRNA from degradation while facilitating cellular uptake and endosomal escape.
The 2010s witnessed accelerated development in LNP design, with innovations in lipid chemistry yielding formulations with enhanced biodistribution profiles and reduced toxicity. Breakthrough discoveries of novel ionizable lipids with optimized pKa values dramatically improved the efficiency of mRNA delivery to target tissues, particularly the liver, which became the initial focus for many therapeutic applications.
The COVID-19 pandemic served as a catalyst for mRNA-LNP technology, demonstrating unprecedented speed from concept to global deployment of vaccines. This real-world validation has significantly accelerated investment and research interest across the pharmaceutical industry, expanding potential applications beyond vaccines to protein replacement therapies, gene editing, and immunomodulation.
The primary objectives for mRNA-LNP technology advancement now focus on several key areas: enhancing tissue-specific targeting beyond hepatic delivery; improving the therapeutic index through reduced immunogenicity and toxicity; extending mRNA half-life and protein expression duration; scaling manufacturing processes for consistent quality and reduced costs; and developing formulations with improved stability profiles that eliminate ultra-cold storage requirements.
Future evolution aims to create programmable delivery systems capable of targeting specific cell types across diverse tissues, potentially revolutionizing treatment approaches for genetic disorders, cancer, and autoimmune diseases. The convergence of mRNA design optimization and LNP engineering represents a frontier with transformative potential for pharmaceutical development and precision medicine.
Pharmaceutical Market Demand for mRNA Delivery Systems
The global mRNA therapeutics market has experienced unprecedented growth following the successful deployment of mRNA-based COVID-19 vaccines, catalyzing significant interest in mRNA delivery technologies. Current market valuations place the mRNA therapeutics sector at approximately $46.7 billion in 2023, with projections indicating a compound annual growth rate (CAGR) of 12.8% through 2030, potentially reaching $109.8 billion by decade's end.
Lipid nanoparticle (LNP) delivery systems represent the dominant technology platform within this market, accounting for over 85% of current mRNA delivery methods in clinical applications. This dominance stems from LNPs' demonstrated ability to protect mRNA from degradation while facilitating cellular uptake and endosomal escape—critical factors for therapeutic efficacy.
The pharmaceutical industry's demand for advanced mRNA-LNP delivery systems extends beyond vaccines into multiple therapeutic areas. Oncology represents the fastest-growing segment, with approximately 37% of mRNA-LNP clinical trials focusing on cancer immunotherapies and personalized cancer vaccines. Rare genetic disorders constitute another significant market segment, with an estimated 23% market share, driven by the precision medicine paradigm and orphan drug incentives.
Infectious disease prevention remains a substantial market driver, accounting for 28% of development efforts, bolstered by the proven success of COVID-19 vaccines and expanding research into influenza, RSV, and emerging pathogens. Additionally, autoimmune disorders and protein replacement therapies are emerging application areas, collectively representing about 12% of the market.
Regional analysis reveals North America commanding approximately 48% of the global market share, followed by Europe (27%) and Asia-Pacific (18%), with the latter showing the highest growth trajectory at 15.2% CAGR. This geographic distribution reflects both research infrastructure concentration and regulatory environment maturity.
Key market demand drivers include improved tissue-specific targeting capabilities, enhanced stability profiles for non-frozen distribution, reduced manufacturing costs, and minimized immunogenicity. Pharmaceutical companies are particularly seeking LNP formulations that can achieve targeted delivery to tissues beyond the liver, which represents a significant technical challenge and market opportunity.
The contract development and manufacturing organization (CDMO) segment is experiencing 18.3% annual growth, indicating strong demand for specialized expertise in LNP formulation and scale-up. This trend underscores the technical complexity of mRNA-LNP manufacturing and the pharmaceutical industry's strategic approach to accessing this technology through partnerships rather than solely through internal development.
Lipid nanoparticle (LNP) delivery systems represent the dominant technology platform within this market, accounting for over 85% of current mRNA delivery methods in clinical applications. This dominance stems from LNPs' demonstrated ability to protect mRNA from degradation while facilitating cellular uptake and endosomal escape—critical factors for therapeutic efficacy.
The pharmaceutical industry's demand for advanced mRNA-LNP delivery systems extends beyond vaccines into multiple therapeutic areas. Oncology represents the fastest-growing segment, with approximately 37% of mRNA-LNP clinical trials focusing on cancer immunotherapies and personalized cancer vaccines. Rare genetic disorders constitute another significant market segment, with an estimated 23% market share, driven by the precision medicine paradigm and orphan drug incentives.
Infectious disease prevention remains a substantial market driver, accounting for 28% of development efforts, bolstered by the proven success of COVID-19 vaccines and expanding research into influenza, RSV, and emerging pathogens. Additionally, autoimmune disorders and protein replacement therapies are emerging application areas, collectively representing about 12% of the market.
Regional analysis reveals North America commanding approximately 48% of the global market share, followed by Europe (27%) and Asia-Pacific (18%), with the latter showing the highest growth trajectory at 15.2% CAGR. This geographic distribution reflects both research infrastructure concentration and regulatory environment maturity.
Key market demand drivers include improved tissue-specific targeting capabilities, enhanced stability profiles for non-frozen distribution, reduced manufacturing costs, and minimized immunogenicity. Pharmaceutical companies are particularly seeking LNP formulations that can achieve targeted delivery to tissues beyond the liver, which represents a significant technical challenge and market opportunity.
The contract development and manufacturing organization (CDMO) segment is experiencing 18.3% annual growth, indicating strong demand for specialized expertise in LNP formulation and scale-up. This trend underscores the technical complexity of mRNA-LNP manufacturing and the pharmaceutical industry's strategic approach to accessing this technology through partnerships rather than solely through internal development.
Current Landscape and Barriers in LNP Technology
The mRNA lipid nanoparticle (LNP) delivery landscape has evolved significantly since the COVID-19 pandemic catalyzed unprecedented investment and research in this field. Currently, LNPs represent the most clinically advanced non-viral vector system for mRNA delivery, with two approved COVID-19 vaccines from Pfizer/BioNTech and Moderna demonstrating their efficacy and safety at scale. These successes have positioned LNPs as the gold standard for mRNA delivery in pharmaceutical applications.
Despite these achievements, the current LNP technology faces several critical barriers that limit broader therapeutic applications beyond vaccines. Delivery efficiency remains a significant challenge, with most LNPs showing preferential accumulation in the liver following systemic administration. This hepatic tropism restricts their utility for targeting other tissues and organs, creating a substantial barrier for treating diseases requiring delivery to specific cell types outside the liver.
Another major limitation is the immunogenicity of LNP formulations. While beneficial for vaccine applications, the inherent immunostimulatory properties of both the lipid components and the mRNA cargo can trigger unwanted inflammatory responses when used for non-vaccine therapeutics. This immune activation can lead to reduced efficacy, increased clearance rates, and potential safety concerns for chronic administration scenarios.
Manufacturing scalability and reproducibility present additional challenges. Current production methods often yield heterogeneous LNP populations with batch-to-batch variations in size, polydispersity, and encapsulation efficiency. These inconsistencies can affect pharmacokinetic profiles, biodistribution patterns, and ultimately therapeutic outcomes, complicating regulatory approval pathways.
Stability issues further constrain the practical application of LNP-mRNA therapeutics. Most formulations require ultra-cold storage conditions (-70°C to -20°C), creating logistical hurdles for global distribution and limiting access in resource-constrained settings. The development of thermostable formulations remains an active area of research but has yet to yield broadly applicable solutions.
The regulatory landscape for LNP-mRNA therapeutics is still evolving, with limited precedents beyond vaccines. Regulatory agencies are developing frameworks to address the unique characteristics of these complex modalities, including considerations for characterization, quality control, and safety assessment. This regulatory uncertainty adds complexity to development timelines and investment decisions.
Cost considerations also present significant barriers to widespread adoption. Current manufacturing processes for GMP-grade LNPs and mRNA are expensive, with estimates suggesting treatment costs could range from thousands to tens of thousands of dollars per dose for complex therapeutics, potentially limiting patient access and commercial viability for certain indications.
Despite these achievements, the current LNP technology faces several critical barriers that limit broader therapeutic applications beyond vaccines. Delivery efficiency remains a significant challenge, with most LNPs showing preferential accumulation in the liver following systemic administration. This hepatic tropism restricts their utility for targeting other tissues and organs, creating a substantial barrier for treating diseases requiring delivery to specific cell types outside the liver.
Another major limitation is the immunogenicity of LNP formulations. While beneficial for vaccine applications, the inherent immunostimulatory properties of both the lipid components and the mRNA cargo can trigger unwanted inflammatory responses when used for non-vaccine therapeutics. This immune activation can lead to reduced efficacy, increased clearance rates, and potential safety concerns for chronic administration scenarios.
Manufacturing scalability and reproducibility present additional challenges. Current production methods often yield heterogeneous LNP populations with batch-to-batch variations in size, polydispersity, and encapsulation efficiency. These inconsistencies can affect pharmacokinetic profiles, biodistribution patterns, and ultimately therapeutic outcomes, complicating regulatory approval pathways.
Stability issues further constrain the practical application of LNP-mRNA therapeutics. Most formulations require ultra-cold storage conditions (-70°C to -20°C), creating logistical hurdles for global distribution and limiting access in resource-constrained settings. The development of thermostable formulations remains an active area of research but has yet to yield broadly applicable solutions.
The regulatory landscape for LNP-mRNA therapeutics is still evolving, with limited precedents beyond vaccines. Regulatory agencies are developing frameworks to address the unique characteristics of these complex modalities, including considerations for characterization, quality control, and safety assessment. This regulatory uncertainty adds complexity to development timelines and investment decisions.
Cost considerations also present significant barriers to widespread adoption. Current manufacturing processes for GMP-grade LNPs and mRNA are expensive, with estimates suggesting treatment costs could range from thousands to tens of thousands of dollars per dose for complex therapeutics, potentially limiting patient access and commercial viability for certain indications.
Current LNP Formulation and Manufacturing Approaches
01 Lipid composition optimization for mRNA delivery
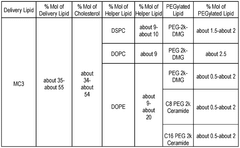
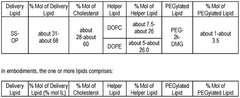
The composition of lipids in nanoparticles significantly affects mRNA delivery efficiency. Optimized ratios of ionizable lipids, helper lipids, cholesterol, and PEG-lipids can enhance cellular uptake and endosomal escape. Specific lipid formulations can be tailored for targeting different tissues and improving the stability of the mRNA cargo during delivery, resulting in better translation efficiency and therapeutic outcomes.- Lipid composition optimization for mRNA delivery: Optimization of lipid compositions in nanoparticles enhances mRNA delivery efficiency. This includes developing specific ratios of ionizable lipids, helper lipids, cholesterol, and PEG-lipids to improve stability, cellular uptake, and endosomal escape. These optimized formulations can increase transfection efficiency while reducing cytotoxicity, making them suitable for various therapeutic applications.
- Novel ionizable lipids for improved mRNA delivery: Development of novel ionizable lipids with unique structural features enhances mRNA delivery capabilities. These lipids possess optimized pKa values, biodegradable linkages, and tailored hydrophobic tails that facilitate efficient endosomal escape and cytosolic release of mRNA. The innovative lipid designs improve transfection efficiency while maintaining favorable safety profiles for therapeutic applications.
- Surface modification strategies for targeted delivery: Surface modifications of lipid nanoparticles with targeting ligands enable tissue-specific mRNA delivery. These modifications include conjugation of antibodies, peptides, aptamers, or small molecules to the nanoparticle surface, allowing for recognition of specific cell types or tissues. Such targeted delivery approaches enhance therapeutic efficacy while reducing off-target effects and required dosages.
- Manufacturing and stability enhancement techniques: Advanced manufacturing processes and formulation strategies improve the stability and shelf-life of mRNA lipid nanoparticles. These include microfluidic mixing techniques, lyophilization methods, and incorporation of stabilizing excipients. Such approaches prevent mRNA degradation, maintain nanoparticle size distribution, and preserve transfection efficiency during storage and administration.
- Delivery systems for specific therapeutic applications: Specialized lipid nanoparticle formulations designed for specific therapeutic applications, such as cancer immunotherapy, genetic disorders, or vaccines. These tailored delivery systems account for the unique challenges of each application, including tissue barriers, immune responses, and pharmacokinetic considerations. Modifications may include tissue-specific targeting components, immune-modulating agents, or enhanced penetration capabilities.
02 Novel ionizable lipids for improved mRNA delivery
Development of novel ionizable lipids with optimized structures enhances the delivery of mRNA to target cells. These lipids can change their charge depending on pH, facilitating endosomal escape after cellular uptake. Structural modifications to the lipid head groups, linkers, and hydrophobic tails can improve biodistribution, reduce toxicity, and increase transfection efficiency of the mRNA payload.Expand Specific Solutions03 Surface modification of lipid nanoparticles for targeted delivery
Surface modifications of lipid nanoparticles with targeting ligands enable tissue-specific delivery of mRNA. These modifications can include antibodies, peptides, aptamers, or small molecules that bind to specific receptors on target cells. Such targeted delivery approaches reduce off-target effects, lower required doses, and improve the therapeutic index of mRNA-based treatments.Expand Specific Solutions04 Manufacturing and stability optimization of mRNA-LNPs
Advanced manufacturing processes and formulation techniques improve the stability and scalability of mRNA-loaded lipid nanoparticles. Microfluidic mixing, controlled precipitation, and lyophilization methods can enhance batch consistency and extend shelf-life. Stabilizing excipients and optimized buffer systems protect the mRNA cargo from degradation during storage and administration.Expand Specific Solutions05 Delivery systems for specific therapeutic applications
Specialized lipid nanoparticle formulations designed for specific therapeutic applications such as cancer immunotherapy, genetic disorders, and infectious diseases. These tailored delivery systems consider the unique requirements of different disease states, administration routes, and target tissues. Modifications to the lipid composition and particle characteristics optimize biodistribution and cellular uptake for each therapeutic context.Expand Specific Solutions
Leading Companies in mRNA LNP Development
The mRNA Lipid Nanoparticle (LNP) Delivery market is currently in a growth phase, with significant expansion following COVID-19 vaccine successes. The global market is projected to reach $5-7 billion by 2028, growing at 25-30% CAGR. Technologically, the field is advancing from first-generation liver-targeting LNPs toward tissue-specific delivery systems. Key players include established companies like Moderna and Sanofi, alongside innovative startups such as NanoVation Therapeutics and Renagade Therapeutics developing proprietary LNP platforms. Academic institutions (University of Pennsylvania, Georgia Tech) continue providing foundational research, while regional expansion is evident with Chinese companies (Shenzhen Regis, Hongxin Biotechnology) rapidly entering the space, creating a competitive landscape balancing commercial development with ongoing technical innovation.
Genevant Sciences GmbH
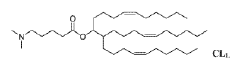
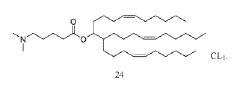
Technical Solution: Genevant Sciences has developed the Lipid Nanoparticle (LNP) platform technology that utilizes proprietary ionizable amino lipids, specifically designed for efficient mRNA delivery. Their LNP system incorporates a unique pH-sensitive cationic lipid (DLin-MC3-DMA) that remains neutral at physiological pH but becomes positively charged in the acidic environment of endosomes, facilitating membrane fusion and cytoplasmic release of mRNA[1][2]. Genevant's technology employs a precise lipid composition with optimized helper lipids that enhance stability and cellular uptake, typically consisting of ionizable lipids, phospholipids, cholesterol, and PEG-lipids in specific molar ratios[3]. Their manufacturing process utilizes controlled ethanol dilution and rapid mixing techniques to achieve consistent nanoparticle size distribution (70-100nm) with high encapsulation efficiency (>95%)[4]. The company has also developed targeted delivery approaches by incorporating ligands such as N-acetylgalactosamine (GalNAc) for hepatocyte-specific delivery[5].
Strengths: Extensive intellectual property portfolio covering fundamental LNP technology; proven clinical validation through partnerships; sophisticated ionizable lipid chemistry optimized for endosomal escape. Weaknesses: Reliance on licensing business model rather than direct product development; potential challenges with tissue targeting beyond liver; ongoing patent litigation with competitors in the LNP space.
ModernaTX, Inc.
Technical Solution: ModernaTX has pioneered proprietary lipid nanoparticle (LNP) delivery systems specifically optimized for mRNA therapeutics. Their platform employs a combination of ionizable lipids, helper phospholipids, cholesterol, and PEG-lipids in precise ratios to create LNPs with high encapsulation efficiency (>90%) and targeted delivery capabilities[1]. Moderna's LNP technology features SM-102, a proprietary ionizable lipid that facilitates endosomal escape through pH-dependent conformational changes, enabling efficient cytosolic mRNA delivery[2]. Their manufacturing process utilizes microfluidic mixing devices that allow for precise control of particle size (typically 80-100nm) and narrow polydispersity indices (<0.2), ensuring batch-to-batch consistency[3]. Moderna has also developed tissue-specific targeting strategies by modifying the lipid composition and surface properties of their LNPs, enabling preferential delivery to liver, lung, or other tissues depending on the therapeutic application[4].
Strengths: Industry-leading manufacturing scale and consistency; extensive clinical validation through COVID-19 vaccines; proprietary ionizable lipids with optimized endosomal escape properties. Weaknesses: Potential cold chain storage requirements; higher production costs compared to traditional therapeutics; limited tissue targeting beyond liver without additional modifications.
Key Patents and Innovations in LNP Design
Lipid nanoparticles
PatentWO2020219941A1
Innovation
- Development of lipid nanoparticles with specific PEG-lipid conjugate structures and compositions, including varying molecular weights and ratios of PEG-lipid, cationic, and non-cationic lipids, to enhance stability, reduce aggregation, and minimize immune response, allowing for effective delivery of nucleic acids like siRNA and mRNA.
Lipid nanoparticle (LNP) formulations
PatentWO2024226779A1
Innovation
- The development of lipid nanoparticle (LNP) formulations comprising specific lipids that associate with nucleic acid-based agents, including modified mRNA and plasmid DNA, to form aggregates or particles that can be delivered to the retina, utilizing a combination of cationic, anionic, and neutral lipids, along with PEGylated lipids to enhance stability and targeting.
Regulatory Framework for mRNA-LNP Therapeutics
The regulatory landscape for mRNA-LNP therapeutics represents a complex and evolving framework that significantly impacts development timelines and market access. Currently, regulatory agencies worldwide are adapting existing pharmaceutical frameworks to address the unique characteristics of mRNA-LNP technologies. The FDA's approach involves classifying these therapeutics under biological products, requiring Biologics License Applications (BLAs) with comprehensive safety and efficacy data.
In Europe, the EMA has established specific guidelines for advanced therapy medicinal products (ATMPs), which include certain mRNA-based therapeutics. These guidelines emphasize quality control measures for lipid components, mRNA purity standards, and stability testing protocols specific to nanoparticle formulations. The accelerated approval pathways created during the COVID-19 pandemic have established important precedents for emergency use authorizations while maintaining rigorous safety standards.
Key regulatory considerations for mRNA-LNP products include characterization requirements for lipid components, which must demonstrate consistent composition, purity, and stability. Regulatory bodies require extensive analytical testing to confirm the structural integrity of encapsulated mRNA and the physical properties of the resulting nanoparticles, including size distribution, zeta potential, and polydispersity index.
Manufacturing process validation presents unique challenges under current regulatory frameworks. Authorities demand robust documentation of critical process parameters affecting LNP formation, encapsulation efficiency, and batch-to-batch consistency. The cold chain requirements for mRNA-LNP products have prompted regulatory agencies to establish specialized guidelines for temperature monitoring, storage conditions, and distribution validation.
Toxicology assessment frameworks for these novel therapeutics focus on potential inflammatory responses, cytokine release, and biodistribution patterns specific to lipid nanoparticles. Regulatory agencies increasingly require advanced immunogenicity testing and long-term follow-up studies to monitor delayed adverse effects, particularly for products intended for repeated administration.
Global harmonization efforts are underway through initiatives like the International Council for Harmonisation (ICH) to standardize regulatory requirements across major markets. However, significant regional differences persist in areas such as required non-clinical studies, acceptable manufacturing processes, and post-marketing surveillance requirements. Companies developing mRNA-LNP therapeutics must navigate these variations through comprehensive regulatory strategies tailored to each target market.
The regulatory framework continues to evolve as scientific understanding of mRNA-LNP technologies advances. Emerging trends include the development of product-specific guidance documents, increased focus on characterizing novel excipients in LNP formulations, and refined approaches to accelerated approval pathways for serious conditions with unmet medical needs.
In Europe, the EMA has established specific guidelines for advanced therapy medicinal products (ATMPs), which include certain mRNA-based therapeutics. These guidelines emphasize quality control measures for lipid components, mRNA purity standards, and stability testing protocols specific to nanoparticle formulations. The accelerated approval pathways created during the COVID-19 pandemic have established important precedents for emergency use authorizations while maintaining rigorous safety standards.
Key regulatory considerations for mRNA-LNP products include characterization requirements for lipid components, which must demonstrate consistent composition, purity, and stability. Regulatory bodies require extensive analytical testing to confirm the structural integrity of encapsulated mRNA and the physical properties of the resulting nanoparticles, including size distribution, zeta potential, and polydispersity index.
Manufacturing process validation presents unique challenges under current regulatory frameworks. Authorities demand robust documentation of critical process parameters affecting LNP formation, encapsulation efficiency, and batch-to-batch consistency. The cold chain requirements for mRNA-LNP products have prompted regulatory agencies to establish specialized guidelines for temperature monitoring, storage conditions, and distribution validation.
Toxicology assessment frameworks for these novel therapeutics focus on potential inflammatory responses, cytokine release, and biodistribution patterns specific to lipid nanoparticles. Regulatory agencies increasingly require advanced immunogenicity testing and long-term follow-up studies to monitor delayed adverse effects, particularly for products intended for repeated administration.
Global harmonization efforts are underway through initiatives like the International Council for Harmonisation (ICH) to standardize regulatory requirements across major markets. However, significant regional differences persist in areas such as required non-clinical studies, acceptable manufacturing processes, and post-marketing surveillance requirements. Companies developing mRNA-LNP therapeutics must navigate these variations through comprehensive regulatory strategies tailored to each target market.
The regulatory framework continues to evolve as scientific understanding of mRNA-LNP technologies advances. Emerging trends include the development of product-specific guidance documents, increased focus on characterizing novel excipients in LNP formulations, and refined approaches to accelerated approval pathways for serious conditions with unmet medical needs.
Safety and Immunogenicity Considerations
The safety profile of mRNA lipid nanoparticle (LNP) delivery systems represents a critical consideration in pharmaceutical development. Current evidence indicates that while generally well-tolerated, these delivery systems can trigger both local and systemic inflammatory responses. Local reactions typically manifest as erythema, pain, and swelling at the injection site, while systemic effects may include fatigue, headache, and low-grade fever. These reactions are predominantly attributed to the innate immune recognition of the lipid components rather than the mRNA cargo itself.
The immunogenicity of LNP-mRNA formulations presents both challenges and opportunities. On one hand, the inherent immunostimulatory properties of certain lipids can serve as built-in adjuvants, enhancing vaccine efficacy. On the other hand, these same properties may limit repeated dosing strategies for therapeutic applications due to accelerated clearance and potential hypersensitivity reactions. Recent research has demonstrated that PEGylated lipids, commonly used to improve LNP stability, can induce anti-PEG antibodies after repeated administration, potentially compromising therapeutic efficacy.
Complement activation represents another significant safety concern. Several LNP formulations have been shown to activate the complement system, potentially leading to complement activation-related pseudoallergy (CARPA). This reaction can manifest as cardiopulmonary distress and hypotension in severe cases. Modifying lipid composition and optimizing particle size have emerged as strategies to mitigate these effects.
Long-term safety considerations remain partially uncharted territory. While acute toxicity profiles are increasingly well-characterized, the biodistribution and potential accumulation of lipid components in tissues following repeated administration require further investigation. Preliminary studies suggest that most lipid components undergo metabolic degradation and elimination, but certain modified lipids may persist longer in specific tissues.
Regulatory frameworks for assessing LNP-mRNA safety continue to evolve. Current guidelines emphasize comprehensive toxicological evaluation, including genotoxicity, reproductive toxicity, and immunogenicity assessments. The unprecedented rapid deployment of mRNA-LNP vaccines during the COVID-19 pandemic has generated substantial real-world safety data, significantly advancing our understanding of these delivery systems in diverse populations.
Future developments in safety optimization focus on designing lipids with improved biodegradability, reduced immunogenicity, and enhanced tissue-specific targeting. Computational approaches and high-throughput screening methodologies are increasingly employed to predict potential toxicities and immunological responses, accelerating the development of safer LNP formulations while maintaining therapeutic efficacy.
The immunogenicity of LNP-mRNA formulations presents both challenges and opportunities. On one hand, the inherent immunostimulatory properties of certain lipids can serve as built-in adjuvants, enhancing vaccine efficacy. On the other hand, these same properties may limit repeated dosing strategies for therapeutic applications due to accelerated clearance and potential hypersensitivity reactions. Recent research has demonstrated that PEGylated lipids, commonly used to improve LNP stability, can induce anti-PEG antibodies after repeated administration, potentially compromising therapeutic efficacy.
Complement activation represents another significant safety concern. Several LNP formulations have been shown to activate the complement system, potentially leading to complement activation-related pseudoallergy (CARPA). This reaction can manifest as cardiopulmonary distress and hypotension in severe cases. Modifying lipid composition and optimizing particle size have emerged as strategies to mitigate these effects.
Long-term safety considerations remain partially uncharted territory. While acute toxicity profiles are increasingly well-characterized, the biodistribution and potential accumulation of lipid components in tissues following repeated administration require further investigation. Preliminary studies suggest that most lipid components undergo metabolic degradation and elimination, but certain modified lipids may persist longer in specific tissues.
Regulatory frameworks for assessing LNP-mRNA safety continue to evolve. Current guidelines emphasize comprehensive toxicological evaluation, including genotoxicity, reproductive toxicity, and immunogenicity assessments. The unprecedented rapid deployment of mRNA-LNP vaccines during the COVID-19 pandemic has generated substantial real-world safety data, significantly advancing our understanding of these delivery systems in diverse populations.
Future developments in safety optimization focus on designing lipids with improved biodegradability, reduced immunogenicity, and enhanced tissue-specific targeting. Computational approaches and high-throughput screening methodologies are increasingly employed to predict potential toxicities and immunological responses, accelerating the development of safer LNP formulations while maintaining therapeutic efficacy.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!