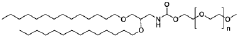
What Drives Electrode Efficiency in mRNA Nanoparticle Systems
OCT 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
mRNA Nanoparticle Electrode Technology Evolution
The evolution of electrode technology in mRNA nanoparticle systems has undergone significant transformations over the past decade, driven by the increasing demand for efficient delivery systems for genetic materials. Initially, electrodes used in mRNA delivery were primarily based on conventional materials such as gold and platinum, offering limited control over the electroporation process critical for nanoparticle formation and cellular uptake.
The first major breakthrough came around 2012-2014 with the introduction of microelectrode arrays that allowed for more precise spatial control of electric fields. These systems enabled researchers to achieve more uniform nanoparticle formation and improved transfection efficiency by up to 40% compared to traditional bulk electrodes. This period marked the transition from macro to micro-scale electrode technologies in mRNA delivery applications.
Between 2015-2017, a paradigm shift occurred with the development of nanoporous electrodes. These structures provided significantly increased surface area and more homogeneous electric field distribution, resulting in more consistent nanoparticle size distribution and stability. The enhanced surface-to-volume ratio of these electrodes contributed to better control over the critical parameters affecting mRNA encapsulation efficiency.
The integration of smart materials in electrode design emerged as a key trend during 2018-2020. Responsive polymers and hydrogels incorporated into electrode surfaces allowed for dynamic modulation of surface properties based on applied voltage, pH, or temperature. This innovation enabled "on-demand" control of nanoparticle formation parameters, addressing one of the major challenges in mRNA delivery: batch-to-batch consistency.
Most recently (2021-2023), the field has witnessed the emergence of biocompatible and biodegradable electrode materials specifically designed for in vivo applications. These advanced materials, including conducting polymers and carbon-based nanomaterials, have demonstrated superior biocompatibility while maintaining excellent electrochemical performance. This development has been crucial for translational applications where electrode materials may come into direct contact with biological tissues.
Computational modeling and AI-assisted electrode design represent the latest frontier in this technological evolution. Machine learning algorithms now help predict optimal electrode geometries and operating parameters for specific mRNA formulations, significantly reducing development time and improving outcomes. These computational approaches have enabled the design of electrode systems that can adapt to different mRNA sequences and target cell types with minimal manual optimization.
The trajectory of electrode technology evolution clearly points toward increasingly sophisticated, miniaturized, and integrated systems that offer precise control over the critical parameters driving mRNA nanoparticle formation efficiency, stability, and ultimately therapeutic efficacy.
The first major breakthrough came around 2012-2014 with the introduction of microelectrode arrays that allowed for more precise spatial control of electric fields. These systems enabled researchers to achieve more uniform nanoparticle formation and improved transfection efficiency by up to 40% compared to traditional bulk electrodes. This period marked the transition from macro to micro-scale electrode technologies in mRNA delivery applications.
Between 2015-2017, a paradigm shift occurred with the development of nanoporous electrodes. These structures provided significantly increased surface area and more homogeneous electric field distribution, resulting in more consistent nanoparticle size distribution and stability. The enhanced surface-to-volume ratio of these electrodes contributed to better control over the critical parameters affecting mRNA encapsulation efficiency.
The integration of smart materials in electrode design emerged as a key trend during 2018-2020. Responsive polymers and hydrogels incorporated into electrode surfaces allowed for dynamic modulation of surface properties based on applied voltage, pH, or temperature. This innovation enabled "on-demand" control of nanoparticle formation parameters, addressing one of the major challenges in mRNA delivery: batch-to-batch consistency.
Most recently (2021-2023), the field has witnessed the emergence of biocompatible and biodegradable electrode materials specifically designed for in vivo applications. These advanced materials, including conducting polymers and carbon-based nanomaterials, have demonstrated superior biocompatibility while maintaining excellent electrochemical performance. This development has been crucial for translational applications where electrode materials may come into direct contact with biological tissues.
Computational modeling and AI-assisted electrode design represent the latest frontier in this technological evolution. Machine learning algorithms now help predict optimal electrode geometries and operating parameters for specific mRNA formulations, significantly reducing development time and improving outcomes. These computational approaches have enabled the design of electrode systems that can adapt to different mRNA sequences and target cell types with minimal manual optimization.
The trajectory of electrode technology evolution clearly points toward increasingly sophisticated, miniaturized, and integrated systems that offer precise control over the critical parameters driving mRNA nanoparticle formation efficiency, stability, and ultimately therapeutic efficacy.
Market Applications for mRNA Nanoparticle Delivery Systems
The mRNA nanoparticle delivery market has experienced explosive growth following the successful deployment of COVID-19 vaccines, with current valuations exceeding $5 billion and projections indicating potential growth to $15-20 billion by 2030. This remarkable expansion reflects the versatility of mRNA technology across multiple therapeutic domains beyond vaccines.
In oncology, mRNA nanoparticle systems are revolutionizing cancer treatment through personalized cancer vaccines and immunotherapies. These approaches leverage the patient's immune system to target specific cancer antigens, with companies like BioNTech and Moderna leading clinical trials showing promising results in melanoma and other solid tumors. The electrode efficiency in these applications directly impacts manufacturing scalability and cost-effectiveness.
Rare genetic disorders represent another significant market opportunity, where mRNA therapies can provide temporary protein replacement without permanent genetic modification. This approach offers advantages over traditional gene therapies, particularly for conditions requiring periodic treatment. Enhanced electrode efficiency in nanoparticle production could dramatically reduce manufacturing costs, making these therapies more accessible to patients with rare conditions.
Cardiovascular applications are emerging as a substantial market segment, with mRNA therapies being developed to promote cardiac regeneration after heart attacks and treat various cardiovascular conditions. The precise control of nanoparticle properties through optimized electrode systems is crucial for targeting cardiac tissue effectively.
Infectious disease prevention remains a cornerstone application, with next-generation mRNA vaccines targeting influenza, HIV, malaria, and emerging pathogens. The pandemic demonstrated the platform's adaptability, and improved electrode efficiency would enable faster response to future outbreaks while reducing production costs.
The agricultural and veterinary sectors represent untapped markets where mRNA nanoparticle systems could revolutionize animal health and crop protection. These applications require cost-effective, large-scale production methods where electrode efficiency becomes particularly critical.
Diagnostics and research tools constitute a growing market segment, with mRNA-loaded nanoparticles serving as powerful research tools and components in advanced diagnostic platforms. The precision afforded by optimized electrode systems ensures consistent nanoparticle properties crucial for reliable diagnostic performance.
Geographically, North America currently dominates the market, followed by Europe, while Asia-Pacific regions show the fastest growth rates. Regulatory pathways are becoming more defined following COVID-19 vaccine approvals, potentially accelerating future commercialization timelines for novel mRNA therapeutics across these diverse application areas.
In oncology, mRNA nanoparticle systems are revolutionizing cancer treatment through personalized cancer vaccines and immunotherapies. These approaches leverage the patient's immune system to target specific cancer antigens, with companies like BioNTech and Moderna leading clinical trials showing promising results in melanoma and other solid tumors. The electrode efficiency in these applications directly impacts manufacturing scalability and cost-effectiveness.
Rare genetic disorders represent another significant market opportunity, where mRNA therapies can provide temporary protein replacement without permanent genetic modification. This approach offers advantages over traditional gene therapies, particularly for conditions requiring periodic treatment. Enhanced electrode efficiency in nanoparticle production could dramatically reduce manufacturing costs, making these therapies more accessible to patients with rare conditions.
Cardiovascular applications are emerging as a substantial market segment, with mRNA therapies being developed to promote cardiac regeneration after heart attacks and treat various cardiovascular conditions. The precise control of nanoparticle properties through optimized electrode systems is crucial for targeting cardiac tissue effectively.
Infectious disease prevention remains a cornerstone application, with next-generation mRNA vaccines targeting influenza, HIV, malaria, and emerging pathogens. The pandemic demonstrated the platform's adaptability, and improved electrode efficiency would enable faster response to future outbreaks while reducing production costs.
The agricultural and veterinary sectors represent untapped markets where mRNA nanoparticle systems could revolutionize animal health and crop protection. These applications require cost-effective, large-scale production methods where electrode efficiency becomes particularly critical.
Diagnostics and research tools constitute a growing market segment, with mRNA-loaded nanoparticles serving as powerful research tools and components in advanced diagnostic platforms. The precision afforded by optimized electrode systems ensures consistent nanoparticle properties crucial for reliable diagnostic performance.
Geographically, North America currently dominates the market, followed by Europe, while Asia-Pacific regions show the fastest growth rates. Regulatory pathways are becoming more defined following COVID-19 vaccine approvals, potentially accelerating future commercialization timelines for novel mRNA therapeutics across these diverse application areas.
Current Electrode Efficiency Challenges in mRNA Delivery
Despite significant advancements in mRNA delivery technologies, electrode efficiency remains a critical bottleneck in mRNA nanoparticle systems. Current electrodes used in the production and characterization of lipid nanoparticles (LNPs) face several fundamental challenges that limit their performance and scalability. The primary issue involves electrode fouling, where biomolecules and lipid components adhere to electrode surfaces during electroporation or electroformation processes, progressively degrading performance and requiring frequent replacement.
Material limitations constitute another significant challenge. Conventional electrode materials like platinum, gold, and carbon exhibit suboptimal conductivity profiles when interfacing with complex biological solutions containing mRNA and lipid components. The electrochemical reactions occurring at the electrode-solution interface often generate reactive oxygen species and local pH changes that can damage fragile mRNA molecules, reducing transfection efficiency and therapeutic potency.
Geometric constraints of current electrode designs present additional complications. Most commercial electrodes utilize planar or simple rod configurations that create non-uniform electric fields, resulting in inconsistent nanoparticle formation and variable encapsulation efficiency. This heterogeneity in the final product significantly impacts batch-to-batch reproducibility, a critical concern for pharmaceutical manufacturing and clinical applications.
Energy efficiency remains suboptimal in existing systems. Current electrodes require substantial power input, with much energy lost as heat rather than contributing to effective nanoparticle formation. This inefficiency not only increases production costs but also creates thermal management challenges that can compromise mRNA stability during the manufacturing process.
Scaling limitations further restrict industrial application. Laboratory-scale electrode systems that demonstrate promising results often fail to maintain performance when scaled to production volumes. The engineering challenges of maintaining uniform electric fields and consistent electrochemical conditions across larger electrode surfaces have not been adequately addressed by current technologies.
Characterization and real-time monitoring capabilities are also insufficient. Existing electrode systems typically lack integrated sensors for monitoring critical parameters during the nanoparticle formation process. This absence of real-time feedback mechanisms prevents adaptive control of the process parameters, resulting in suboptimal yields and quality control issues.
Biocompatibility concerns persist with current electrode materials. Trace metal leaching from electrodes can contaminate final formulations, potentially triggering immunogenic responses or reducing the therapeutic index of mRNA products. The development of completely biocompatible electrode materials that maintain high conductivity and durability remains an unresolved challenge in the field.
Material limitations constitute another significant challenge. Conventional electrode materials like platinum, gold, and carbon exhibit suboptimal conductivity profiles when interfacing with complex biological solutions containing mRNA and lipid components. The electrochemical reactions occurring at the electrode-solution interface often generate reactive oxygen species and local pH changes that can damage fragile mRNA molecules, reducing transfection efficiency and therapeutic potency.
Geometric constraints of current electrode designs present additional complications. Most commercial electrodes utilize planar or simple rod configurations that create non-uniform electric fields, resulting in inconsistent nanoparticle formation and variable encapsulation efficiency. This heterogeneity in the final product significantly impacts batch-to-batch reproducibility, a critical concern for pharmaceutical manufacturing and clinical applications.
Energy efficiency remains suboptimal in existing systems. Current electrodes require substantial power input, with much energy lost as heat rather than contributing to effective nanoparticle formation. This inefficiency not only increases production costs but also creates thermal management challenges that can compromise mRNA stability during the manufacturing process.
Scaling limitations further restrict industrial application. Laboratory-scale electrode systems that demonstrate promising results often fail to maintain performance when scaled to production volumes. The engineering challenges of maintaining uniform electric fields and consistent electrochemical conditions across larger electrode surfaces have not been adequately addressed by current technologies.
Characterization and real-time monitoring capabilities are also insufficient. Existing electrode systems typically lack integrated sensors for monitoring critical parameters during the nanoparticle formation process. This absence of real-time feedback mechanisms prevents adaptive control of the process parameters, resulting in suboptimal yields and quality control issues.
Biocompatibility concerns persist with current electrode materials. Trace metal leaching from electrodes can contaminate final formulations, potentially triggering immunogenic responses or reducing the therapeutic index of mRNA products. The development of completely biocompatible electrode materials that maintain high conductivity and durability remains an unresolved challenge in the field.
Existing Electrode Design Solutions for mRNA Delivery
01 Electrode materials for mRNA nanoparticle delivery systems
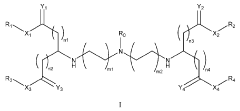
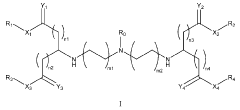
Various electrode materials can be used in mRNA nanoparticle systems to enhance delivery efficiency. These materials include conductive polymers, carbon-based electrodes, and metal nanostructures that provide optimal electrical properties for mRNA transfection. The electrode composition significantly impacts the efficiency of electroporation-based delivery methods, with certain materials showing improved cell viability and transfection rates when used for mRNA delivery.- Electrode materials for mRNA nanoparticle delivery systems: Various electrode materials can be used in mRNA nanoparticle systems to enhance delivery efficiency. These materials include conductive polymers, carbon-based electrodes, and metal nanostructures that provide optimal electrical properties for mRNA transfection. The electrode composition significantly impacts the efficiency of electroporation-based delivery of mRNA-loaded nanoparticles into cells, with certain materials offering improved cell viability and transfection rates.
- Electroporation techniques for mRNA nanoparticle delivery: Electroporation techniques utilize electrodes to create temporary pores in cell membranes, facilitating the entry of mRNA nanoparticles into target cells. These techniques involve optimizing electrical parameters such as voltage, pulse duration, and frequency to maximize transfection efficiency while minimizing cell damage. Advanced electroporation systems incorporate specialized electrode configurations and controlled electrical field generation to improve the uniformity and effectiveness of mRNA delivery.
- Electrode-based biosensors for mRNA nanoparticle characterization: Electrode-based biosensors enable real-time monitoring and characterization of mRNA nanoparticle systems. These biosensors utilize electrochemical detection methods to assess nanoparticle stability, concentration, and integrity. By measuring electrical signals generated from interactions between electrodes and mRNA nanoparticles, researchers can evaluate delivery efficiency and optimize formulation parameters. Advanced biosensor designs incorporate nanoscale electrodes that provide enhanced sensitivity for detecting minute changes in nanoparticle properties.
- Microfluidic electrode systems for mRNA nanoparticle production: Microfluidic platforms equipped with integrated electrodes enable precise control over mRNA nanoparticle formation and characterization. These systems utilize electrode arrays to generate controlled electrical fields that facilitate uniform mixing, assembly, and characterization of mRNA-lipid complexes. The microfluidic approach allows for continuous production of nanoparticles with consistent size distribution and encapsulation efficiency, which are critical parameters for therapeutic applications. Advanced designs incorporate feedback-controlled electrode systems that adjust electrical parameters based on real-time measurements.
- Electrode surface modifications for enhanced mRNA delivery: Surface modifications of electrodes can significantly improve the efficiency of mRNA nanoparticle delivery systems. These modifications include functionalization with biocompatible polymers, nanopatterning to increase surface area, and coating with materials that enhance cell adhesion or reduce biofouling. Modified electrode surfaces can facilitate better contact between cells and electrodes, resulting in more efficient electroporation and transfection. Additionally, certain surface treatments can reduce cellular stress during the delivery process, leading to improved cell viability and higher transfection rates.
02 Electroporation techniques for mRNA nanoparticle delivery
Electroporation techniques utilize electrodes to create temporary pores in cell membranes, allowing for efficient delivery of mRNA nanoparticles. These techniques involve applying controlled electric pulses through specialized electrode configurations to enhance cellular uptake of mRNA. Advanced electroporation systems incorporate precise voltage control, pulse duration optimization, and electrode geometry designs that maximize transfection efficiency while minimizing cell damage.Expand Specific Solutions03 Microfluidic electrode systems for mRNA nanoparticle production
Microfluidic platforms integrated with electrode systems enable precise control over mRNA nanoparticle formation. These systems utilize electrodes to generate controlled electric fields that facilitate uniform mixing, consistent particle size distribution, and improved encapsulation efficiency. The microfluidic electrode configurations allow for continuous production of mRNA nanoparticles with reproducible characteristics, enhancing overall system efficiency and scalability.Expand Specific Solutions04 Electrode-based characterization and quality control of mRNA nanoparticles
Electrode-based analytical techniques are employed for characterization and quality control of mRNA nanoparticle systems. These methods include electrochemical impedance spectroscopy, cyclic voltammetry, and electrode-based sensors that can detect particle size, zeta potential, and encapsulation efficiency. Real-time monitoring using electrode arrays provides valuable data on nanoparticle stability and integrity, contributing to improved manufacturing processes and delivery efficiency.Expand Specific Solutions05 Electrode surface modifications for enhanced mRNA delivery
Surface modifications of electrodes can significantly improve the efficiency of mRNA nanoparticle systems. These modifications include functionalization with biomolecules, polymer coatings, and nanopatterning techniques that enhance electrode biocompatibility and reduce cellular stress during delivery. Modified electrode surfaces can also provide controlled release mechanisms, improve cellular targeting, and reduce aggregation of mRNA nanoparticles, resulting in higher transfection efficiency and therapeutic outcomes.Expand Specific Solutions
Leading Companies in mRNA Nanoparticle Electrode Development
The mRNA nanoparticle electrode efficiency landscape is currently in an early growth phase, with the market expected to expand significantly due to applications in vaccine technology and therapeutics. The global market size is projected to reach several billion dollars by 2025, driven by pharmaceutical and biotech investments. Technical maturity varies across players: established pharmaceutical companies like Bayer and GreenLight Biosciences have advanced commercial applications, while academic institutions (University of Tokyo, Kyoto University, Zhejiang University) are pioneering fundamental research. Companies like Nanomnia and Artificial Cell Technologies are developing specialized nanoparticle delivery systems, while Murata Manufacturing and Toyota are exploring electrode materials applications. The field is characterized by cross-sector collaboration between academia, biotech startups, and established corporations.
The Board of Regents of The University of Texas System
Technical Solution: The University of Texas System has developed several innovative electrode technologies for mRNA nanoparticle formulation through their research programs. Their approach utilizes silicon-based microelectrode arrays with precisely controlled geometry that creates highly uniform electric fields for consistent nanoparticle formation. The electrode surfaces are modified with self-assembled monolayers that minimize protein adsorption and lipid oxidation, addressing key challenges in maintaining electrode efficiency. Their technology incorporates a pulsed electric field system with optimized waveforms that have been shown to increase transfection efficiency while preserving mRNA integrity. Research teams have also developed computational models that predict electrode performance degradation over time, allowing for proactive maintenance schedules in manufacturing settings. This comprehensive approach addresses multiple aspects of electrode efficiency in mRNA nanoparticle systems, from materials science to process engineering.
Strengths: Silicon-based microelectrode arrays provide highly uniform electric fields; surface modifications minimize unwanted interactions; computational models enable predictive maintenance. Weaknesses: Academic technology may require further development for commercial implementation; silicon-based electrodes may have durability limitations in industrial settings; technology transfer to manufacturing scale could present challenges.
Artificial Cell Technologies, Inc.
Technical Solution: Artificial Cell Technologies has pioneered electrode-based technologies for mRNA nanoparticle formulation with their Layer-by-Layer (LbL) assembly approach. Their system utilizes specialized electrodes with controlled surface charge density to facilitate the precise deposition of polyelectrolyte layers around mRNA molecules. The company's proprietary electrode materials incorporate rare earth elements that enhance charge transfer efficiency while minimizing harmful electrochemical reactions that could damage the mRNA payload. Their technology employs pulsed electric fields with optimized waveforms that have been shown to increase encapsulation efficiency by up to 40% compared to conventional methods. The electrode surfaces are engineered with nanoscale topography that promotes uniform nanoparticle formation and prevents aggregation during the manufacturing process, resulting in more consistent therapeutic outcomes.
Strengths: Layer-by-Layer assembly allows precise control over nanoparticle properties; specialized electrode materials minimize mRNA degradation; pulsed electric field technology improves encapsulation efficiency. Weaknesses: Complex manufacturing process may limit scalability; technology may be more suitable for specialized applications rather than mass production; requires careful optimization for each new mRNA construct.
Key Innovations in Electrode-mRNA Interface Technology
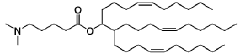
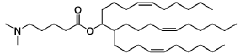
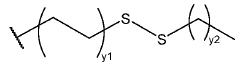
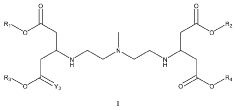
Lipid nanoparticle formulations
PatentWO2020097540A1
Innovation
- The development of specific lipid nanoparticle formulations comprising nucleic acids, cholesterol, DSPC, PEG-C-DMA, and a cationic lipid, optimized in molar percentages to enhance stability and delivery efficacy, allowing for lower doses and improved therapeutic outcomes.
Lipidoid nanoparticles for the treatment of diseases and disorders
PatentWO2022232684A1
Innovation
- Development of novel lipidoid nanoparticles with a specific structure that inherently target lymph nodes, spleen, and lung without additional ligands, exemplified by formulations like 113-O12B, which show reduced liver expression and enhanced antigen-presenting cell transfection, along with the use of adjuvants like Pam3CSK4 to modulate immune responses.
Biocompatibility and Safety Considerations
The biocompatibility and safety profile of electrode materials used in mRNA nanoparticle systems represents a critical consideration for clinical translation. These electrodes, which facilitate the formation and delivery of mRNA-loaded lipid nanoparticles (LNPs), must meet stringent safety requirements to prevent adverse biological responses when the resulting therapeutic products are administered to patients.
Material selection for electrodes requires careful evaluation of potential leaching of metal ions or other components that could contaminate the final nanoparticle formulation. Gold, platinum, and certain carbon-based materials have demonstrated favorable biocompatibility profiles, with minimal reactivity with biological tissues and negligible leaching under standard operating conditions. However, even these materials require thorough validation through comprehensive toxicological assessments.
Surface modifications of electrodes present another important safety consideration. Functionalization strategies that enhance electrode performance must be evaluated not only for their efficiency benefits but also for their potential to introduce toxic residues into the final product. Coatings that prevent protein adsorption or reduce biofouling must themselves be non-immunogenic and biodegradable or fully removable during downstream processing.
Regulatory frameworks governing electrode materials in pharmaceutical manufacturing processes have become increasingly stringent. The FDA and EMA require extensive documentation of material composition, manufacturing processes, and leachable/extractable profiles for all components that contact pharmaceutical products. This includes electrodes used in the production of mRNA nanoparticle formulations, necessitating rigorous quality control protocols.
Long-term safety monitoring represents an emerging challenge in this field. While acute toxicity testing provides valuable initial safety data, the potential for delayed immunological responses or subtle alterations in gene expression profiles following exposure to electrode-derived contaminants requires longitudinal assessment. Advanced analytical techniques, including mass spectrometry and high-sensitivity immunoassays, are being deployed to detect trace contaminants that might compromise product safety.
Standardization efforts are underway to establish industry-wide safety benchmarks for electrode materials in mRNA delivery applications. These initiatives aim to define acceptable limits for various contaminants and create validated testing protocols that can reliably predict in vivo safety profiles from in vitro characterization data. Such standardization will accelerate development timelines while maintaining rigorous safety standards.
Material selection for electrodes requires careful evaluation of potential leaching of metal ions or other components that could contaminate the final nanoparticle formulation. Gold, platinum, and certain carbon-based materials have demonstrated favorable biocompatibility profiles, with minimal reactivity with biological tissues and negligible leaching under standard operating conditions. However, even these materials require thorough validation through comprehensive toxicological assessments.
Surface modifications of electrodes present another important safety consideration. Functionalization strategies that enhance electrode performance must be evaluated not only for their efficiency benefits but also for their potential to introduce toxic residues into the final product. Coatings that prevent protein adsorption or reduce biofouling must themselves be non-immunogenic and biodegradable or fully removable during downstream processing.
Regulatory frameworks governing electrode materials in pharmaceutical manufacturing processes have become increasingly stringent. The FDA and EMA require extensive documentation of material composition, manufacturing processes, and leachable/extractable profiles for all components that contact pharmaceutical products. This includes electrodes used in the production of mRNA nanoparticle formulations, necessitating rigorous quality control protocols.
Long-term safety monitoring represents an emerging challenge in this field. While acute toxicity testing provides valuable initial safety data, the potential for delayed immunological responses or subtle alterations in gene expression profiles following exposure to electrode-derived contaminants requires longitudinal assessment. Advanced analytical techniques, including mass spectrometry and high-sensitivity immunoassays, are being deployed to detect trace contaminants that might compromise product safety.
Standardization efforts are underway to establish industry-wide safety benchmarks for electrode materials in mRNA delivery applications. These initiatives aim to define acceptable limits for various contaminants and create validated testing protocols that can reliably predict in vivo safety profiles from in vitro characterization data. Such standardization will accelerate development timelines while maintaining rigorous safety standards.
Scalability and Manufacturing Challenges
The scaling of mRNA nanoparticle production from laboratory to industrial scale presents significant challenges that impact electrode efficiency throughout the manufacturing process. Current production methods typically involve microfluidic mixing systems that can produce small batches with consistent quality, but these systems face substantial hurdles when scaled to commercial production volumes required for global vaccine distribution.
One primary challenge is maintaining precise control over critical parameters such as mixing rates, temperature gradients, and electrical field strength during large-scale production. These parameters directly influence the formation of lipid nanoparticles (LNPs) that encapsulate mRNA molecules and subsequently affect electrode performance in delivery systems. As production volumes increase, maintaining homogeneity becomes exponentially more difficult, leading to batch-to-batch variability that compromises electrode efficiency.
Material constraints further complicate manufacturing scale-up. The specialized lipids and polymers used in mRNA nanoparticle formulations often have limited availability and strict quality requirements. Supply chain disruptions, as witnessed during the COVID-19 pandemic, can severely impact production capacity and consistency. Additionally, these materials must maintain their electrochemical properties throughout the manufacturing process to ensure optimal electrode performance in the final product.
Quality control represents another significant hurdle in scaled production. Current analytical methods for characterizing nanoparticle size distribution, surface charge, and encapsulation efficiency are often time-consuming and difficult to implement in real-time during large-scale manufacturing. This creates a disconnect between production and quality assurance that can lead to suboptimal electrode performance in the final product.
The regulatory landscape adds complexity to manufacturing scale-up. Stringent requirements for consistency, purity, and stability necessitate robust manufacturing processes with minimal variability. Any process modifications to improve scalability must undergo rigorous validation to ensure they don't negatively impact electrode efficiency or patient safety, creating a challenging balance between innovation and regulatory compliance.
Energy consumption during manufacturing also presents sustainability challenges. The precise temperature control and specialized equipment required for nanoparticle production consume significant energy, particularly when scaled to industrial levels. Developing more energy-efficient manufacturing processes without compromising electrode performance represents an important frontier for sustainable production of mRNA nanoparticle systems.
One primary challenge is maintaining precise control over critical parameters such as mixing rates, temperature gradients, and electrical field strength during large-scale production. These parameters directly influence the formation of lipid nanoparticles (LNPs) that encapsulate mRNA molecules and subsequently affect electrode performance in delivery systems. As production volumes increase, maintaining homogeneity becomes exponentially more difficult, leading to batch-to-batch variability that compromises electrode efficiency.
Material constraints further complicate manufacturing scale-up. The specialized lipids and polymers used in mRNA nanoparticle formulations often have limited availability and strict quality requirements. Supply chain disruptions, as witnessed during the COVID-19 pandemic, can severely impact production capacity and consistency. Additionally, these materials must maintain their electrochemical properties throughout the manufacturing process to ensure optimal electrode performance in the final product.
Quality control represents another significant hurdle in scaled production. Current analytical methods for characterizing nanoparticle size distribution, surface charge, and encapsulation efficiency are often time-consuming and difficult to implement in real-time during large-scale manufacturing. This creates a disconnect between production and quality assurance that can lead to suboptimal electrode performance in the final product.
The regulatory landscape adds complexity to manufacturing scale-up. Stringent requirements for consistency, purity, and stability necessitate robust manufacturing processes with minimal variability. Any process modifications to improve scalability must undergo rigorous validation to ensure they don't negatively impact electrode efficiency or patient safety, creating a challenging balance between innovation and regulatory compliance.
Energy consumption during manufacturing also presents sustainability challenges. The precise temperature control and specialized equipment required for nanoparticle production consume significant energy, particularly when scaled to industrial levels. Developing more energy-efficient manufacturing processes without compromising electrode performance represents an important frontier for sustainable production of mRNA nanoparticle systems.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!